
We could not find any results for:
Make sure your spelling is correct or try broadening your search.
| Share Name | Share Symbol | Market | Type |
|---|---|---|---|
| AbbVie Inc | NYSE:ABBV | NYSE | Common Stock |
| Price Change | % Change | Share Price | High Price | Low Price | Open Price | Shares Traded | Last Trade | |
|---|---|---|---|---|---|---|---|---|
| 2.98 | 1.85% | 163.79 | 164.25 | 160.74 | 161.85 | 5,499,576 | 21:31:23 |
MONTREAL, Dec. 6, 2019 /CNW/ - AbbVie (NYSE: ABBV), a global, research and development-based biopharmaceutical company, announced an agreement was reached with the pan-Canadian Pharmaceutical Alliance (pCPA) regarding SKYRIZI™ (risankizumab) for the treatment of moderate to severe plaque psoriasis in patients who are candidates for systemic therapy or phototherapy.
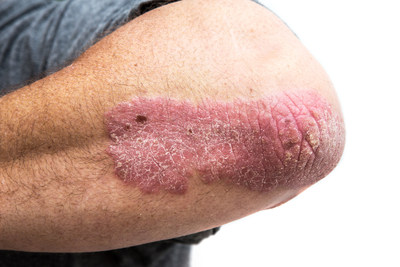
"Although the introduction of biologics has improved treatment outcomes, many patients continue to live with their needs unmet. Patients strive for therapies that provide predictable and durable full skin clearance with convenient dosing. Ultimately, they want a therapy that improves their quality of life,'' Dr. Kim Papp, Probity Medical Research Inc. ''It is great news to know that more Canadians will have access to a new treatment option."
SKYRIZI™ received Health Canada approval in April 2019 based on results from four pivotal Phase 3 studies, ultIMMa-1, ultIMMa-2, IMMvent and IMMhance evaluating more than 2,000 patients with moderate to severe plaque psoriasis.4 SKYRIZI™ is part of a collaboration between Boehringer Ingelheim and AbbVie, with AbbVie leading development and commercialization globally.
Canadians living with moderate to severe plaque psoriasis were well represented in all four of the pivotal clinical trials leading to Health Canada's approval, showing the Canadian leadership in this clinical development program. In clinical studies, SKYRIZI™ significantly improved levels of skin clearance after just 16 weeks and maintained clearance at one year (52 weeks).4
"We are committed to continuing to improve the lives of those living with psoriasis where there is still much to be done," explains Stéphane Lassignardie, Vice-President and General Manager, AbbVie Canada. "We are extremely proud that SKYRIZI™ is the first IL-23 inhibitor to reach an agreement with the pCPA. This is a great step forward for Canadians to obtain access to this innovative therapy."
About AbbVie Care
The AbbVie Care program is designed to provide a wide range of customized services including reimbursement and financial support, pharmacy services, lab work reminders and coordination, personalized education and ongoing disease management support throughout the treatment journey. For more information, consult www.abbviecare.ca.
About AbbVie
AbbVie is a global, research and development-based biopharmaceutical company committed to developing innovative advanced therapies for some of the world's most complex and critical conditions. The company's mission is to use its expertise, dedicated people and unique approach to innovation to markedly improve treatments across four primary therapeutic areas: immunology, oncology, virology and neuroscience. In more than 75 countries, AbbVie employees are working every day to advance health solutions for people around the world. For more information about AbbVie, please visit us at www.abbvie.ca and www.abbvie.com. Follow @abbvieCanada and @abbvie on Twitter or view careers on our Facebook or LinkedIn page.
References: | |
1. | International Federation of Psoriasis Associations. Available at: https://ifpa-pso.com/wp-content/uploads/2017/01/Brochure-Psoriasis-is-a-serious-disease-deserving-global-attention.pdf. Accessed March 22, 2019. |
2. | Mroweitz, U., et al. Definition of treatment goals for moderate to severe psoriasis: a European consensus. Arch Dermatol Res. 2011 Jan; 303(1): 1–10. |
3. | Levin, et al. Biologic fatigue in psoriasis. J Dermatolog Treat. 2014 Feb;25(1):78-82. doi: 10.3109/09546634.2013.826341. |
4. | SKYRIZI™ (risankizumab) [Canadian Product Monograph]. AbbVie Corporation, 2019. |
SOURCE AbbVie

Copyright 2019 Canada NewsWire
1 Year AbbVie Chart |
1 Month AbbVie Chart |

It looks like you are not logged in. Click the button below to log in and keep track of your recent history.
Support: +44 (0) 203 8794 460 | support@advfn.com
By accessing the services available at ADVFN you are agreeing to be bound by ADVFN's Terms & Conditions