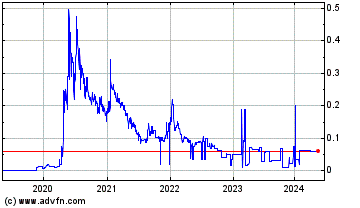
Medmira (PK) (USOTC:MMIRF)
Historical Stock Chart
From Mar 2020 to Mar 2025