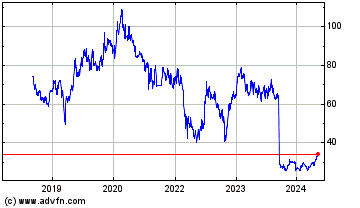
Prosus NV (EU:PRX)
Historical Stock Chart
From Mar 2020 to Mar 2025