
We could not find any results for:
Make sure your spelling is correct or try broadening your search.
| Share Name | Share Symbol | Market | Type |
|---|---|---|---|
| Homology Medicines Inc | NASDAQ:FIXX | NASDAQ | Common Stock |
| Price Change | % Change | Share Price | Bid Price | Offer Price | High Price | Low Price | Open Price | Shares Traded | Last Trade | |
|---|---|---|---|---|---|---|---|---|---|---|
| 0.00 | 0.00% | 0.9347 | 0.8911 | 0.9293 | 0 | 00:00:00 |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported):
(Exact name of registrant as specified in its charter)
| (State or other jurisdiction of incorporation or organization) |
(Commission File Number) |
(I.R.S. Employer Identification No.) | ||
| |
||||
| (Address of principal executive offices) | (Zip Code) | |||
(Registrant’s telephone number, including area code)
Not Applicable
(Former name or former address, if changed since last report.)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class |
Trading |
Name of each exchange on which registered | ||
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act.
| Item 7.01. | Regulation FD Disclosure. |
On October 10, 2023, Homology Medicines, Inc. (the “Company”) posted a corporate slide presentation in the “Investor Relations” portion of its website at investors.homologymedicines.com. A copy of its current corporate slide presentation is attached to this Current Report on Form 8-K as Exhibit 99.1. The Company undertakes no obligation to update, supplement or amend the materials attached hereto as Exhibit 99.1.
The information contained in Item 7.01 of this Form 8-K (including Exhibit 99.1 attached hereto) shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference in any filing under the Securities Act of 1933, as amended, or the Exchange Act, except as expressly provided by specific reference in such a filing.
| Item 9.01 | Financial Statements and Exhibits. |
(d) Exhibits
The following Exhibit 99.1 relating to Item 7.01 shall be deemed to be furnished, and not filed:
| Exhibit No. |
Description | |
| 99.1 | Corporate Slide Presentation of Homology Medicines, Inc. dated October 10, 2023. | |
| 104 | Cover Page Interactive Data File (embedded within the Inline XBRL document). | |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| HOMOLOGY MEDICINES, INC. | ||||||
| Date: October 10, 2023 | By: | /s/ Paul Alloway | ||||
| Paul Alloway | ||||||
| Chief Legal Officer and Secretary | ||||||

Corporate Presentation October 2023 Exhibit 99.1

© Copyright 2023 Homology Medicines, Inc. All rights reserved. Forward-Looking Statements This presentation contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. We intend such forward-looking statements to be covered by the safe harbor provisions for forward-looking statements contained in Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. All statements contained in this presentation that do not relate to matters of historical fact should be considered forward-looking statements, including, without limitation, statements regarding: our expectations surrounding the potential, safety, efficacy, and regulatory and clinical progress of our product candidates; the potential of our gene therapy and gene editing platforms, including our GTx-mAb platform; our plans and timing for the release of additional preclinical and clinical data; our plans to progress our pipeline of genetic medicine candidates and the anticipated timing for these milestones; our expectations surrounding our relationship with Oxford Biomedica Solutions; our competitive position, business strategy, prospective products, timing, design, results and likelihood of success of studies and/or clinical trials; our position as a leader in the development of genetic medicines; and our plans to engage in future collaborations and strategic partnerships. The words “believe,” “may,” “will,” “estimate,” “potential,” “continue,” “anticipate,” “intend,” “expect,” “could,” “would,” “project,” “plan,” “target,” and similar expressions are intended to identify forward-looking statements, though not all forward-looking statements use these words or expressions. These statements are neither promises nor guarantees, but involve known and unknown risks, uncertainties and other important factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements, including, but not limited to, the following: the impact of the COVID-19 pandemic on our business and operations, including our preclinical studies and clinical trials, and on general economic conditions; we have and expect to continue to incur significant losses; our need for additional funding, which may not be available; failure to identify additional product candidates and develop or commercialize marketable products; the early stage of our development efforts; potential unforeseen events during clinical trials could cause delays or other adverse consequences; risks relating to the regulatory approval process; interim, topline and preliminary data may change as more patient data become available, and are subject to audit and verification procedures that could result in material changes in the final data; our product candidates may cause serious adverse side effects; inability to maintain our collaborations, or the failure of these collaborations; our reliance on third parties, including for the manufacture of materials for our research programs, preclinical and clinical studies; failure to obtain U.S. or international marketing approval; ongoing regulatory obligations; effects of significant competition; unfavorable pricing regulations, third-party reimbursement practices or healthcare reform initiatives; product liability lawsuits; securities class action litigation; failure to attract, retain and motivate qualified personnel; the possibility of system failures or security breaches; risks relating to intellectual property; and significant costs incurred as a result of operating as a public company. These and other important factors discussed under the caption “Risk Factors” in our Quarterly Report on Form 10-Q for the quarter ended June 30, 2023 and our other filings with the Securities and Exchange Commission could cause actual results to differ materially from those indicated by the forward-looking statements made in this presentation. Any such forward-looking statements represent management’s estimates as of the date of this presentation. While we may elect to update such forward-looking statements at some point in the future, we disclaim any obligation to do so, even if subsequent events cause our views to change. This presentation also includes statistical and other industry and market data that we obtained from industry publications and research, surveys and studies conducted by third parties or us. Industry publications and third-party research, surveys and studies generally indicate that their information has been obtained from sources believed to be reliable, although they do not guarantee the accuracy or completeness of such information. All of the market data used in this presentation involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. While we believe these industry publications and third-party research, surveys and studies are reliable, we have not independently verified such data. The industry in which we operate is subject to a high degree of uncertainty, change and risk due to a variety of factors, which could cause results to differ materially from those expressed in the estimates made by the independent parties and by us.

© Copyright 2023 Homology Medicines, Inc. All rights reserved. Clinical-Stage Fully Integrated Gene Therapy and Gene Editing Company Rare Disease Experience Team’s prior experience includes developing and/or launching 11 rare disease drugs with >$2B in annual revenue Technology 15 novel AAVHSCs; potential to expand Equity investments from Pfizer and Novartis Extensive I.P. portfolio AAV Process Development and Manufacturing Expertise Co-owned Manufacturing and Innovation Business, Oxford Biomedica Solutions Research and Development 3 programs advanced to the clinic since 2019 Discovery, Research & Development 5 development candidates a
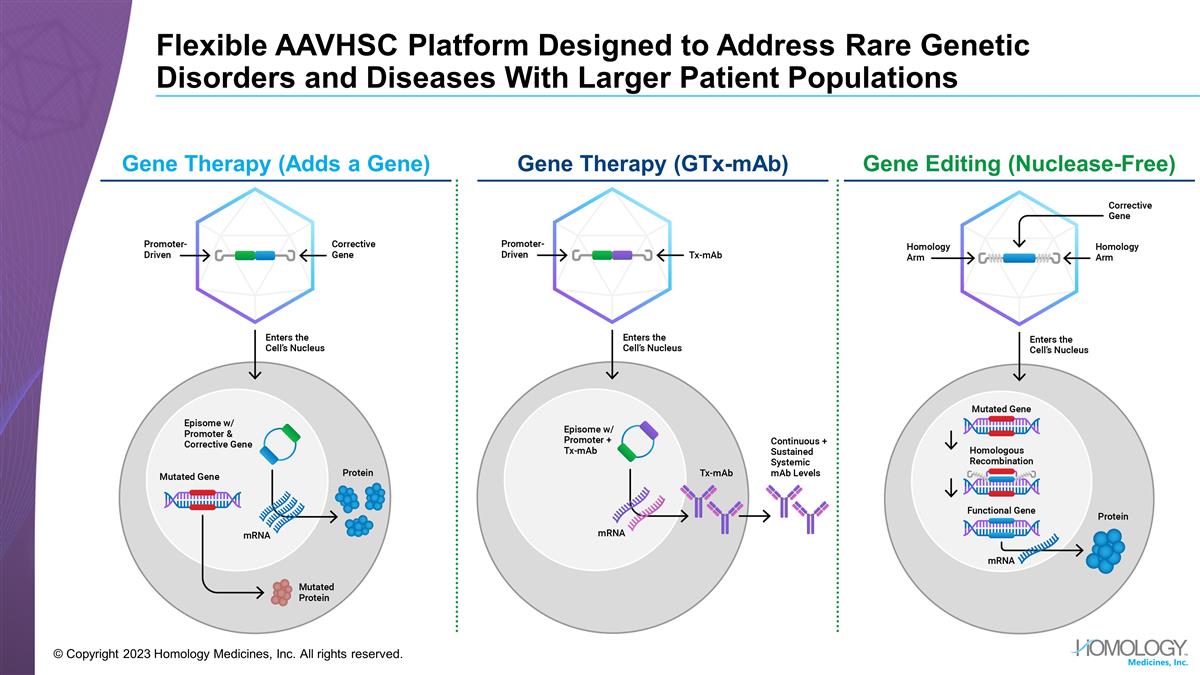
Flexible AAVHSC Platform Designed to Address Rare Genetic Disorders and Diseases With Larger Patient Populations Gene Therapy (Adds a Gene) Gene Therapy (GTx-mAb) Gene Editing (Nuclease-Free) © Copyright 2023 Homology Medicines, Inc. All rights reserved.

Gene Editing Candidate HMI-103 for PKU
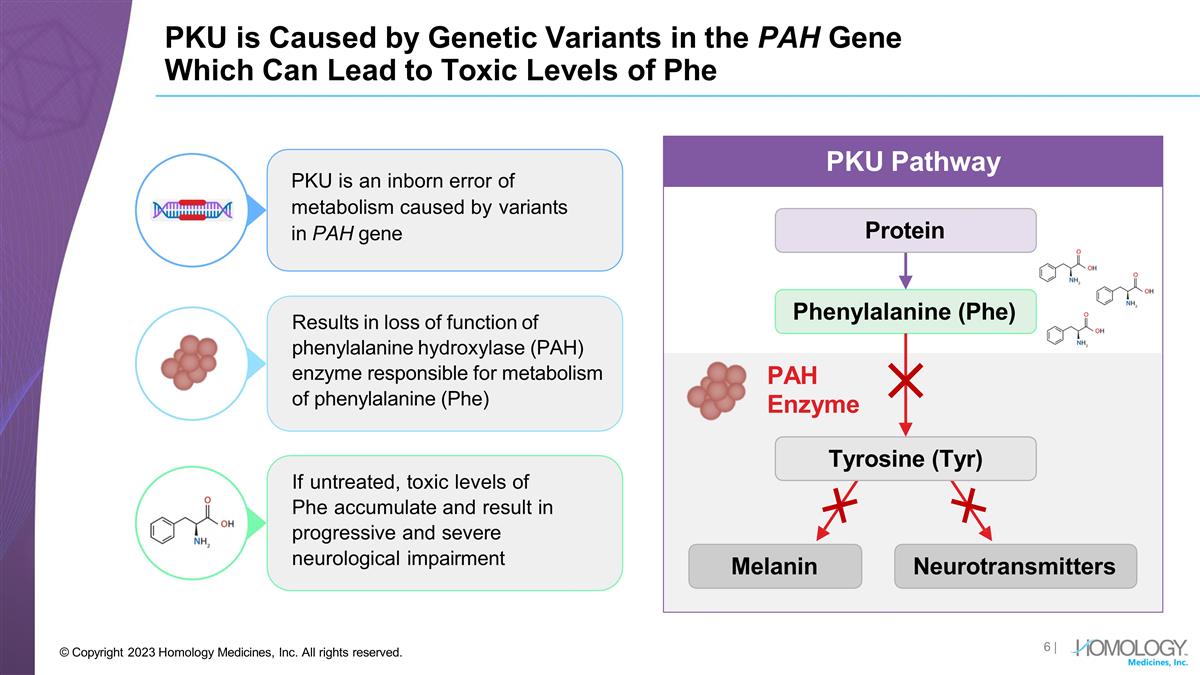
Results in loss of function of phenylalanine hydroxylase (PAH) enzyme responsible for metabolism of phenylalanine (Phe) If untreated, toxic levels of Phe accumulate and result in progressive and severe neurological impairment PKU is an inborn error of metabolism caused by variants in PAH gene PKU is Caused by Genetic Variants in the PAH Gene Which Can Lead to Toxic Levels of Phe Protein Phenylalanine (Phe) PAH Enzyme Melanin Neurotransmitters PKU Pathway Tyrosine (Tyr) | © Copyright 2023 Homology Medicines, Inc. All rights reserved.
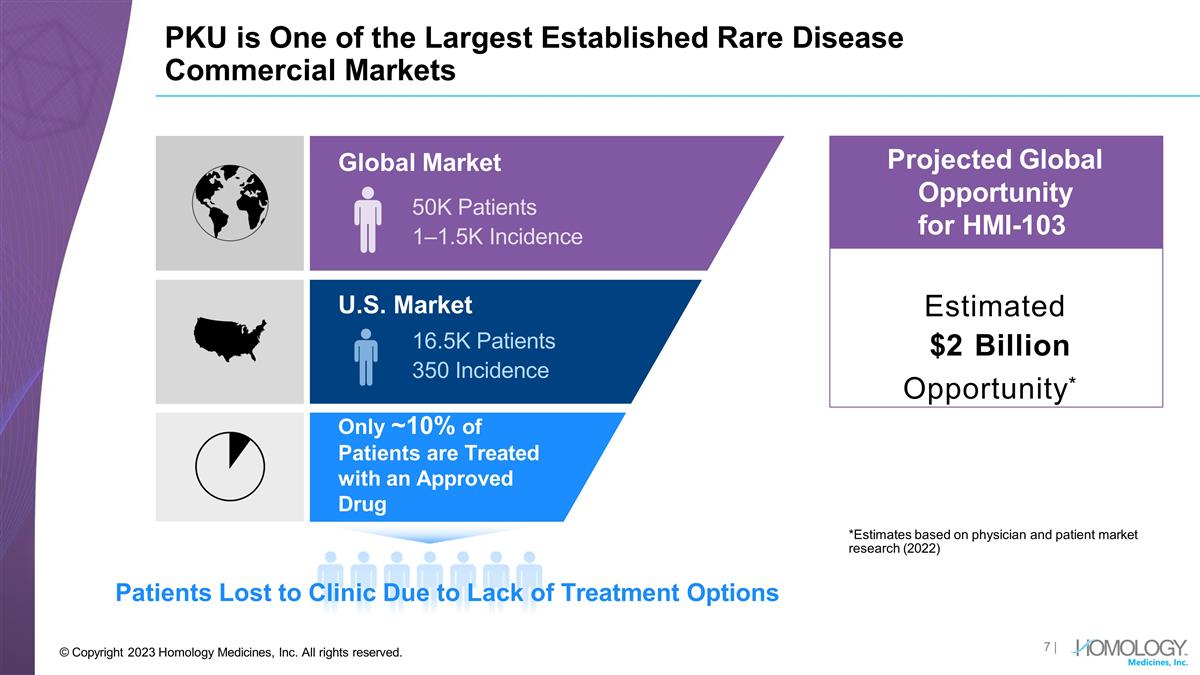
PKU is One of the Largest Established Rare Disease Commercial Markets Patients Lost to Clinic Due to Lack of Treatment Options Estimated $2 Billion Opportunity* Projected Global Opportunity for HMI-103 Global Market 50K Patients 1–1.5K Incidence U.S. Market 16.5K Patients 350 Incidence Only ~10% of Patients are Treated with an Approved Drug *Estimates based on physician and patient market research (2022) | © Copyright 2023 Homology Medicines, Inc. All rights reserved.
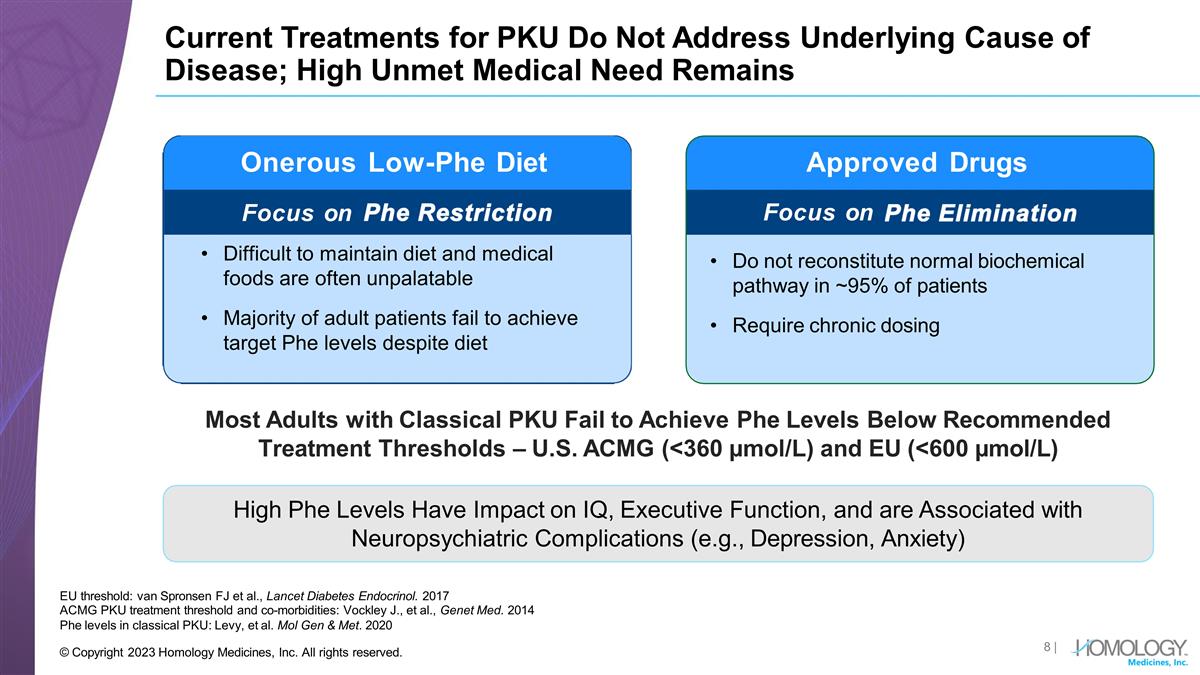
Current Treatments for PKU Do Not Address Underlying Cause of Disease; High Unmet Medical Need Remains EU threshold: van Spronsen FJ et al., Lancet Diabetes Endocrinol. 2017 ACMG PKU treatment threshold and co-morbidities: Vockley J., et al., Genet Med. 2014 Phe levels in classical PKU: Levy, et al. Mol Gen & Met. 2020 Approved Drugs Generally ineffective Does not reconstitute normal biochemical pathway Very poor compliance Do not reconstitute normal biochemical pathway in ~95% of patients Require chronic dosing Onerous Low-Phe Diet Focus on Most Adults with Classical PKU Fail to Achieve Phe Levels Below Recommended Treatment Thresholds – U.S. ACMG (<360 µmol/L) and EU (<600 µmol/L) High Phe Levels Have Impact on IQ, Executive Function, and are Associated with Neuropsychiatric Complications (e.g., Depression, Anxiety) Focus on | © Copyright 2023 Homology Medicines, Inc. All rights reserved. Difficult to maintain diet and medical foods are often unpalatable Majority of adult patients fail to achieve target Phe levels despite diet
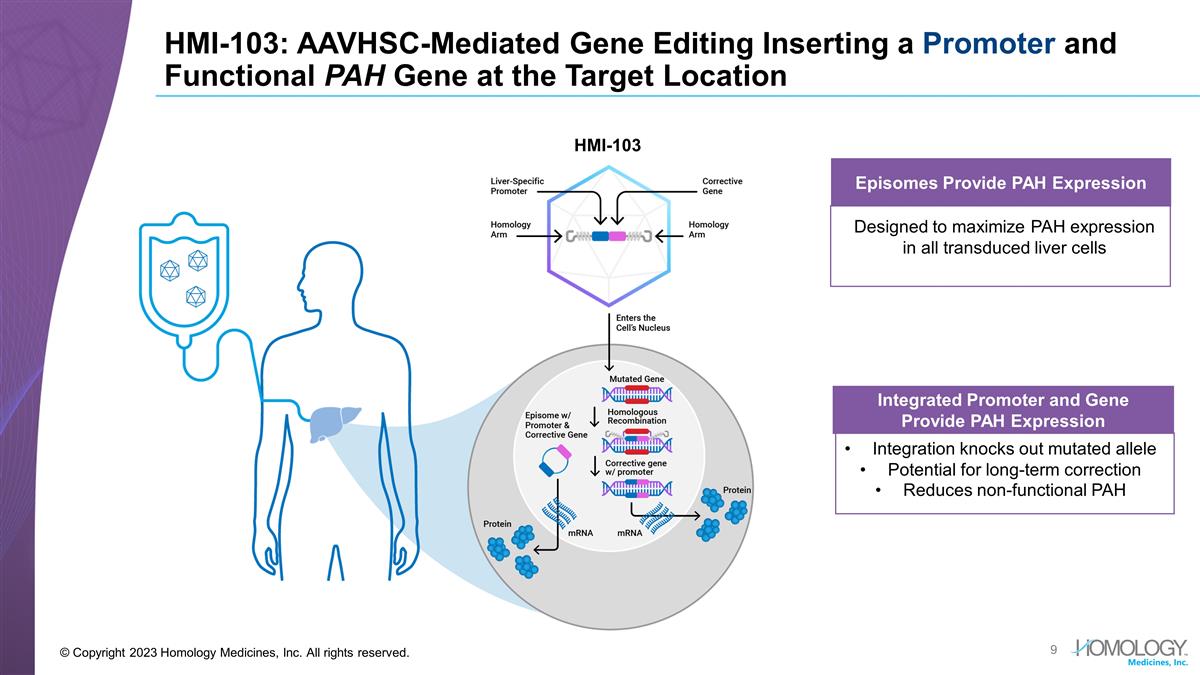
HMI-103: AAVHSC-Mediated Gene Editing Inserting a Promoter and Functional PAH Gene at the Target Location © Copyright 2023 Homology Medicines, Inc. All rights reserved. Integrated Promoter and Gene Provide PAH Expression Integration knocks out mutated allele Potential for long-term correction Reduces non-functional PAH Episomes Provide PAH Expression Designed to maximize PAH expression in all transduced liver cells HMI-103
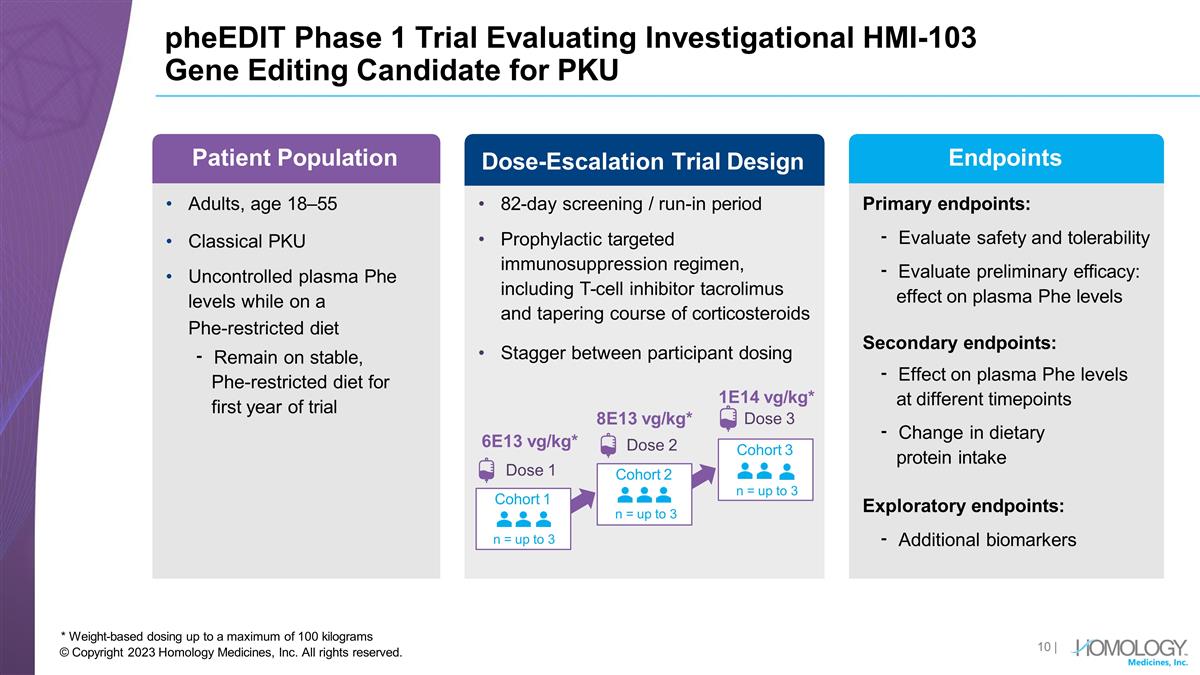
pheEDIT Phase 1 Trial Evaluating Investigational HMI-103 Gene Editing Candidate for PKU * Weight-based dosing up to a maximum of 100 kilograms Adults, age 18–55 Classical PKU Uncontrolled plasma Phe levels while on a Phe-restricted diet ⁃ Remain on stable, Phe-restricted diet for first year of trial Primary endpoints: ⁃ Evaluate safety and tolerability ⁃ Evaluate preliminary efficacy: effect on plasma Phe levels Secondary endpoints: ⁃ Effect on plasma Phe levels at different timepoints ⁃ Change in dietary protein intake Exploratory endpoints: ⁃ Additional biomarkers 82-day screening / run-in period Prophylactic targeted immunosuppression regimen, including T-cell inhibitor tacrolimus and tapering course of corticosteroids Stagger between participant dosing Cohort 1 n = up to 3 Cohort 2 n = up to 3 Cohort 3 n = up to 3 6E13 vg/kg* Dose 1 Patient Population Dose-Escalation Trial Design 8E13 vg/kg* Dose 2 1E14 vg/kg* Dose 3 Endpoints | © Copyright 2023 Homology Medicines, Inc. All rights reserved.
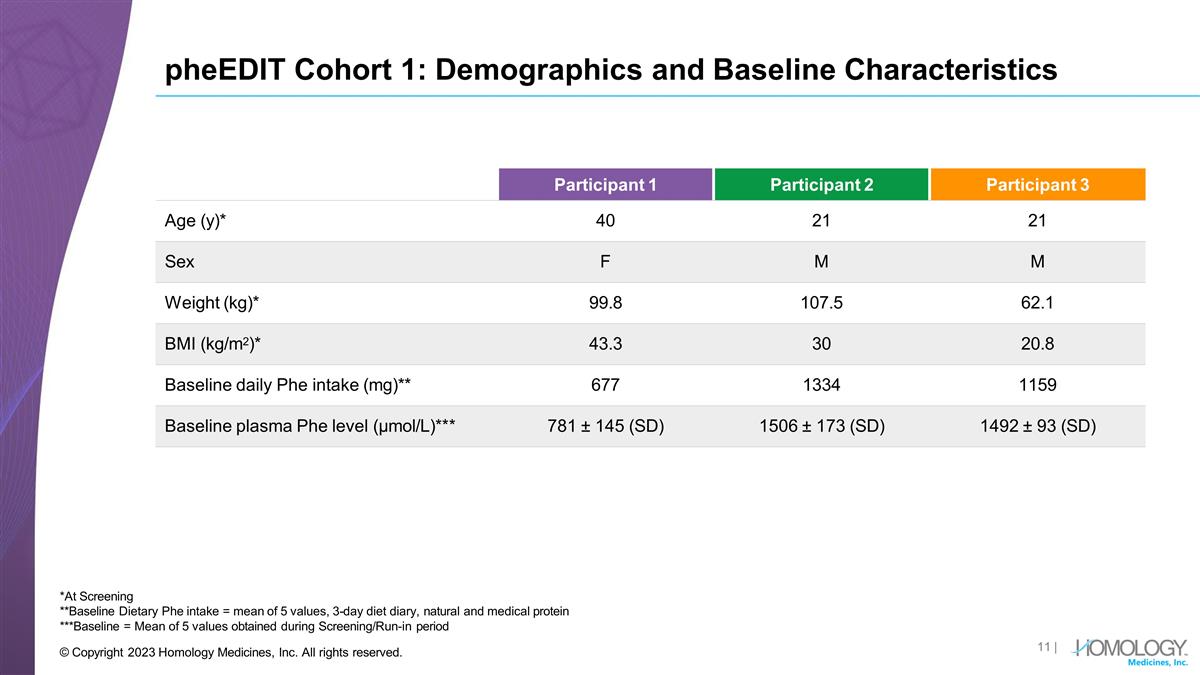
pheEDIT Cohort 1: Demographics and Baseline Characteristics | © Copyright 2023 Homology Medicines, Inc. All rights reserved. *At Screening **Baseline Dietary Phe intake = mean of 5 values, 3-day diet diary, natural and medical protein ***Baseline = Mean of 5 values obtained during Screening/Run-in period Participant 1 Participant 2 Participant 3 Age (y)* 40 21 21 Sex F M M Weight (kg)* 99.8 107.5 62.1 BMI (kg/m2)* 43.3 30 20.8 Baseline daily Phe intake (mg)** 677 1334 1159 Baseline plasma Phe level (µmol/L)*** 781 ± 145 (SD) 1506 ± 173 (SD) 1492 ± 93 (SD)
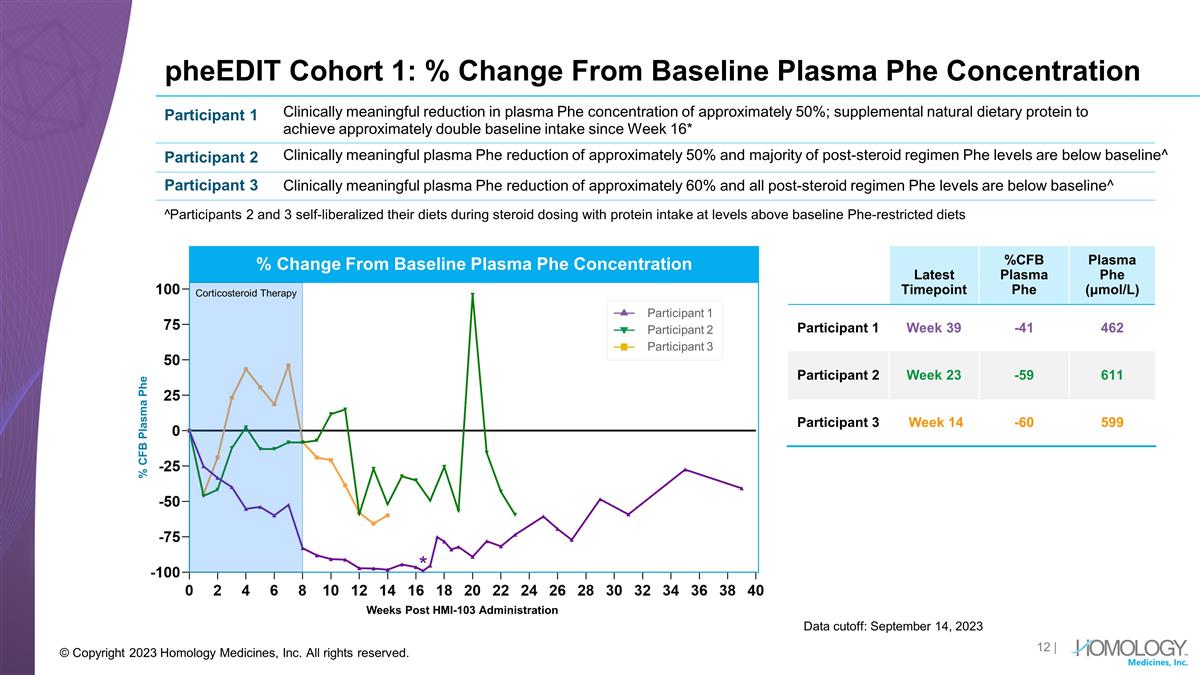
pheEDIT Cohort 1: % Change From Baseline Plasma Phe Concentration Data cutoff: September 14, 2023 © Copyright 2023 Homology Medicines, Inc. All rights reserved. Latest Timepoint %CFB Plasma Phe Plasma Phe (μmol/L) Participant 1 Week 39 -41 462 Participant 2 Week 23 -59 611 Participant 3 Week 14 -60 599 Participant 1 Participant 2 Participant 3 Clinically meaningful reduction in plasma Phe concentration of approximately 50%; supplemental natural dietary protein to achieve approximately double baseline intake since Week 16* Clinically meaningful plasma Phe reduction of approximately 50% and majority of post-steroid regimen Phe levels are below baseline^ Clinically meaningful plasma Phe reduction of approximately 60% and all post-steroid regimen Phe levels are below baseline^ ^Participants 2 and 3 self-liberalized their diets during steroid dosing with protein intake at levels above baseline Phe-restricted diets Weeks Post HMI-103 Administration Participant 1 Participant 2 Participant 3 % Change From Baseline Plasma Phe Concentration % CFB Plasma Phe Corticosteroid Therapy
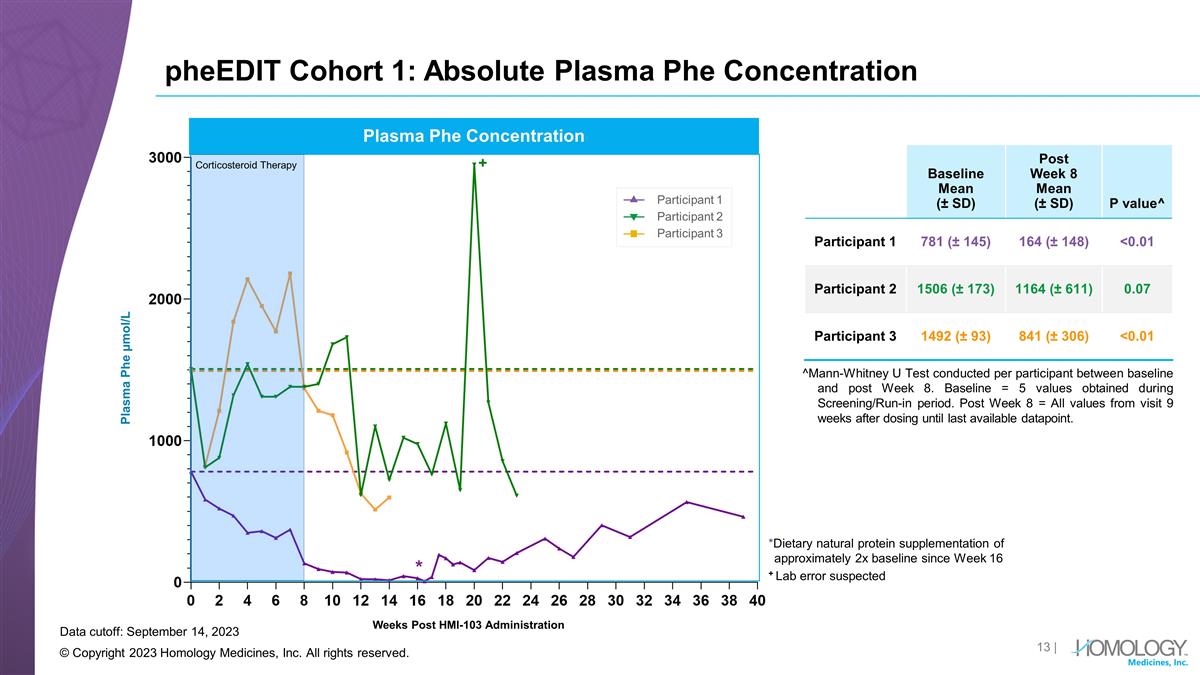
pheEDIT Cohort 1: Absolute Plasma Phe Concentration © Copyright 2023 Homology Medicines, Inc. All rights reserved. Baseline Mean (± SD) Post Week 8 Mean (± SD) P value^ Participant 1 781 (± 145) 164 (± 148) <0.01 Participant 2 1506 (± 173) 1164 (± 611) 0.07 Participant 3 1492 (± 93) 841 (± 306) <0.01 Weeks Post HMI-103 Administration Participant 1 Participant 2 Participant 3 Plasma Phe Concentration Plasma Phe µmol/L Corticosteroid Therapy Data cutoff: September 14, 2023 ^Mann-Whitney U Test conducted per participant between baseline and post Week 8. Baseline = 5 values obtained during Screening/Run-in period. Post Week 8 = All values from visit 9 weeks after dosing until last available datapoint. *Dietary natural protein supplementation of approximately 2x baseline since Week 16 + Lab error suspected +
pheEDIT Cohort 1: Safety Data Observations LFT = liver function tests HMI-103 has been generally well-tolerated in all three participants No serious adverse events (SAEs) The majority of treatment-related adverse events (AEs) have been mild and transient No LFT abnormalities while on prophylactic, targeted immunosuppression regimen of T-cell inhibitor tacrolimus and corticosteroids Data cut-off date: Sept 14, 2023 © Copyright 2023 Homology Medicines, Inc. All rights reserved. |
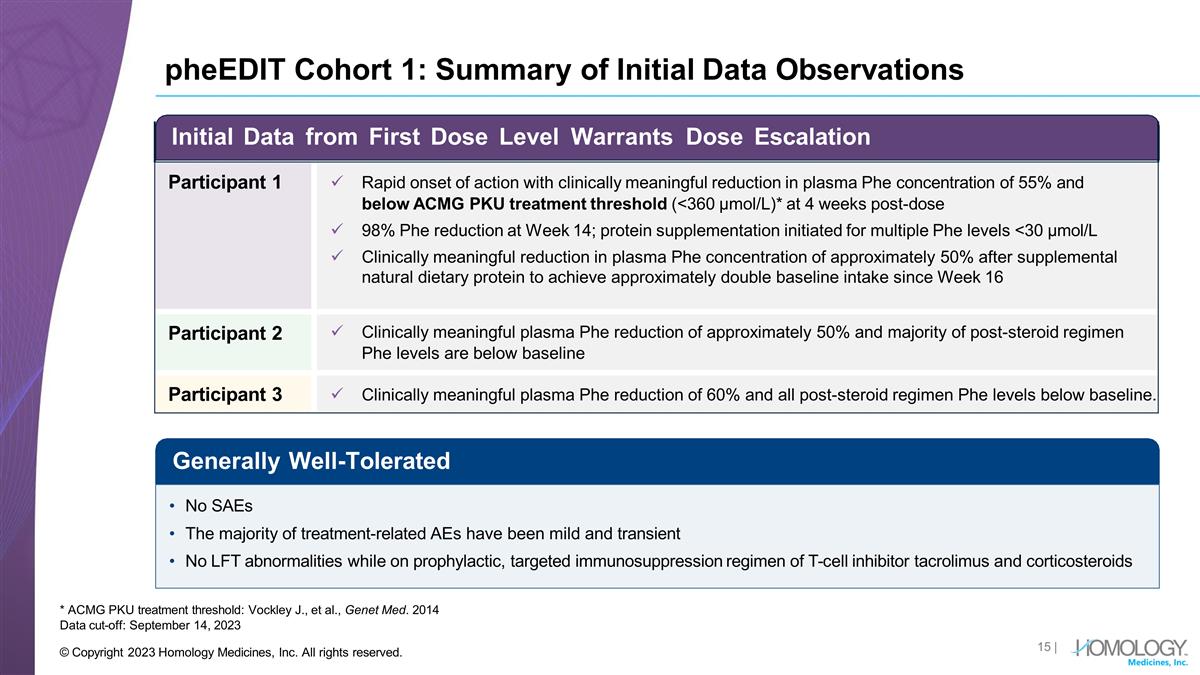
pheEDIT Cohort 1: Summary of Initial Data Observations * ACMG PKU treatment threshold: Vockley J., et al., Genet Med. 2014 Data cut-off: September 14, 2023 Generally Well-Tolerated No SAEs The majority of treatment-related AEs have been mild and transient No LFT abnormalities while on prophylactic, targeted immunosuppression regimen of T-cell inhibitor tacrolimus and corticosteroids Initial Data from First Dose Level Warrants Dose Escalation Participant 1 Rapid onset of action with clinically meaningful reduction in plasma Phe concentration of 55% and below ACMG PKU treatment threshold (<360 µmol/L)* at 4 weeks post-dose 98% Phe reduction at Week 14; protein supplementation initiated for multiple Phe levels <30 µmol/L Clinically meaningful reduction in plasma Phe concentration of approximately 50% after supplemental natural dietary protein to achieve approximately double baseline intake since Week 16 Participant 2 Clinically meaningful plasma Phe reduction of approximately 50% and majority of post-steroid regimen Phe levels are below baseline Participant 3 Clinically meaningful plasma Phe reduction of 60% and all post-steroid regimen Phe levels below baseline. | © Copyright 2023 Homology Medicines, Inc. All rights reserved.

Gene Therapy Candidate HMI-203 for Hunter Syndrome

High Unmet Need for Hunter Syndrome Treatment That Addresses Peripheral and Cognitive Effects *WORLDSymposium™ 2022. Haroldson J, et al. **National MPS Society. “A Guide to Understanding MPS II.” © Copyright 2023 Homology Medicines, Inc. All rights reserved. Patients on existing ERT continue to experience*: Increased mortality, sleep apnea, chronic and joint pain, lung and cardiac conditions, hearing loss, limited mobility/range of motion Anxiety caused by uncertainty of disease progression Shortened life expectancy ERT does not cross blood-brain-barrier (BBB); Neuronopathic patients experience**: Decreased cognitive function, seizures, cerebrospinal fluid accumulation, carpal tunnel syndrome Life expectancy into the second decade Caused primarily by IDS gene mutations Leads to toxic lysosomal accumulation of glycosaminoglycans (GAGs) Severe form includes progressive debilitation and intellectual decline followed by death in 10–20 years Prevalence: 1 in 100,000 to 1 in 170,000; primarily males MPS II (Hunter syndrome) All People with MPS II Experience Peripheral Disease Manifestations
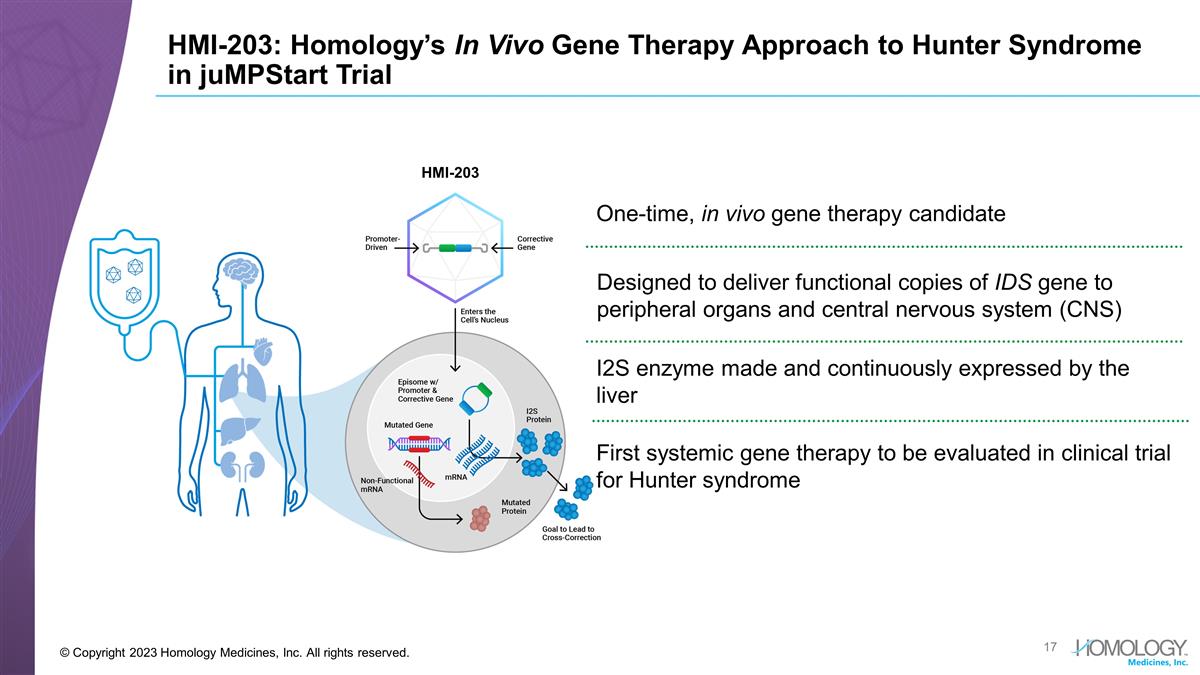
HMI-203: Homology’s In Vivo Gene Therapy Approach to Hunter Syndrome in juMPStart Trial © Copyright 2023 Homology Medicines, Inc. All rights reserved. One-time, in vivo gene therapy candidate Designed to deliver functional copies of IDS gene to peripheral organs and central nervous system (CNS) First systemic gene therapy to be evaluated in clinical trial for Hunter syndrome I2S enzyme made and continuously expressed by the liver HMI-203
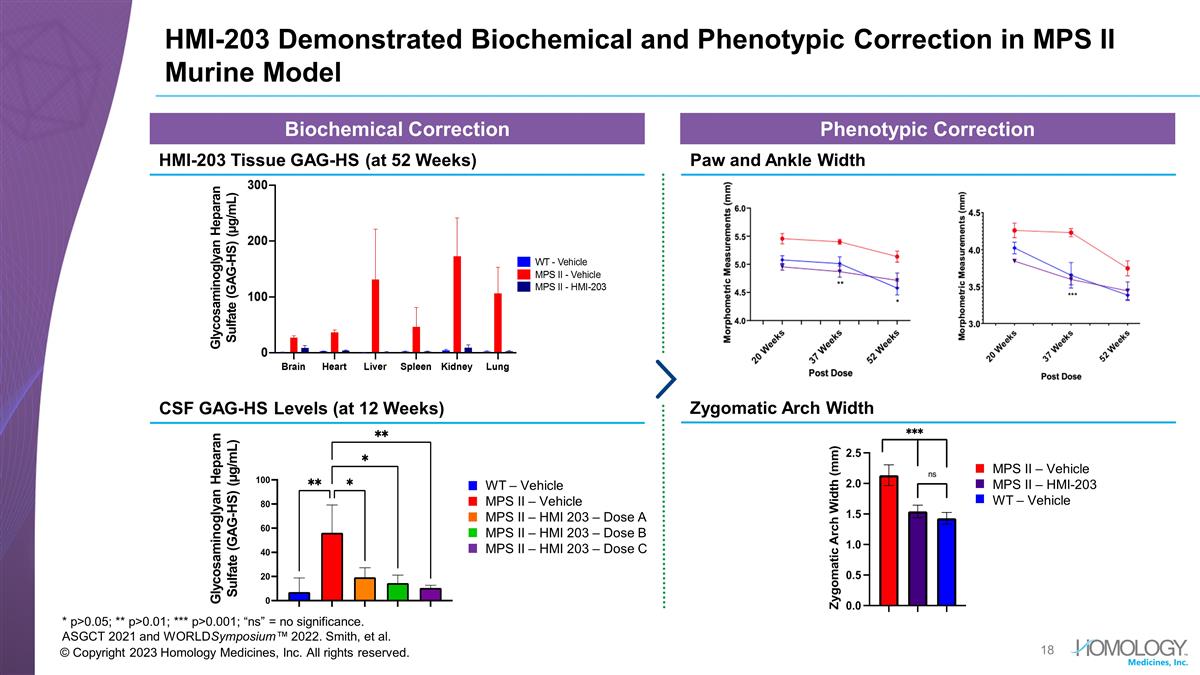
© Copyright 2023 Homology Medicines, Inc. All rights reserved. HMI-203 Demonstrated Biochemical and Phenotypic Correction in MPS II Murine Model * p>0.05; ** p>0.01; *** p>0.001; “ns” = no significance. ASGCT 2021 and WORLDSymposium™ 2022. Smith, et al. Glycosaminoglyan Heparan Sulfate (GAG-HS) (µg/mL) Biochemical Correction Phenotypic Correction HMI-203 Tissue GAG-HS (at 52 Weeks) Paw and Ankle Width CSF GAG-HS Levels (at 12 Weeks) Glycosaminoglyan Heparan Sulfate (GAG-HS) (µg/mL) WT – Vehicle MPS II – Vehicle MPS II – HMI 203 – Dose A MPS II – HMI 203 – Dose B MPS II – HMI 203 – Dose C MPS II – Vehicle MPS II – HMI-203 WT – Vehicle Zygomatic Arch Width
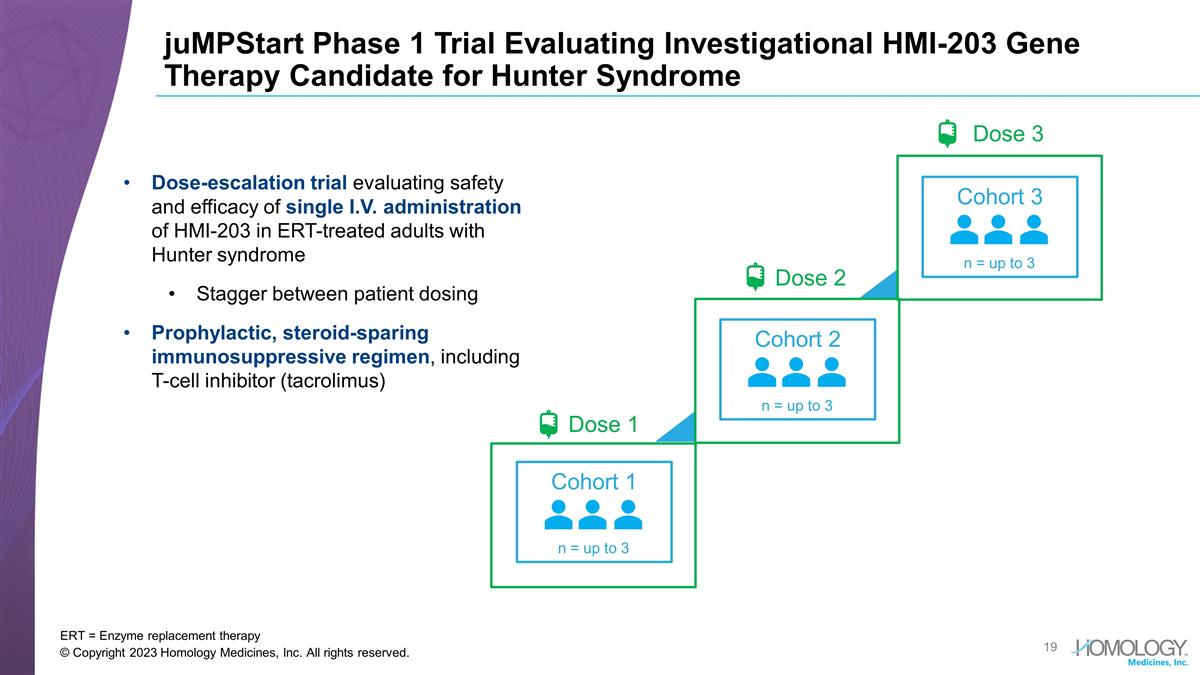
juMPStart Phase 1 Trial Evaluating Investigational HMI-203 Gene Therapy Candidate for Hunter Syndrome ERT = Enzyme replacement therapy © Copyright 2023 Homology Medicines, Inc. All rights reserved. Dose-escalation trial evaluating safety and efficacy of single I.V. administration of HMI-203 in ERT-treated adults with Hunter syndrome Stagger between patient dosing Prophylactic, steroid-sparing immunosuppressive regimen, including T-cell inhibitor (tacrolimus) Dose 3 Dose 2 Cohort 2 n = up to 3 Dose 1 Cohort 1 n = up to 3 Cohort 3 n = up to 3

Pipeline Updates and 2023 Milestones
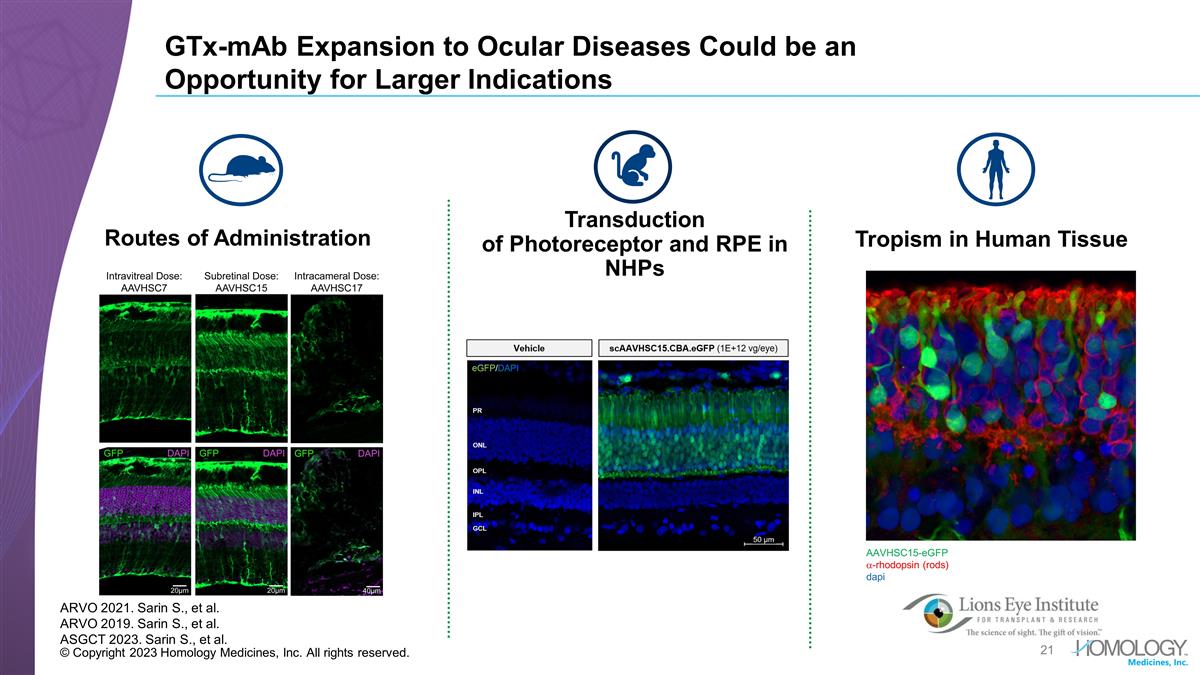
© Copyright 2023 Homology Medicines, Inc. All rights reserved. GTx-mAb Expansion to Ocular Diseases Could be an Opportunity for Larger Indications Routes of Administration AAVHSC15-eGFP a-rhodopsin (rods) dapi ARVO 2021. Sarin S., et al. ARVO 2019. Sarin S., et al. ASGCT 2023. Sarin S., et al. Tropism in Human Tissue Transduction of Photoreceptor and RPE in NHPs
© Copyright 2023 Homology Medicines, Inc. All rights reserved. Optimized, In Vivo Gene Therapy Candidate, HMI-204 for the Treatment of MLD *Clin Chem. 2016 Jan; 62(1): 279–286. **Prevalence: MLD Foundation Single I.V. administration of HMI-204 in murine model of MLD resulted in: Broad biodistribution to peripheral organs and the CNS Human ARSA (hARSA) expression levels in multiple brain regions and cell types well-above minimum levels of enzyme needed to correct the phenotype* hARSA activity levels in the brain that are predictive of functional improvements Detection of active ARSA in the serum Expression of near-normal ARSA activity levels following active liver growth Optimizations also led to significant improvements in vector yield and superior packaging Actively Seeking a Partner to Advance Development Candidate MLD Caused Primarily by ARSA Gene Mutations Results in destruction of myelin-producing cells Late infantile form includes rapidly progressive motor and cognitive decline followed by death in 5–10 years Prevalence: 1 in 40,000**
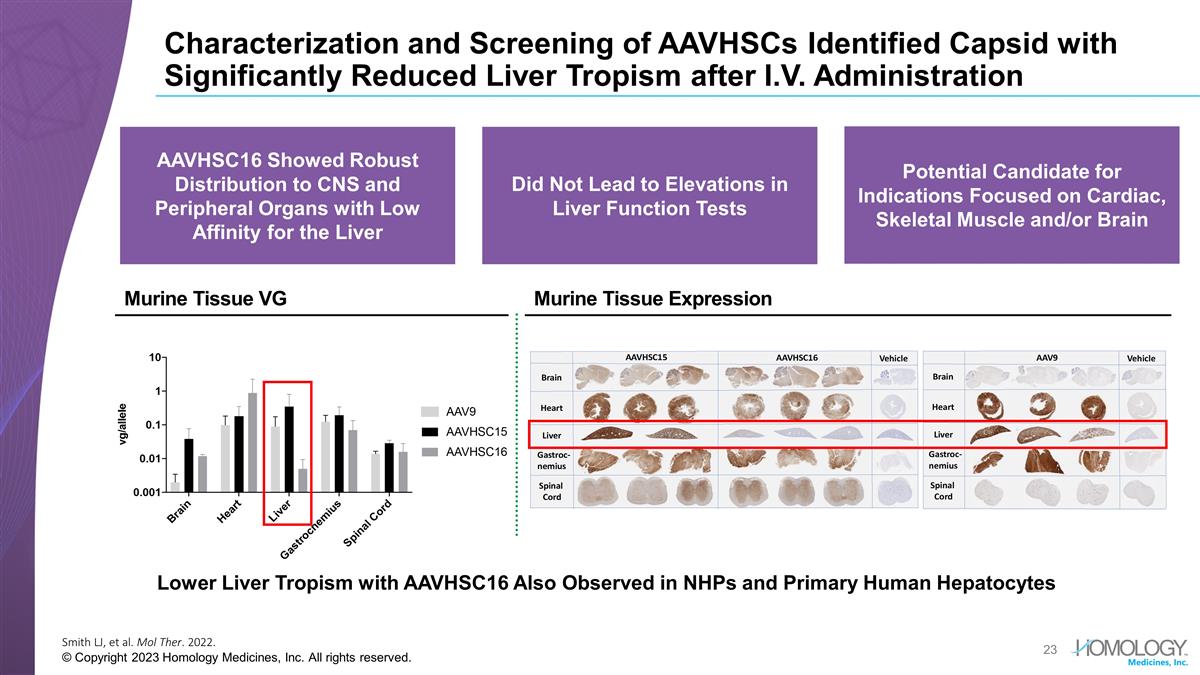
Characterization and Screening of AAVHSCs Identified Capsid with Significantly Reduced Liver Tropism after I.V. Administration Smith LJ, et al. Mol Ther. 2022. © Copyright 2023 Homology Medicines, Inc. All rights reserved. Lower Liver Tropism with AAVHSC16 Also Observed in NHPs and Primary Human Hepatocytes Murine Tissue Expression Murine Tissue VG Potential Candidate for Indications Focused on Cardiac, Skeletal Muscle and/or Brain Did Not Lead to Elevations in Liver Function Tests AAVHSC16 Showed Robust Distribution to CNS and Peripheral Organs with Low Affinity for the Liver

Next Steps: Evaluating Strategic Options © Copyright 2023 Homology Medicines, Inc. All rights reserved. Completed a review of our business and will be evaluating strategic alternatives for the Company and its clinical and preclinical assets to maximize shareholder value Approved by the Board of Directors Based on anticipated clinical development timelines and current financing environment, stopping further program development efforts with the exception of required actions, including continued monitoring of participants in its clinical trials Related reduction in force of 87% Retained TD Cowen as strategic financial advisor Strategic options may include, but are not limited to, an acquisition, merger, reverse merger, sale of assets, strategic partnerships, or other transactions

Document and Entity Information |
Oct. 10, 2023 |
|---|---|
| Cover [Abstract] | |
| Amendment Flag | false |
| Entity Central Index Key | 0001661998 |
| Document Type | 8-K |
| Document Period End Date | Oct. 10, 2023 |
| Entity Registrant Name | HOMOLOGY MEDICINES, INC. |
| Entity Incorporation State Country Code | DE |
| Entity File Number | 001-38433 |
| Entity Tax Identification Number | 47-3468154 |
| Entity Address, Address Line One | One Patriots Park |
| Entity Address, City or Town | Bedford |
| Entity Address, State or Province | MA |
| Entity Address, Postal Zip Code | 01730 |
| City Area Code | (781) |
| Local Phone Number | 301-7277 |
| Written Communications | false |
| Soliciting Material | false |
| Pre Commencement Tender Offer | false |
| Pre Commencement Issuer Tender Offer | false |
| Security 12b Title | Common Stock, $0.0001 par value per share |
| Trading Symbol | FIXX |
| Security Exchange Name | NASDAQ |
| Entity Emerging Growth Company | true |
| Entity Ex Transition Period | false |
1 Year Homology Medicines Chart |
1 Month Homology Medicines Chart |

It looks like you are not logged in. Click the button below to log in and keep track of your recent history.
Support: +44 (0) 203 8794 460 | support@advfn.com
By accessing the services available at ADVFN you are agreeing to be bound by ADVFN's Terms & Conditions