
We could not find any results for:
Make sure your spelling is correct or try broadening your search.
| Share Name | Share Symbol | Market | Type |
|---|---|---|---|
| Lyra Therapeutics Inc | NASDAQ:LYRA | NASDAQ | Common Stock |
| Price Change | % Change | Share Price | Bid Price | Offer Price | High Price | Low Price | Open Price | Shares Traded | Last Trade | |
|---|---|---|---|---|---|---|---|---|---|---|
| -0.0069 | -4.01% | 0.1651 | 0.1651 | 0.1652 | 0.18 | 0.163 | 0.1761 | 993,726 | 16:23:21 |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): |
(Exact name of Registrant as Specified in Its Charter)
(State or Other Jurisdiction |
(Commission File Number) |
(IRS Employer |
||
|
|
|
|
|
|
||||
|
||||
(Address of Principal Executive Offices) |
|
(Zip Code) |
||
Registrant’s Telephone Number, Including Area Code: |
|
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
Securities registered pursuant to Section 12(b) of the Act:
|
|
Trading |
|
|
|
|
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act.
Item 2.02 Results of Operations and Financial Condition.
On November 12, 2024, Lyra Therapeutics, Inc. (the “Company”) announced its financial results for the quarter ended September 30, 2024. The full text of the press release issued in connection with the announcement is furnished as Exhibit 99.1 to this Current Report on Form 8-K.
The information contained in Item 2.02 of this Current Report on Form 8-K (including Exhibit 99.1 attached hereto) shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference in any filing under the Securities Act of 1933, as amended, or the Exchange Act, except as expressly provided by specific reference in such a filing.
Item 9.01 Financial Statements and Exhibits.
(d) Exhibits
The following exhibit relating to Item 2.02 of this Current Report on Form 8-K shall be deemed to be furnished, and not filed:
Exhibit No. |
|
Description |
|
|
|
99.1 |
|
|
104 |
|
Cover Page Interactive Data File (embedded within the Inline XBRL document). |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
|
|
|
Lyra Therapeutics, Inc. |
|
|
|
|
Date: |
November 12, 2024 |
By: |
/s/ Jason Cavalier |
|
|
|
Jason Cavalier, Chief Financial Officer |
Lyra Therapeutics Reports Third Quarter 2024 Financial Results and Provides Corporate Update
WATERTOWN, Mass., November 12, 2024 – Lyra Therapeutics, Inc. (Nasdaq: LYRA) (“Lyra” or the “Company”), a clinical-stage biotechnology company developing long-acting, anti-inflammatory sinonasal implants for the treatment of chronic rhinosinusitis (CRS), today reported its financial results for the third quarter ended September 30, 2024 and provided a corporate update.
“We look forward to key milestones in the coming months from the two ongoing ENLIGHTEN Phase 3 trials that will provide us with a more complete data set and greater insight into determining a potential pathway to approval for LYR-210 in CRS patients with and without nasal polyps. The topline 52-week safety data from the ENLIGHTEN 1 safety extension study was in-line with the primary treatment phase, with no product-related serious adverse events, including for those patients that received a repeat dose, resulting in a 12-month treatment period. We are anticipating additional data from the ENLIGHTEN 1 safety extension study in the coming months, which will be presented at an upcoming medical conference, as well as topline results from the ENLIGHTEN 2 pivotal trial expected in Q2 2025,” said Maria Palasis, Ph.D., President and CEO of Lyra Therapeutics.
Dr. Palasis continued, “We eagerly await the upcoming data readouts, and they will guide us in making data-driven evaluations as we determine the potential path for LYR-210 to add value for CRS patients, investors and other stakeholders.”
The ENLIGHTEN program consists of two pivotal Phase 3 clinical trials, ENLIGHTEN 1 and ENLIGHTEN 2, to evaluate the efficacy and safety of LYR-210 for the treatment of CRS. Each ENLIGHTEN trial has enrolled approximately 180 CRS patients who have failed medical management and have not had prior ethmoid sinus surgery, randomized 2:1 to either LYR-210 (7500µg mometasone furoate) or sham control for 24 weeks.
Topline Results from the ENLIGHTEN 1 52-week extension study
Today, Lyra reported topline 52-week safety data from the ENLIGHTEN 1 safety extension study:
Clinical Program Highlights
Enrollment in ENLIGHTEN 2 completed
Milestones for Ongoing ENLIGHTEN Pivotal Program of LYR-210 in CRS
Third Quarter 2024 Financial Highlights
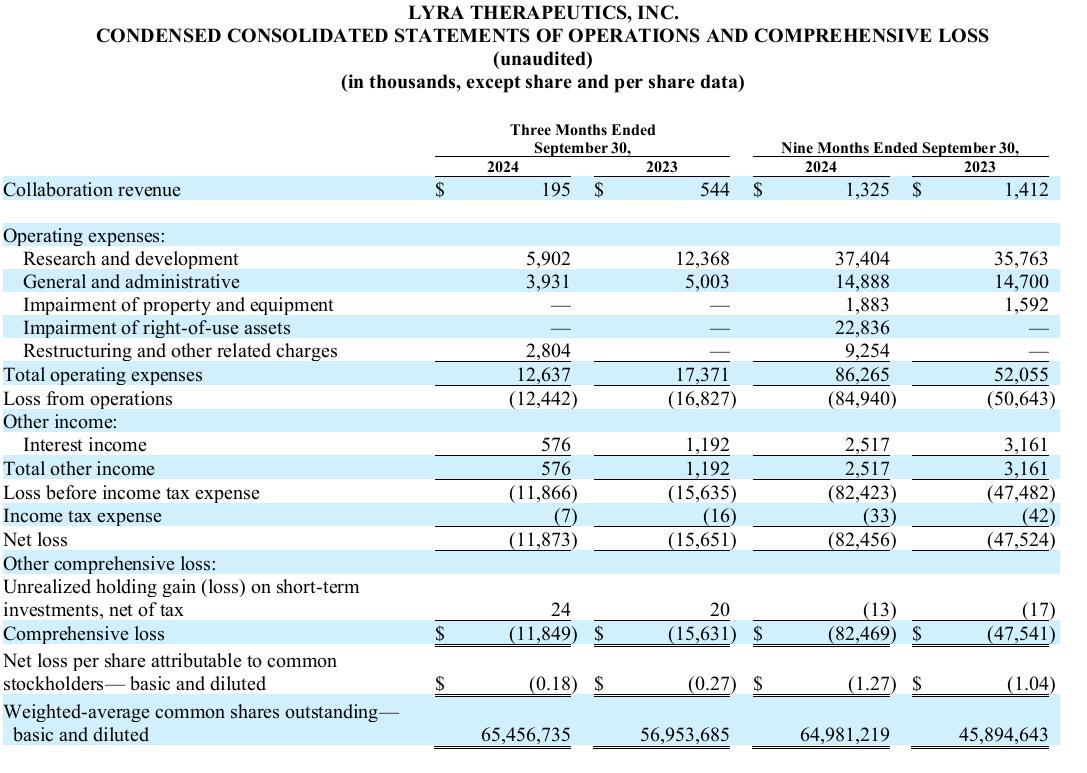
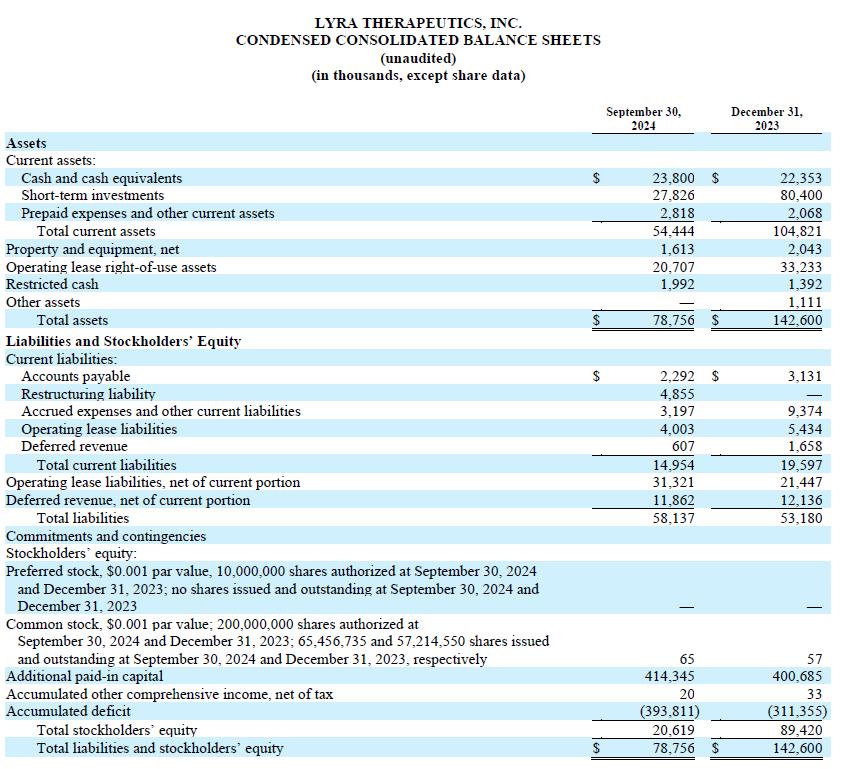
Cash, cash equivalents and short-term investments as of September 30, 2024 were $51.6 million, compared with $67.5 million at June 30, 2024. Based on our current business plan, we anticipate that our cash, cash equivalents and short-term investment balance is sufficient to fund our operating expenses and capital expenditures into the first quarter of 2026.
Research and development expenses for the quarter ended September 30, 2024 were $5.9 million compared to $12.4 million for the same period in 2023, representing a decrease of $6.5 million. The decrease in research and development expenses for the three months ended September 30, 2024 was primarily attributable to a $3.8 million decrease in clinical related costs as we completed both the BEACON trial for LYR-220 and the primary study phase of the ENLIGHTEN 1 trial for LYR-210, a decrease of $2.5 million in employee related costs primarily driven by the reduction in force which occurred in May 2024, a decrease in professional and consulting costs of $0.4 million and a decrease in product development and manufacturing costs of $0.4 million. This decrease in costs was partially offset by an increase in allocated costs and depreciation of $0.6 million.
General and administrative expenses for the quarter ended September 30, 2024 were $3.9 million compared to $5.0 million for the same period in 2023, representing a decrease of $1.1 million. The decrease in general and administrative expenses for the three months ended September 30, 2024 was primarily driven by a decrease in professional and consulting fees of $1.0 million as we scaled back activities subsequent to announcing in May 2024 that the ENLIGHTEN 1 trial did not meet its primary endpoint, in addition to a decrease in employee related costs of $0.5 million primarily due to the reduction in force which occurred in May 2024. These cost decreases were partially offset by an increase in allocation and support costs of $0.4 million primarily due to the increased rent and facilities expenses for the Company’s three leased facilities for the three months ended September 30, 2024 compared to the three months ended September 30, 2023.
Net loss for the quarter ended September 30, 2024 was $11.9 million compared to $15.7 million for the same period in 2023.
About LYR-210
LYR-210 is an investigational product candidate for the treatment of chronic rhinosinusitis (CRS) in patients who have failed current therapies and require further intervention. LYR-210 is a bioresorbable nasal implant designed to be inserted in a simple, in-office procedure. LYR-210 is intended to deliver six months of continuous anti-inflammatory therapy, mometasone furoate, to the sinonasal passages to treat CRS. LYR-210 is being evaluated in the ENLIGHTEN pivotal Phase 3 clinical program.
About Lyra Therapeutics
Lyra Therapeutics, Inc. is a clinical-stage biotechnology company developing long‑acting, anti-inflammatory sinonasal implants for the treatment of chronic rhinosinusitis (CRS). Lyra Therapeutics is developing therapies for CRS, a highly prevalent inflammatory disease of the paranasal sinuses which leads to debilitating symptoms and significant morbidities. LYR-210, the company’s lead product, is a bioabsorbable nasal implant designed to be administered in a simple, in‑office procedure and is intended to deliver six months of continuous anti‑inflammatory drug therapy (7500µg mometasone
furoate) to the sinonasal passages for the treatment of CRS with a single administration. LYR-210, being evaluated in the ENLIGHTEN Phase 3 clinical program, is intended for patients with and without nasal polyps. The company’s therapies are intended to treat the estimated four million CRS patients in the United States who fail medical management each year. For more information, please visit www.lyratx.com and follow us on LinkedIn.
Forward-Looking Statements
This press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. All statements contained in this press release that do not relate to matters of historical fact should be considered forward-looking statements, including statements regarding whether LYR-210 could potentially benefit patients with CRS, the completion of the Company’s ENLIGHTEN 2 Phase 3 clinical trial, and the timing of the release of topline data from the ENLIGHTEN 2 Phase 3 clinical trial. These statements are neither promises nor guarantees, but involve known and unknown risks, uncertainties and other important factors that may cause the Company's actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements. These and other important factors discussed under the caption "Risk Factors" in the Company's Quarterly Report on Form 10-Q filed with the SEC on November 12, 2024 and its other filings with the SEC could cause actual results to differ materially from those indicated by the forward-looking statements made in this press release. Any such forward-looking statements represent management's estimates as of the date of this press release. While the Company may elect to update such forward-looking statements at some point in the future, it disclaims any obligation to do so, even if subsequent events cause its views to change.
Contact Information:
Jason Cavalier, Chief Financial Officer
917.584.7668
jcavalier@lyratx.com
Media Contact:
Kathryn Morris, The Yates Network LLC
914.204.6412
kathryn@theyatesnetwork.com
Document And Entity Information |
Nov. 12, 2024 |
|---|---|
| Cover [Abstract] | |
| Document Type | 8-K |
| Amendment Flag | false |
| Document Period End Date | Nov. 12, 2024 |
| Entity Registrant Name | Lyra Therapeutics, Inc. |
| Entity Central Index Key | 0001327273 |
| Entity Emerging Growth Company | true |
| Entity File Number | 001-39273 |
| Entity Incorporation, State or Country Code | DE |
| Entity Tax Identification Number | 84-1700838 |
| Entity Address, Address Line One | 480 Arsenal Way |
| Entity Address, City or Town | Watertown |
| Entity Address, State or Province | MA |
| Entity Address, Postal Zip Code | 02472 |
| City Area Code | 617 |
| Local Phone Number | 393-4600 |
| Written Communications | false |
| Soliciting Material | false |
| Pre-commencement Tender Offer | false |
| Pre-commencement Issuer Tender Offer | false |
| Entity Ex Transition Period | false |
| Title of 12(b) Security | Common Stock, $0.001 par value per share |
| Trading Symbol | LYRA |
| Security Exchange Name | NASDAQ |
1 Year Lyra Therapeutics Chart |
1 Month Lyra Therapeutics Chart |

It looks like you are not logged in. Click the button below to log in and keep track of your recent history.
Support: +44 (0) 203 8794 460 | support@advfn.com
By accessing the services available at ADVFN you are agreeing to be bound by ADVFN's Terms & Conditions