
We could not find any results for:
Make sure your spelling is correct or try broadening your search.
| Share Name | Share Symbol | Market | Type |
|---|---|---|---|
| NeuroBo Pharmaceuticals Inc | NASDAQ:NRBO | NASDAQ | Common Stock |
| Price Change | % Change | Share Price | Bid Price | Offer Price | High Price | Low Price | Open Price | Shares Traded | Last Trade | |
|---|---|---|---|---|---|---|---|---|---|---|
| -0.05 | -1.04% | 4.78 | 4.74 | 4.97 | 5.30 | 4.65 | 4.83 | 150,687 | 00:00:05 |
DA-1726 Also Exhibited Superior Glucose Lowering Compared to Survodutide
Lipid-Lowering Effect of DA-1726 Shown to be Superior Compared to Tirzepatide
Data Presented at the ADA 84th Scientific Sessions
CAMBRIDGE, Mass., June 22, 2024 /PRNewswire/ -- NeuroBo Pharmaceuticals, Inc. (Nasdaq: NRBO), a clinical-stage biotechnology company focused on the transformation of cardiometabolic diseases, today announced pre-clinical data which indicates that DA-1726, a novel, dual oxyntomodulin (OXM) analog agonist that functions as a glucagon-like peptide-1 receptor (GLP1R) and glucagon receptor (GCGR), demonstrated superiority in weight loss, retention of lean body mass, and lipid-lowering effects compared to survodutide, in pre-clinical models. Tae-Hyoung Kim, Lead research scientist, Dong-A ST Research Center, will present the data today, in a poster at the American Diabetes Association (ADA) 84th Scientific Sessions, taking place June 21-24, in Orlando, Florida.
"The data being presented at the ADA further differentiates DA-1726 from obesity drugs in the same class, potentially due to its GLP-1 and glucagon receptor activity ratio," stated Hyung Heon Kim, President and Chief Executive Officer of NeuroBo. "DA-1726, in obese mouse models, significantly lowered cholesterol levels and induced superior weight loss, compared to survodutide, a drug with the same mechanism of action, while also exhibiting superior glucose lowering. Most notably, DA-1726 demonstrated superior weight loss and retention of relative lean body mass preservation compared to survodutide. Further, as previously disclosed pre-clinical studies showed, DA-1726 resulted in similar weight reduction while consuming more food compared to tirzepatide and this additional data, being presented at the ADA, in a hypercholesterolemia rat model, confirmed that DA-1726 is more effective than tirzepatide in suppressing cholesterol levels, due to its glucagon action, alongside its GLP-1 effect, while also inhibiting weight gain. We believe these distinguishing factors can potentially position DA-1726 as a best-in-class obesity drug with superior efficacy and better tolerability profile. The Phase 1 trial of DA-1726 is progressing well, and we expect to both dose the first patient in the multiple ascending dose (MAD) Part 2 and read-out top-line data from the single ascending dose (SAD) Part 1 in the third quarter of this year, with top-line data from the MAD Part 2 expected in the first quarter of 2025."
DA-1726 is currently in a Phase 1 clinical trial. This study focuses on the pharmacological effects of this novel oxyntomodulin analogue, which has been effective at improving lipid profiles and reducing weight in rodent models. In an obese mouse model, DA-1726 showed superior weight loss compared to survodutide (-24.7%, -18.2%; P<0.05 vs. control) while demonstrating superior body fat mass reduction and relative lean body mass preservation versus survodutide (body fat change -31.4%, -15.1% vs. -8.7% control). DA-1726 also significantly lowered T-CHO (-67.7%, -49.6%; P<0.05 vs. control) and TG (-49.5%, -41.2%; P<0.05 vs. control) while showing superior glucose lowering compared to survodutide (-54.7%, -30.4% vs. control; P<0.05). Interestingly, despite the same mechanism of action, DA-1726-treated mice showed superior weight loss, fat mass reduction and glucose lowering efficacy. This effect might stem from DA-1726's GLP-1 and glucagon receptor activity ratio. DA-1726's glucagon action could further enhance energy expenditure (EE), and it is believed to have significantly increased the expression of EE-related genes in brown adipose tissue.
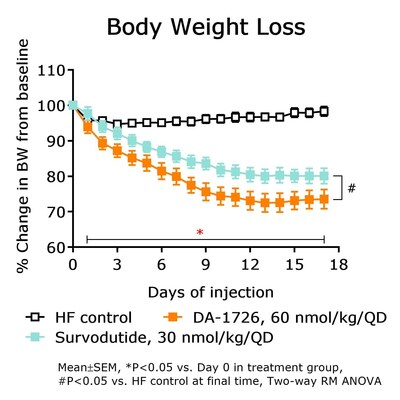
![DA-1726 [W0], DA-1726 [W2], Survodutide [W0], Survodutide [W2] DA-1726 [W0], DA-1726 [W2], Survodutide [W0], Survodutide [W2]](https://mma.prnewswire.com/media/2445098/pie_chart.jpg)
In a prior study, DA-1726 showed a difference in improving lipid levels, despite similar weight loss to tirzepatide. Therefore, the direct lipid-regulating effect of DA-1726 was assessed in a hypercholesterolemia rat model compared with tirzepatide. As a result, DA-1726 was more effective than tirzepatide in suppressing the elevation of T-CHO (-33.5%, -25.5% vs. control; P<0.05) and LDL-C (-53.2%, -41.5% vs. control; P<0.05) despite the two groups of rats consuming the same amount of food. This differentiated impact is thought to arise from DA-1726's glucagon action, alongside its GLP-1 effect. The study evaluated whether these differential effects could also be distinguished from drugs of the same class.
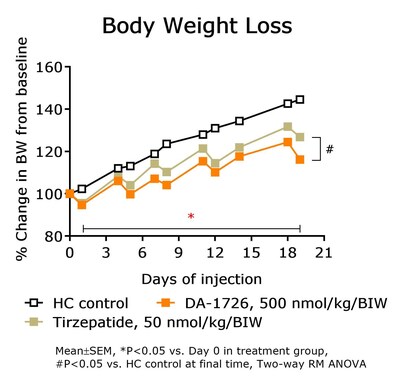
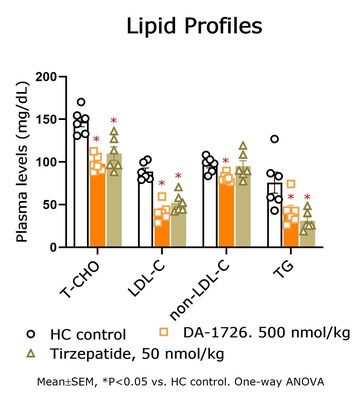
In summary, it was confirmed that DA-1726 has differentiating characteristics in the competition of obesity treatment drugs with similar or identical mechanisms in its effect on improving cholesterol metabolism through glucagon action.
After the presentations, the poster will be accessible within the "Posters" section of NeuroBo's website at: https://www.neurobopharma.com/posters.
About DA-1726
DA-1726 is a novel oxyntomodulin (OXM) analogue functioning as a GLP1R/GCGR dual agonist for the treatment of obesity and Metabolic Dysfunction-Associated Steatohepatitis (MASH) that is to be administered once weekly subcutaneously. DA-1726 acts as a dual agonist of GLP-1 receptors (GLP1R) and glucagon receptors (GCGR), leading to weight loss through reduced appetite and increased energy expenditure. DA-1726 has a well understood mechanism and, in pre-clinical mice models, resulted in improved weight loss compared to semaglutide and cotadutide (another OXM analogue). Additionally, in pre-clinical mouse models, DA-1726 elicited similar weight reduction, while consuming more food, compared to tirzepatide and survodutide, while also preserving lean body mass and demonstrating lipid-lowering effects compared to survodutide.
About NeuroBo Pharmaceuticals
NeuroBo Pharmaceuticals, Inc. is a clinical-stage biotechnology company focused on transforming cardiometabolic diseases. The company is currently developing DA-1241 for the treatment of Metabolic Dysfunction-Associated Steatohepatitis (MASH) and is developing DA-1726 for the treatment of obesity. DA-1241 is a novel G-protein-coupled receptor 119 (GPR119) agonist that promotes the release of key gut peptides GLP-1, GIP, and PYY. In pre-clinical studies, DA-1241 demonstrated a positive effect on liver inflammation, lipid metabolism, weight loss, and glucose metabolism, reducing hepatic steatosis, hepatic inflammation, and liver fibrosis, while also improving glucose control. DA-1726 is a novel oxyntomodulin (OXM) analogue that functions as a glucagon-like peptide-1 receptor (GLP1R) and glucagon receptor (GCGR) dual agonist. OXM is a naturally-occurring gut hormone that activates GLP1R and GCGR, thereby decreasing food intake while increasing energy expenditure, thus potentially resulting in superior body weight loss compared to selective GLP1R agonists.
For more information, please visit www.neurobopharma.com.
Forward Looking Statements
Certain statements in this press release may be considered forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. Words such as "believes", "expects", "anticipates", "may", "will", "should", "seeks", "approximately", "intends", "projects", "plans", "estimates" or the negative of these words or other comparable terminology (as well as other words or expressions referencing future events, conditions or circumstances) are intended to identify forward-looking statements. Forward-looking statements are predictions, projections and other statements about future events that are based on current expectations and assumptions and, as a result, are subject to risks and uncertainties. Many factors could cause actual future events to differ materially from the forward-looking statements in this press release, including, without limitation, those risks associated with NeuroBo's ability to execute on its commercial strategy; the timeline for regulatory submissions; the ability to obtain regulatory approval through the development steps of NeuroBo's current and future product candidates, the ability to realize the benefits of the license agreement with Dong-A ST Co. Ltd., including the impact on future financial and operating results of NeuroBo; the cooperation of NeuroBo's contract manufacturers, clinical study partners and others involved in the development of NeuroBo's current and future product candidates; potential negative interactions between NeuroBo's product candidates and any other products with which they are combined for treatment; NeuroBo's ability to initiate and complete clinical trials on a timely basis; NeuroBo's ability to recruit subjects for its clinical trials; whether NeuroBo receives results from NeuroBo's clinical trials that are consistent with the results of pre-clinical and previous clinical trials; impact of costs related to the license agreement, known and unknown, including costs of any litigation or regulatory actions relating to the license agreement; the effects of changes in applicable laws or regulations; the effects of changes to NeuroBo's stock price on the terms of the license agreement and any future fundraising; and other risks and uncertainties described in NeuroBo's filings with the Securities Exchange Commission, including NeuroBo's most recent Annual Report on Form 10-K. Forward-looking statements speak only as of the date when made. NeuroBo does not assume any obligation to publicly update or revise any forward-looking statements, whether as a result of new information, future events or otherwise, except as required by law.
Contacts:
NeuroBo Pharmaceuticals
Marshall H. Woodworth
Chief Financial Officer
+1-857-299-1033
marshall.woodworth@neurobopharma.com
Rx Communications Group
Michael Miller
+1-917-633-6086
mmiller@rxir.com
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/neurobo-pharmaceuticals-da-1726-demonstrated-superiority-in-weight-loss-retention-of-lean-body-mass-and-lipid-lowering-effects-compared-to-survodutide-in-pre-clinical-models-302179186.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/neurobo-pharmaceuticals-da-1726-demonstrated-superiority-in-weight-loss-retention-of-lean-body-mass-and-lipid-lowering-effects-compared-to-survodutide-in-pre-clinical-models-302179186.html
SOURCE NeuroBo Pharmaceuticals, Inc.

Copyright 2024 PR Newswire
1 Year NeuroBo Pharmaceuticals Chart |
1 Month NeuroBo Pharmaceuticals Chart |

It looks like you are not logged in. Click the button below to log in and keep track of your recent history.
Support: +44 (0) 203 8794 460 | support@advfn.com
By accessing the services available at ADVFN you are agreeing to be bound by ADVFN's Terms & Conditions