
We could not find any results for:
Make sure your spelling is correct or try broadening your search.
| Share Name | Share Symbol | Market | Type |
|---|---|---|---|
| Eyenovia Inc | NASDAQ:EYEN | NASDAQ | Common Stock |
| Price Change | % Change | Share Price | Bid Price | Offer Price | High Price | Low Price | Open Price | Shares Traded | Last Trade | |
|---|---|---|---|---|---|---|---|---|---|---|
| 0.0275 | 4.23% | 0.6775 | 0.6497 | 0.678 | 1,043 | 13:27:12 |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (Date
of earliest event reported):
(Exact Name of Registrant as Specified in its Charter)
| (State or other jurisdiction of incorporation) |
(Commission File Number) |
(IRS Employer Identification No.) |
(Address of Principal Executive Offices, and Zip Code)
(
Registrant’s Telephone Number, Including Area Code
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
Securities registered pursuant to Section 12(b) of the Act:
| (Title of each class) | (Trading Symbol) |
(Name of each exchange on which registered) | ||
(Nasdaq Capital Market) |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (17 CFR §230.405) or Rule 12b-2 of the Securities Exchange Act of 1934 (17 CFR §240.12b-2).
Emerging
growth company
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ¨
| Item 7.01. | Regulation FD Disclosure. |
On June 4, 2024, Eyenovia, Inc. (the “Company”) will begin using an updated corporate presentation with various investors and analysts. A copy of the presentation is furnished as Exhibit 99.1 to this Current Report on Form 8-K and is incorporated herein by reference.
The information contained in this Item 7.01, including Exhibit 99.1, is being “furnished” and shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liability of that Section or Sections 11 and 12(a)(2) of the Securities Act of 1933, as amended (the “Securities Act”). The information contained in this Item 7.01, including Exhibit 99.1, shall not be incorporated by reference into any registration statement or other document pursuant to the Securities Act or into any filing or other document pursuant to the Exchange Act, except as otherwise expressly stated in any such filing.
| Item 9.01. | Financial Statements and Exhibits. |
(d) Exhibits
| Exhibit No. | Description | |
| 99.1 | Eyenovia, Inc. Updated Corporate Presentation, dated June 2024. | |
| 104 | Cover Page Interactive Data File (embedded within the Inline XBRL document). |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| EYENOVIA, INC. | |
| Date: June 4, 2024 | /s/ John Gandolfo |
| John Gandolfo | |
| Chief Financial Officer |
Exhibit 99.1

June 2024 We Are the Optejet® Company Developing and commercializing ophthalmic therapeutics with Optecare Ρ services in large markets with high unmet needs EYEN - COM - V2 - 0021

Forward - looking Statements Except for historical information, all the statements, expectations and assumptions contained in this presentation are forwar d - l ooking statements. Forward - looking statements include, but are not limited to, statements that express our intentions, beliefs, expecta tions, strategies, predictions or any other statements relating to our future activities or other future events or conditions, inclu din g estimated market opportunities for our products, product candidates and platform technology. These statements are based on current expe cta tions, estimates and projections about our business based, in part, on assumptions made by management. These statements are not guar ant ees of future performance and involve risks, uncertainties and assumptions that are difficult to predict. Therefore, actual outco mes and results may, and in some cases are likely to, differ materially from what is expressed or forecasted in the forward - looking statements d ue to numerous factors discussed from time to time in documents which we file with the U.S. Securities and Exchange Commission. In addition, such statements could be affected by risks and uncertainties related to, among other things: risks of our and ou r l icenses’ clinical trials, including, but not limited to, the costs, design, initiation and enrollment, timing, progress and results of su ch trials; the timing and our licensees’ ability to submit applications for, obtaining and maintaining regulatory approvals for Mydcombi, clobetaso l p ropionate and our product candidates; the potential advantages of Mydcombi, clobetasol propionate and our product candidates and platform technology and potential revenues from licensing transactions; the rate and degree of market acceptance and clinical utility of Mydcombi, clobetasol propionate and our product candidates; our estimates regarding the potential market opportunity for Mydcombi, clob eta sol propionate and our product candidates; reliance on third parties to develop and commercialize Mydcombi Ρ , clobetasol propionate and certain of our product candidates; the ability of us and our partners to timely develop, implement and maintain manufacturing, commercialization and marketing capabilities and strategies for Mydcombi, clobetasol propionate and our product candidates; i nte llectual property risks; changes in legal, regulatory, legislative and geopolitical environments in the markets in which we operate an d t he impact of these changes on our ability to obtain regulatory approval for our products; and our competitive position. Any forward - looking statements speak only as of the date on which they are made, and except as may be required under applicable securities laws, Eyenovia does not undertake any obligation to update any forward - looking statements. 2
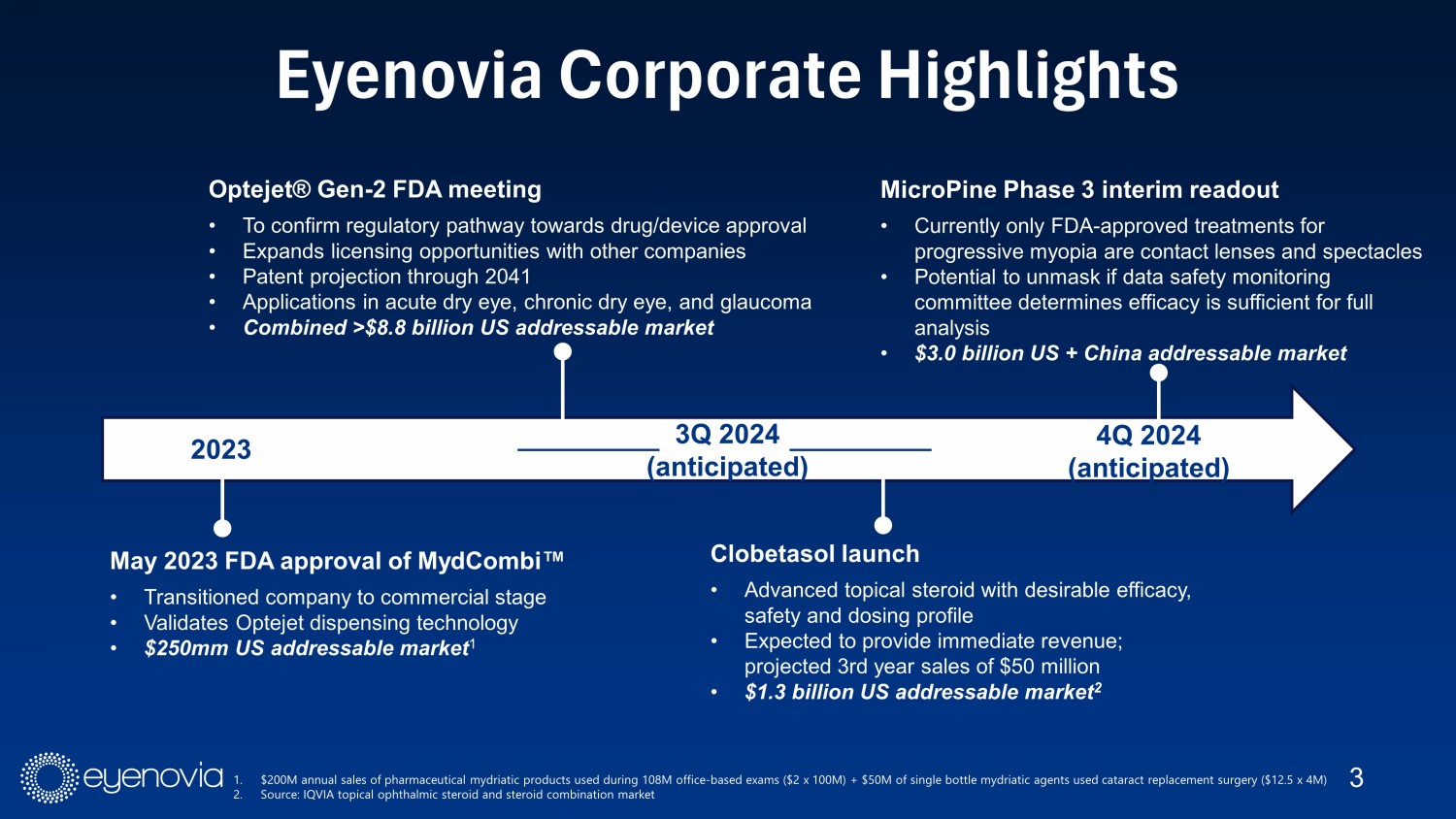
3 Eyenovia Corporate Highlights 2023 May 2023 FDA approval of MydCombi Œ • Transitioned company to commercial stage • Validates Optejet dispensing technology • $250mm US addressable market 1 3Q 2024 (anticipated) Optejet® Gen - 2 FDA meeting • To confirm regulatory pathway towards drug/device approval • Expands licensing opportunities with other companies • Patent projection through 2041 • Applications in acute dry eye, chronic dry eye, and glaucoma • Combined >$8.8 billion US addressable market Clobetasol launch • Advanced topical steroid with desirable efficacy, safety and dosing profile • Expected to provide immediate revenue; projected 3rd year sales of $50 million • $1.3 billion US addressable market 2 MicroPine Phase 3 interim readout • Currently only FDA - approved treatments for progressive myopia are contact lenses and spectacles • Potential to unmask if data safety monitoring committee determines efficacy is sufficient for full analysis • $3.0 billion US + China addressable market 4Q 2024 (anticipated) 1. $200M annual sales of pharmaceutical mydriatic products used during 108M office - based exams ($2 x 100M) + $50M of single bottle mydriatic agents used cataract replacement surgery ($12.5 x 4M) 2. Source: IQVIA topical ophthalmic steroid and steroid combination market

MicroPine Atropine Ophthalmic Metered Spray Our Premier Near - Term Opportunity in the Multi - Billion Dollar Pediatric Progressive Myopia Market 4

Unmet Medical Need Current options are not appropriate for all patients and do not eliminate progression risk $3.0B market in the U.S. and China One of the largest markets in global eyecare Major Clinical Milestone expected in 5 Months CHAPERONE Data Monitoring Committee Review expected in 4Q 2024 5 Asset Highlights Manufacturing CMO manufactures drug products; Device and Sterile Fill and Finish by Eyenovia Strong IP, Non - Substitutable Unique FDA form with design and method patents through 2041 Optejet Technology Easy to use and self - administer with digital capability to track adherence and compliance
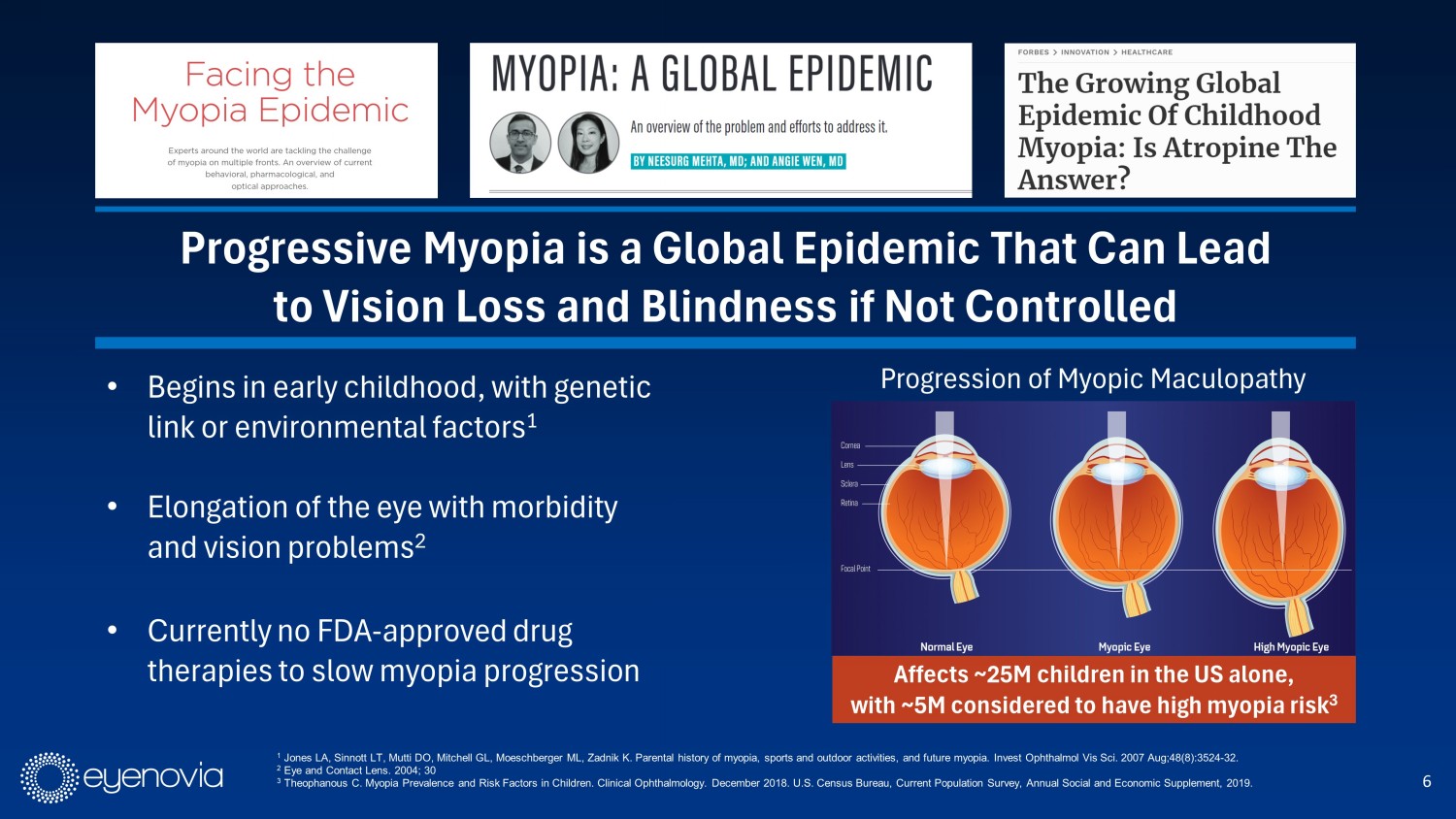
6 • Begins in early childhood, with genetic link or environmental factors 1 • Elongation of the eye with morbidity and vision problems 2 • Currently no FDA - approved drug therapies to slow myopia progression Affects ~25M children in the US alone, with ~5M considered to have high myopia risk 3 1 Jones LA, Sinnott LT, Mutti DO, Mitchell GL, Moeschberger ML, Zadnik K. Parental history of myopia, sports and outdoor activities, and future myopia. Invest Ophthalmol Vis Sci. 2007 Aug;48(8):3524 - 32. 2 Eye and Contact Lens. 2004; 30 3 Theophanous C. Myopia Prevalence and Risk Factors in Children. Clinical Ophthalmology. December 2018. U.S. Census Bureau, Cur re nt Population Survey, Annual Social and Economic Supplement, 2019. Progressive Myopia is a Global Epidemic That Can Lead to Vision Loss and Blindness if Not Controlled Progression of Myopic Maculopathy
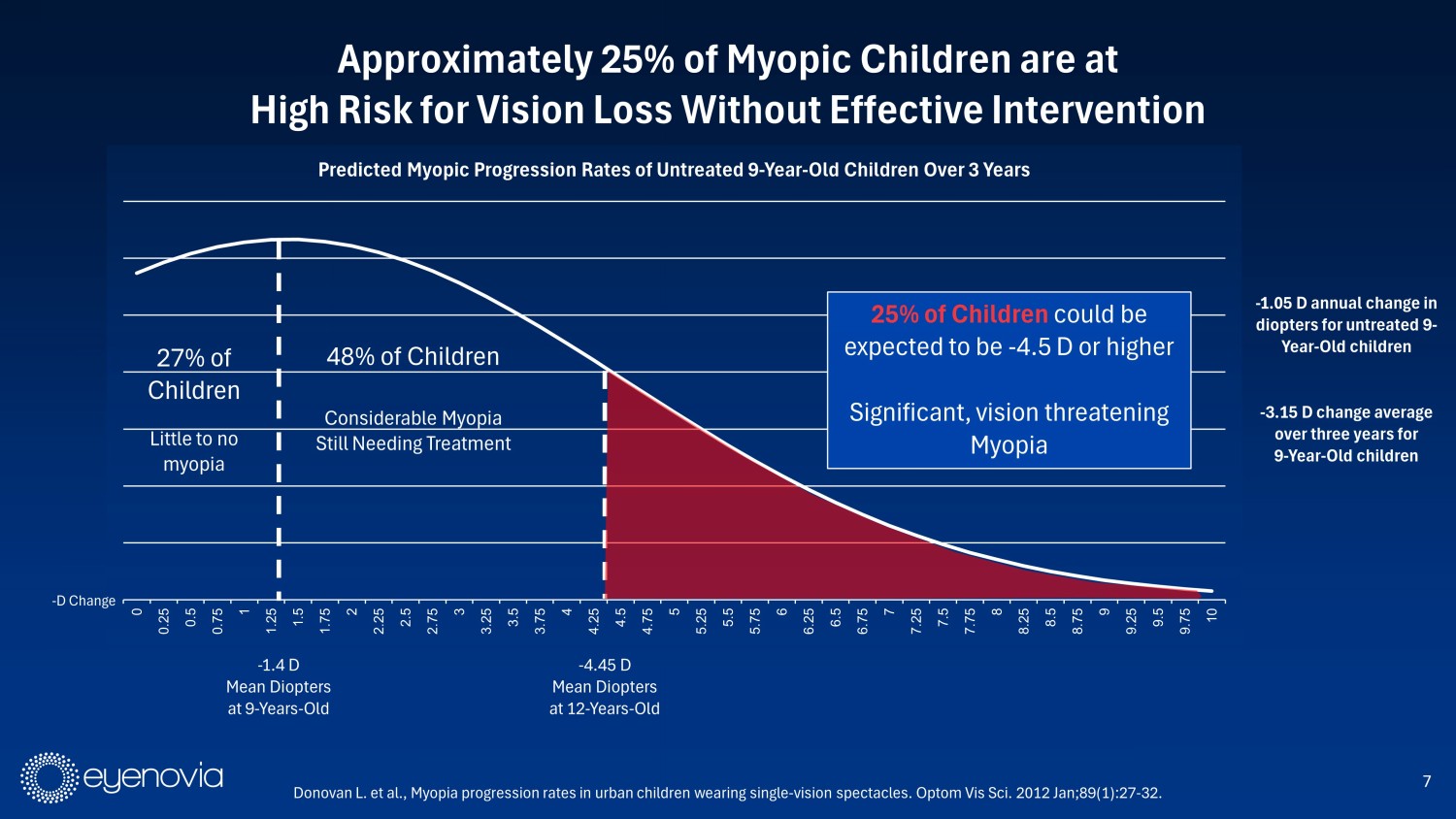
7 Approximately 25% of Myopic Children are at High Risk for Vision Loss Without Effective Intervention - 1.4 D Mean Diopters at 9 - Years - Old Donovan L. et al., Myopia progression rates in urban children wearing single - vision spectacles. Optom Vis Sci. 2012 Jan;89(1):27 - 32. 0 0.25 0.5 0.75 1 1.25 1.5 1.75 2 2.25 2.5 2.75 3 3.25 3.5 3.75 4 4.25 4.5 4.75 5 5.25 5.5 5.75 6 6.25 6.5 6.75 7 7.25 7.5 7.75 8 8.25 8.5 8.75 9 9.25 9.5 9.75 10 Predicted Myopic Progression Rates of Untreated 9 - Year - Old Children Over 3 Years - 4.45 D Mean Diopters at 12 - Years - Old 25% of Children could be expected to be - 4.5 D or higher Significant, vision threatening Myopia 48% of Children Considerable Myopia Still Needing Treatment 27% of Children Little to no myopia - 1.05 D annual change in diopters for untreated 9 - Year - Old children - 3.15 D change average over three years for 9 - Year - Old children - D Change
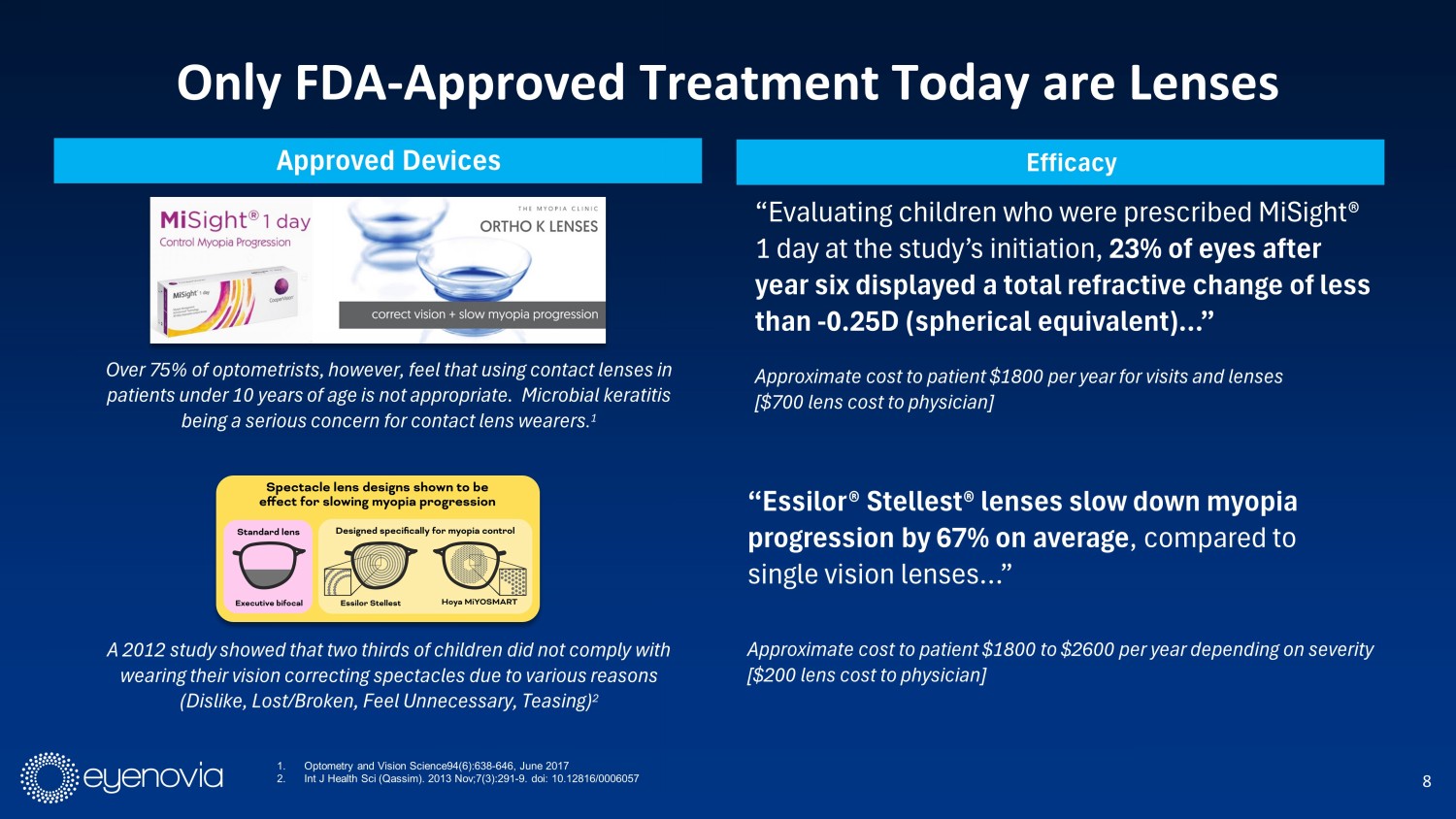
8 Only FDA - Approved Treatment Today are Lenses 1. Optometry and Vision Science94(6):638 - 646, June 2017 2. Int J Health Sci (Qassim). 2013 Nov;7(3):291 - 9. doi : 10.12816/0006057 Approved Devices Over 75% of optometrists, however, feel that using contact lenses in patients under 10 years of age is not appropriate. Microbial keratitis being a serious concern for contact lens wearers . 1 A 2012 study showed that two thirds of children did not comply with wearing their vision correcting spectacles due to various reasons (Dislike, Lost/Broken, Feel Unnecessary, Teasing) 2 Efficacy “Evaluating children who were prescribed MiSight ® 1 day at the study’s initiation, 23% of eyes after year six displayed a total refractive change of less than - 0.25D (spherical equivalent)…” “Essilor® Stellest ® lenses slow down myopia progression by 67% on average , compared to single vision lenses…” Approximate cost to patient $1800 per year for visits and lenses [$700 lens cost to physician] Approximate cost to patient $1800 to $2600 per year depending on severity [$200 lens cost to physician]
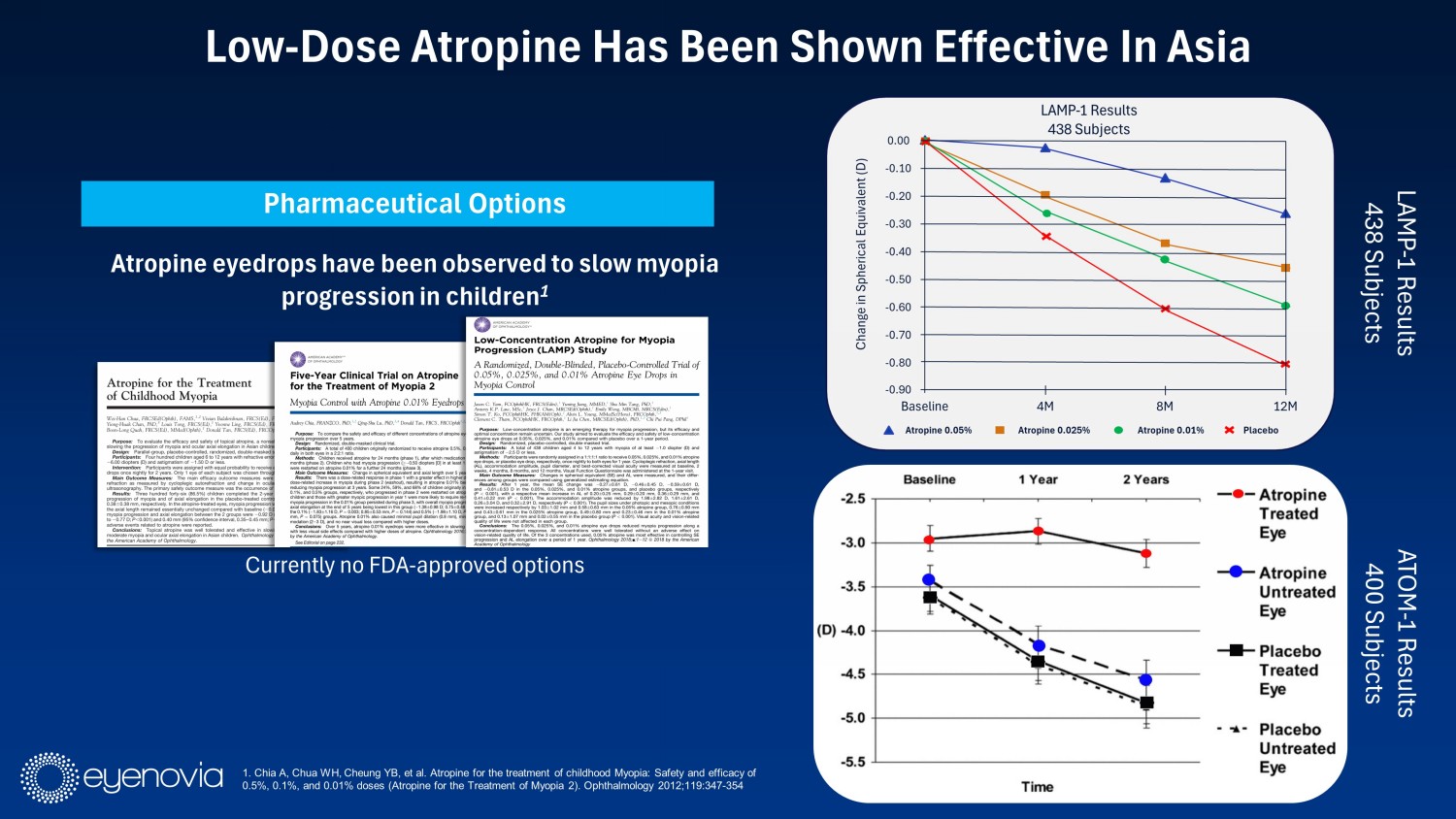
Pharmaceutical Options Atropine eyedrops have been observed to slow myopia progression in children 1 Currently no FDA - approved options Low - Dose Atropine Has Been Shown Effective In Asia 1. Chia A, Chua WH, Cheung YB, et al. Atropine for the treatment of childhood Myopia: Safety and efficacy of 0.5%, 0.1%, and 0.01% doses (Atropine for the Treatment of Myopia 2). Ophthalmology 2012;119:347 - 354 ATOM - 1 Results 400 Subjects LAMP - 1 Results 438 Subjects
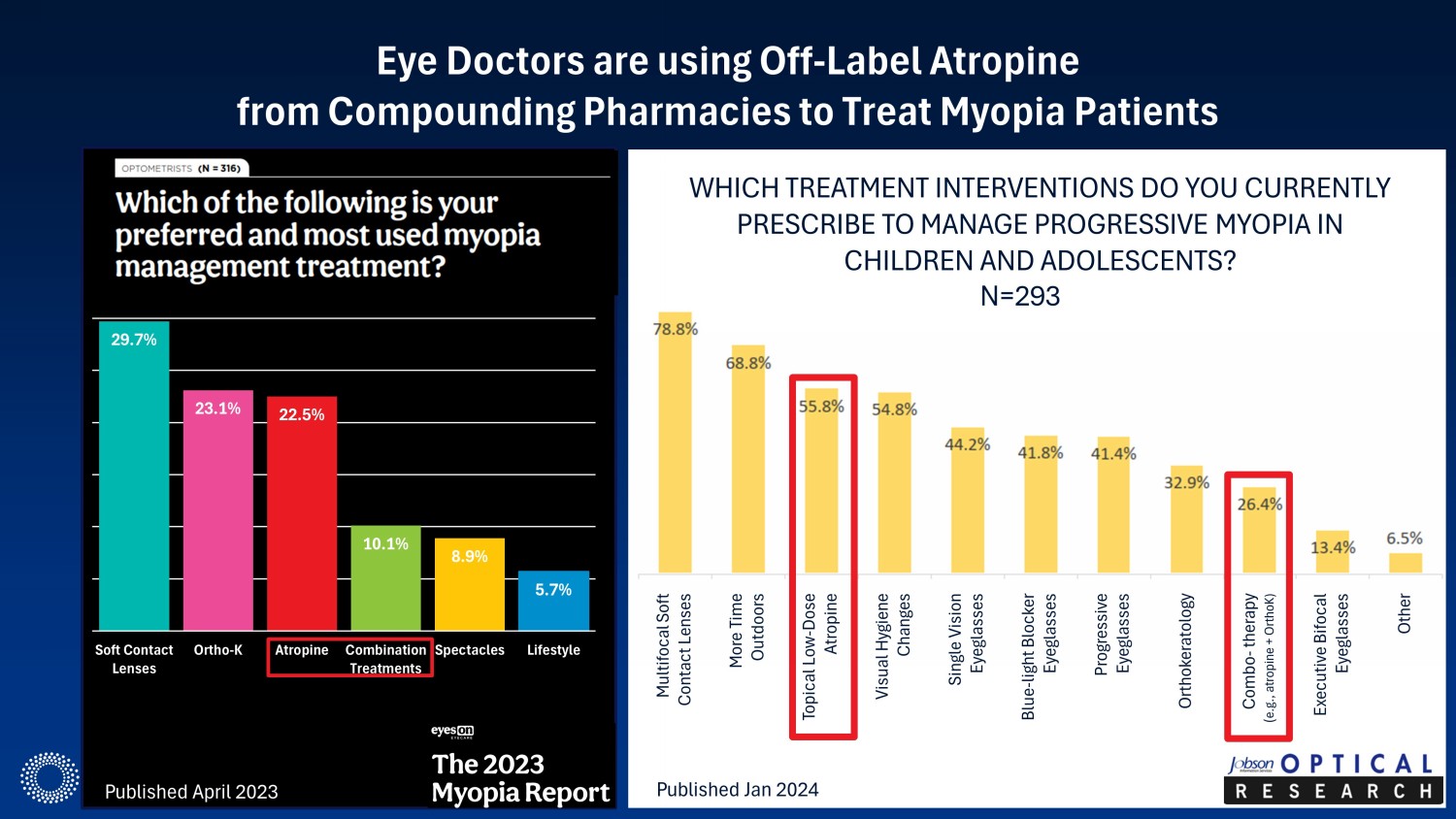
Eye Doctors are using Off - Label Atropine from Compounding Pharmacies to Treat Myopia Patients Multifocal Soft Contact Lenses WHICH TREATMENT INTERVENTIONS DO YOU CURRENTLY PRESCRIBE TO MANAGE PROGRESSIVE MYOPIA IN CHILDREN AND ADOLESCENTS? More Time Outdoors Topical Low - Dose Atropine Visual Hygiene Changes Single Vision Eyeglasses Blue - light Blocker Eyeglasses Progressive Eyeglasses Orthokeratology Combo - therapy ( e.g., atropine + OrthoK) Executive Bifocal Eyeglasses Other Published April 2023 Published Jan 2024 N=293 29.7% 23.1% 22.5% 10.1% 8.9% 5.7% Soft Contact Lenses Ortho-K Atropine Combination Treatments Spectacles Lifestyle

MicroPine The Premier Drug+Device Product for Progressive Myopia Target Product Profile • 60% reduction in myopia progression with minimal rebound after one year • One spray per each eye daily; easy enough for children to use without supervision • Comfortable to instill with minimal impact on the ocular surface • Minimal local side effects and very low systemic exposure • Optecare Ρ compliance system provides dosing reminders and product use history for doctors to improve treatment success • Estimated NSP of $200/month with COGS below $20/month

CHAPERONE The Single Phase 3 Trial Required for FDA Approval • Three arms dosed with 8 microliter ophthalmic spray: placebo, 0.01% and 0.1% atropine • Myopic children in the U.S. between the ages of 3 and 13 at risk for progression • MicroPine given as one spray in each eye at night • Three years to efficacy endpoint – myopia progression of less than 0.5 diopters • Masked data to date indicates very low rate of SAEs (fewer than 1 per 1,000 patient - months of therapy); all SAEs were judged not related to treatment by investigators. Therapy compliance appears higher than what has been seen historically with eye drop studies.
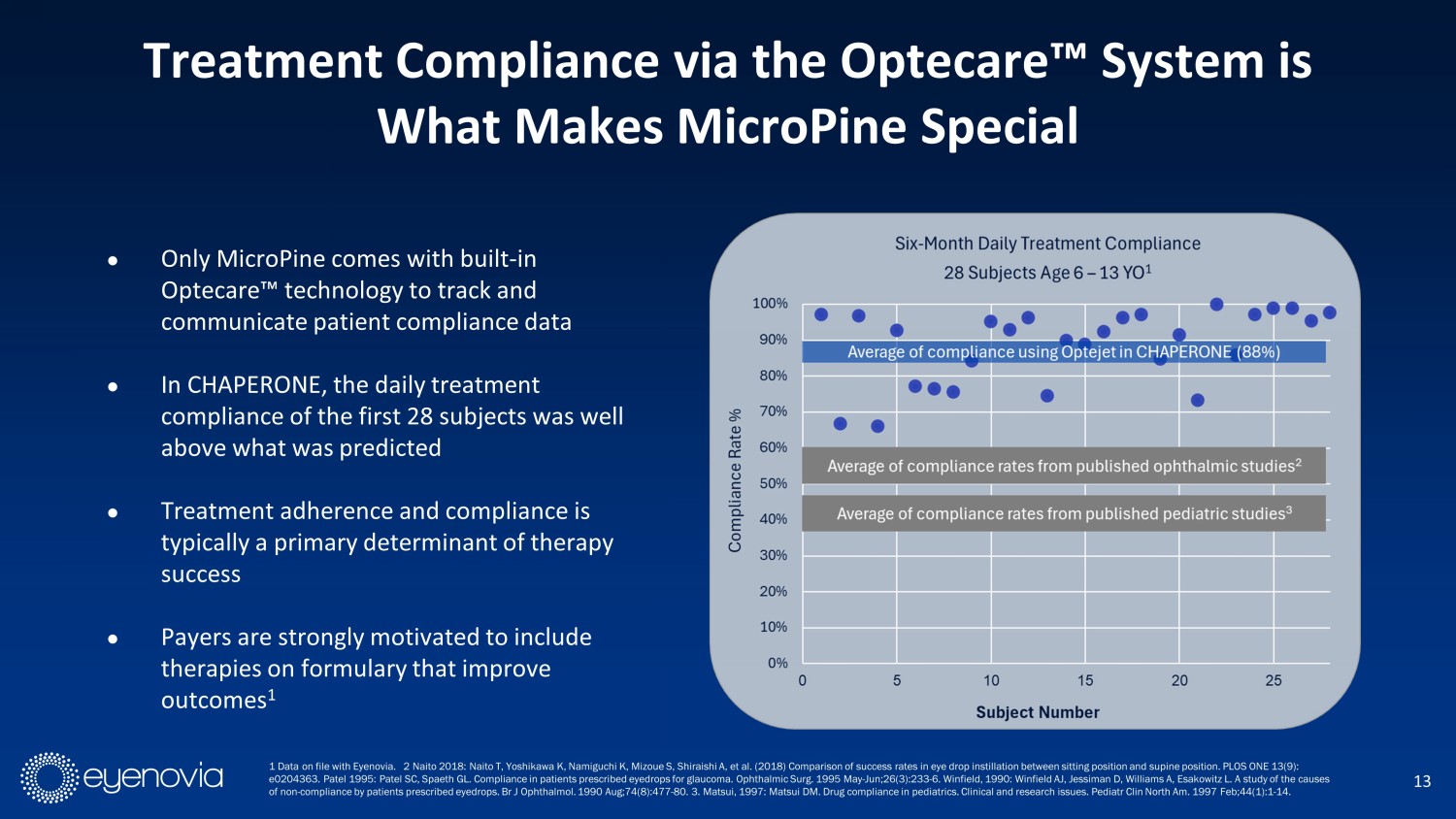
● Only MicroPine comes with built - in Optecare Ρ technology to track and communicate patient compliance data ● In CHAPERONE, the daily treatment compliance of the first 28 subjects was well above what was predicted ● Treatment adherence and compliance is typically a primary determinant of therapy success ● Payers are strongly motivated to include therapies on formulary that improve outcomes 1 13 Treatment Compliance via the Optecare Ρ System is What Makes MicroPine Special 1 Data on file with Eyenovia. 2 Naito 2018: Naito T, Yoshikawa K, Namiguchi K, Mizoue S, Shiraishi A, et al. (2018) Comparison of success rates in eye drop instillation between sitting position and supine position. PLOS ONE 13 (9): e0204363. Patel 1995: Patel SC, Spaeth GL. Compliance in patients prescribed eyedrops for glaucoma. Ophthalmic Surg. 1995 May - Ju n;26(3):233 - 6. Winfield, 1990: Winfield AJ, Jessiman D, Williams A, Esakowitz L. A study of the causes of non - compliance by patients prescribed eyedrops. Br J Ophthalmol . 1990 Aug;74(8):477 - 80. 3. Matsui, 1997: Matsui DM. Drug compliance in pediatrics. Clinical and research issues. Pediatr Clin North Am. 1997 Feb;44(1):1 - 14.
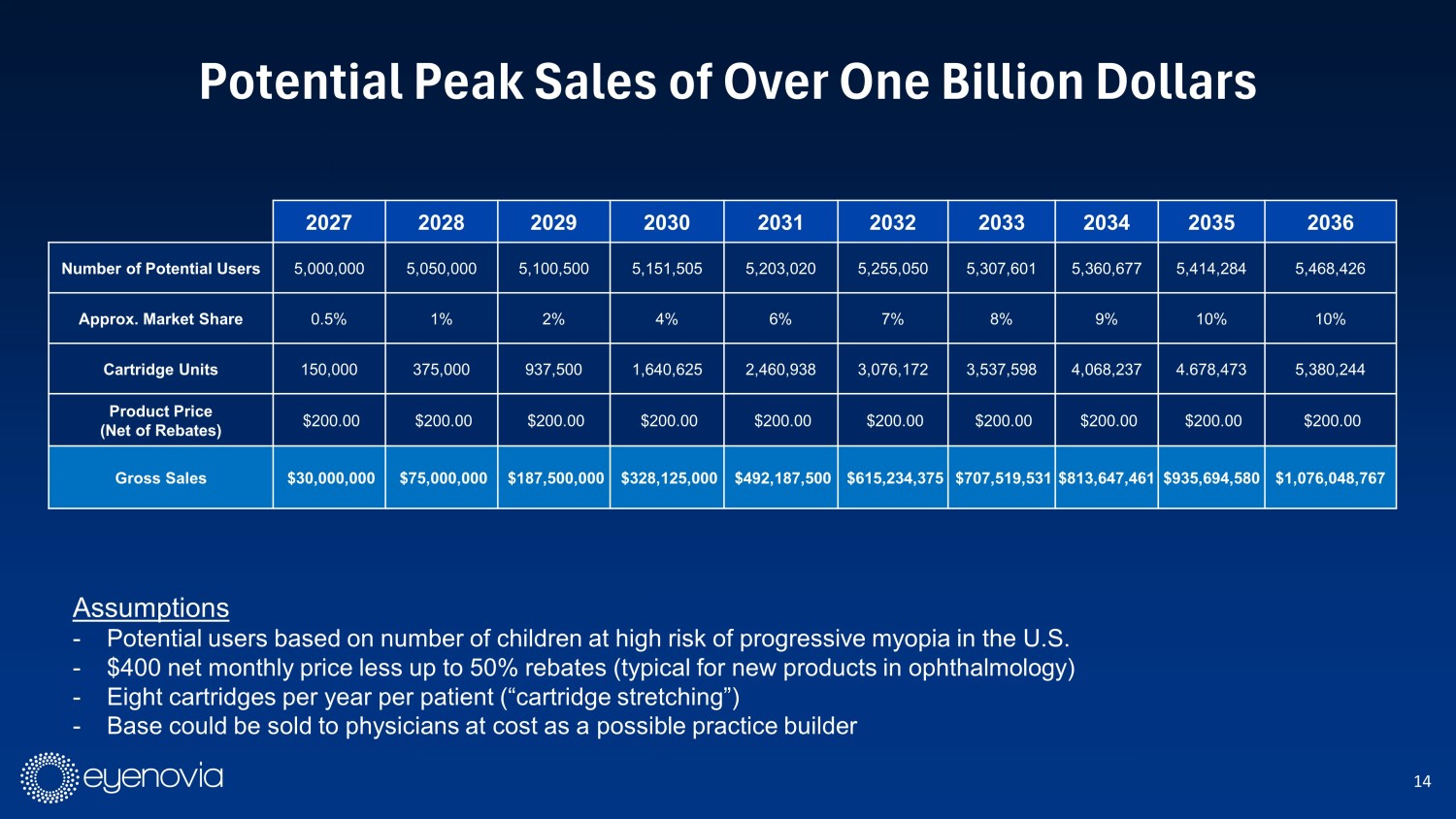
14 Potential Peak Sales of Over One Billion Dollars 2027 2028 2029 2030 2031 2032 2033 2034 2035 2036 Number of Potential Users 5,000,000 5,050,000 5,100,500 5,151,505 5,203,020 5,255,050 5,307,601 5,360,677 5,414,284 5,468,426 Approx. Market Share 0.5% 1% 2% 4% 6% 7% 8% 9% 10% 10% Cartridge Units 150,000 375,000 937,500 1,640,625 2,460,938 3,076,172 3,537,598 4,068,237 4.678,473 5,380,244 Product Price (Net of Rebates) $200.00 $200.00 $200.00 $200.00 $200.00 $200.00 $200.00 $200.00 $200.00 $200.00 Gross Sales $30,000,000 $75,000,000 $187,500,000 $328,125,000 $492,187,500 $615,234,375 $707,519,531 $813,647,461 $935,694,580 $1,076,048,767 Assumptions - Potential users based on number of children at high risk of progressive myopia in the U.S. - $400 net monthly price less up to 50% rebates (typical for new products in ophthalmology) - Eight cartridges per year per patient (“cartridge stretching”) - Base could be sold to physicians at cost as a possible practice builder
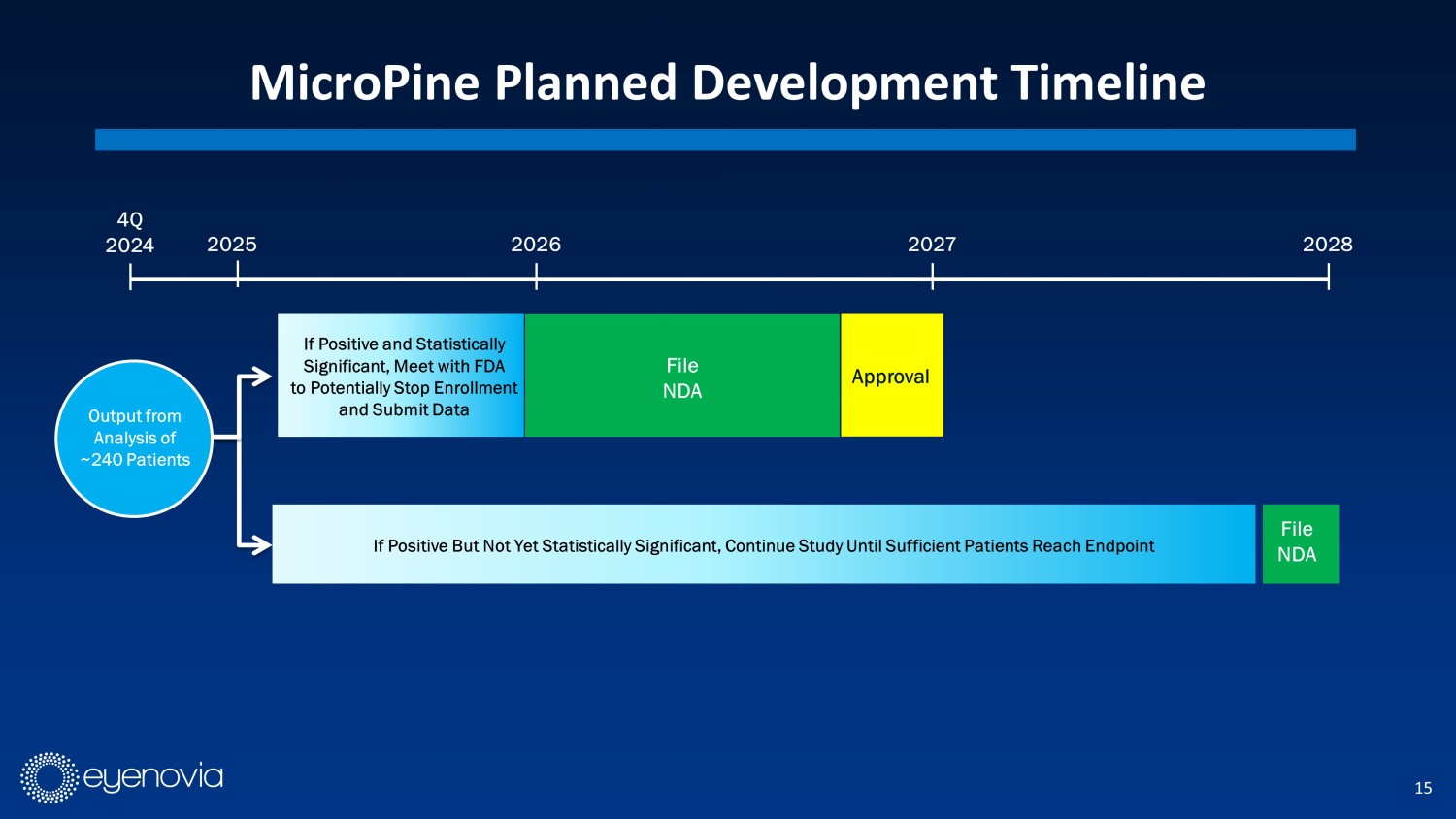
15 MicroPine Planned Development Timeline 4Q 2024 2026 2027 2028 2025 I f Positive But Not Yet Statistically Significant, Continue Study Until Sufficient Patients Reach Endpoint File NDA File NDA If Positive and Statistically Significant, Meet with FDA to Potentially Stop Enrollment and Submit Data Output from Analysis of ~240 Patients Approval

Optejet® Digital Ophthalmic Metered Spray Device The Only FDA - Approved Ophthalmic Digital Drug Delivery Platform June 2024

17 • Designed to address issues with ease - of - use and dosing precision • Delivers efficacy while improving tolerability and reducing side effects 1 • Digital Optecare Œ capabilities 2 Optejet ® with microdose array print technology • Patented digital device platform technology • Unique, class - leading drug products • High - value product pipeline addressing areas of significant medical and market need • Multi - faceted business model with revenue from direct sales and licensing agreements Introducing the Optejet® Gen - 2 1. Wirta DL, Walters TR, Flynn WJ, Rathi S, Ianchulev T. Mydriasis with micro - array print touch - free tropicamide - phenylephrine f ixed combination MIST: pooled randomized Phase III trials. Ther Deliv. 2021 Mar;12(3):201 - 214. 2. Optecare is Eyenovia’s suite of digital compliance and adherence capabilities

18 The Optejet ® Consists of a Replaceable Cartridge (COGS of $20) and Durable Base Spray nozzle with 109 laser - drilled ports Shutter Activation button Ergonomic design Proprietary, pre - filled drug cartridge manufactured by Eyenovia Optejet uses technology similar to that of Inkjet printers to “print” microdroplets of drug onto the eye, using ¼ of the dose of conventional eye drops to maintain efficacy and minimize tolerability issues

19 Ergonomic Design to Improve Usability Horizontal delivery, push - button dosing and no protruding tip Eye Dropper Bottle administration requires head - tilting, squeezing, and reliance on gravity Eye Dropper Bottle tips can touch the patient’s eye surface and medication can drip down their face Optejet administration can be done horizontally with the push of a button Optejet has a recessed nozzle, protected by a shutter when not in use to prevent cross - contamination
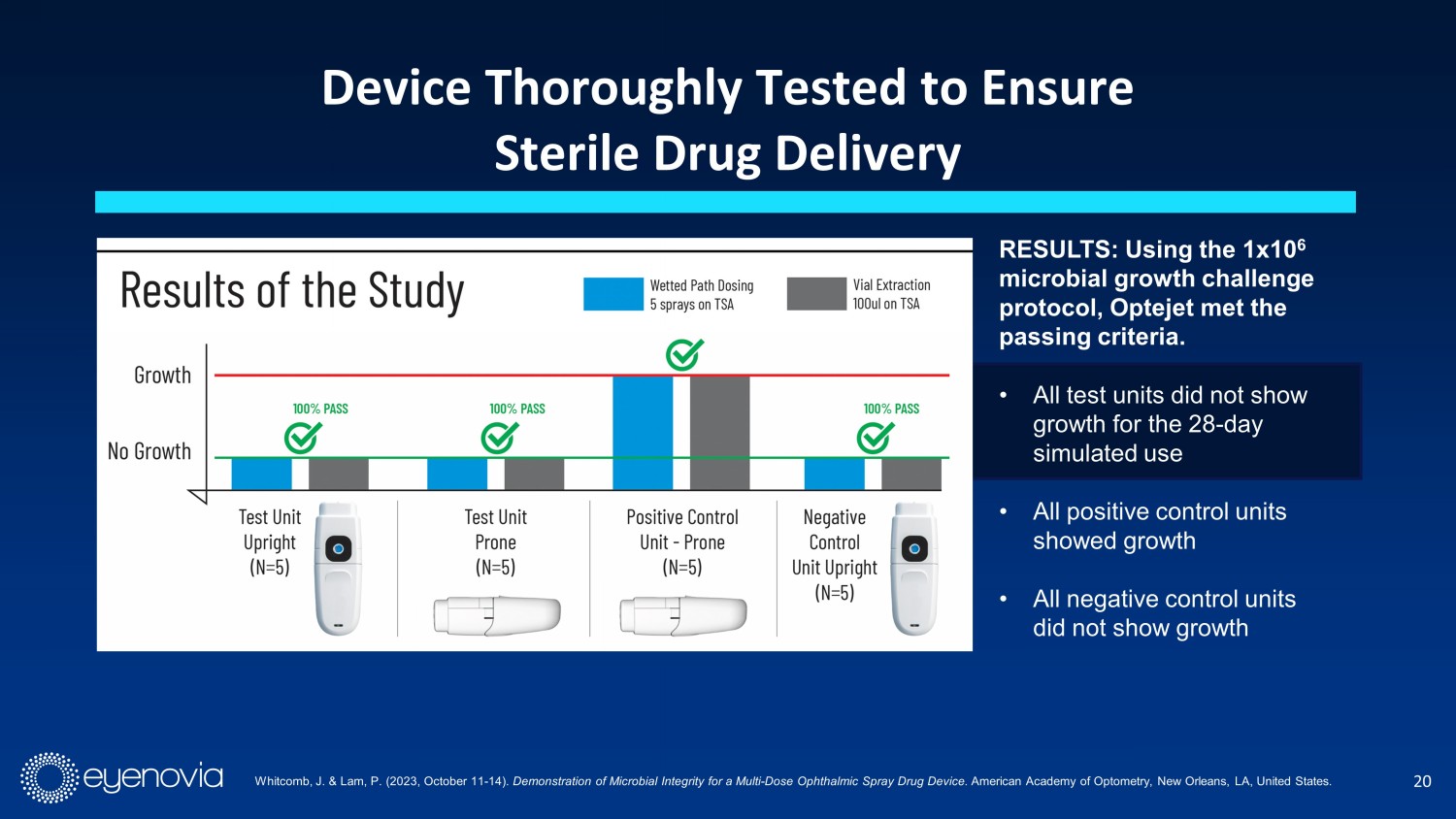
20 Device Thoroughly Tested to Ensure Sterile Drug Delivery RESULTS: Using the 1x10 6 microbial growth challenge protocol, Optejet met the passing criteria. • All test units did not show growth for the 28 - day simulated use • All positive control units showed growth • All negative control units did not show growth Whitcomb, J. & Lam, P. (2023, October 11 - 14). Demonstration of Microbial Integrity for a Multi - Dose Ophthalmic Spray Drug Device. American Academy of Optometry, New Orleans, LA, United States.
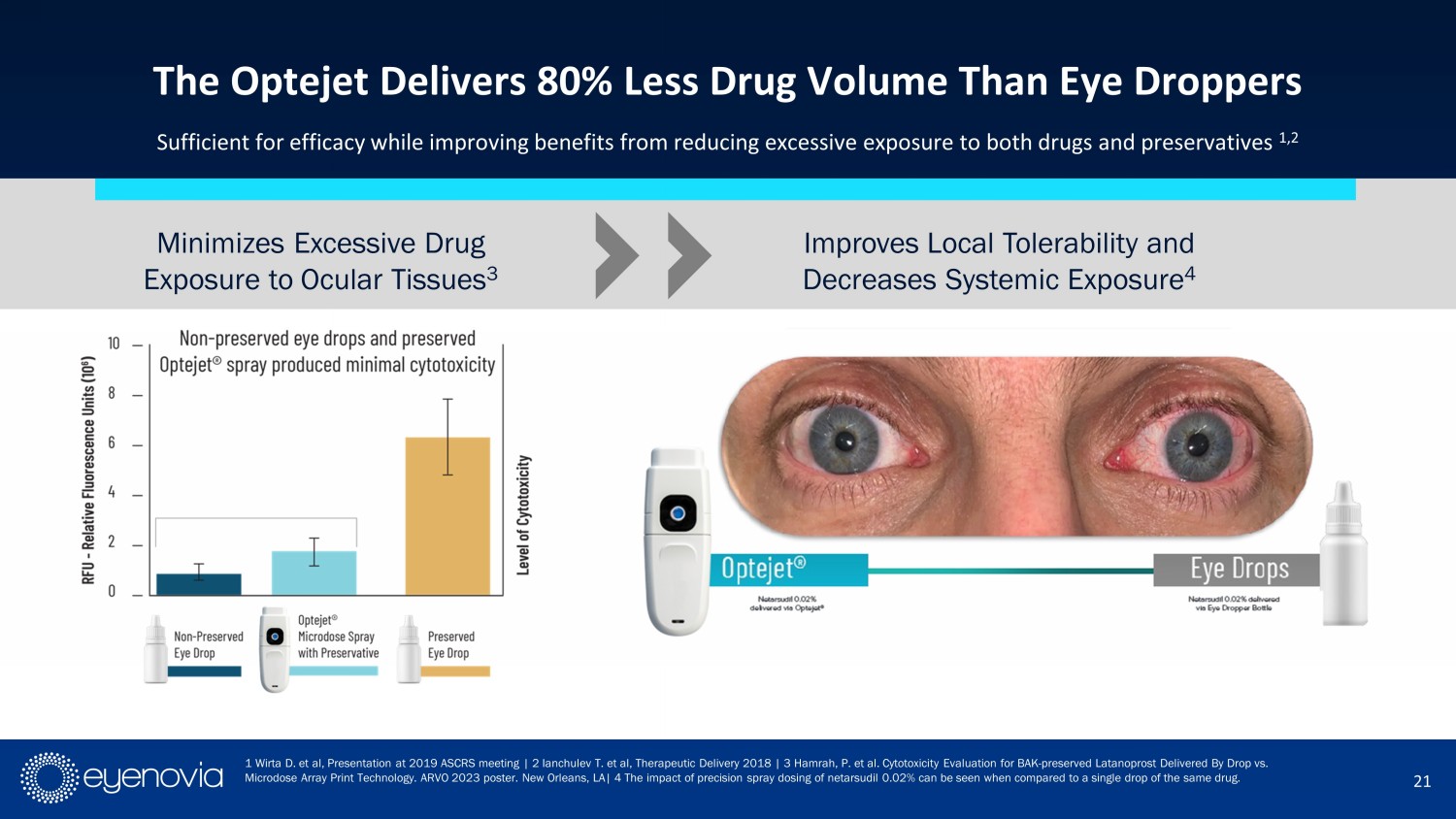
21 The Optejet Delivers 80% Less Drug Volume Than Eye Droppers Sufficient for efficacy while improving benefits from reducing excessive exposure to both drugs and preservatives 1,2 1 Wirta D. et al, Presentation at 2019 ASCRS meeting | 2 Ianchulev T. et al, Therapeutic Delivery 2018 | 3 Hamrah , P. et al. Cytotoxicity Evaluation for BAK - preserved Latanoprost Delivered By Drop vs. Microdose Array Print Technology. ARVO 2023 poster. New Orleans, LA | 4 The impact of precision spray dosing of netarsudil 0.02% can be seen when compared to a single drop of the same drug. Improves Local Tolerability and Decreases Systemic Exposure 4 Minimizes Excessive Drug Exposure to Ocular Tissues 3 When tolerability is poor, patients are very likely to discontinue their medication or put pressure on the ophthalmologist to change their treatment 5 3 4
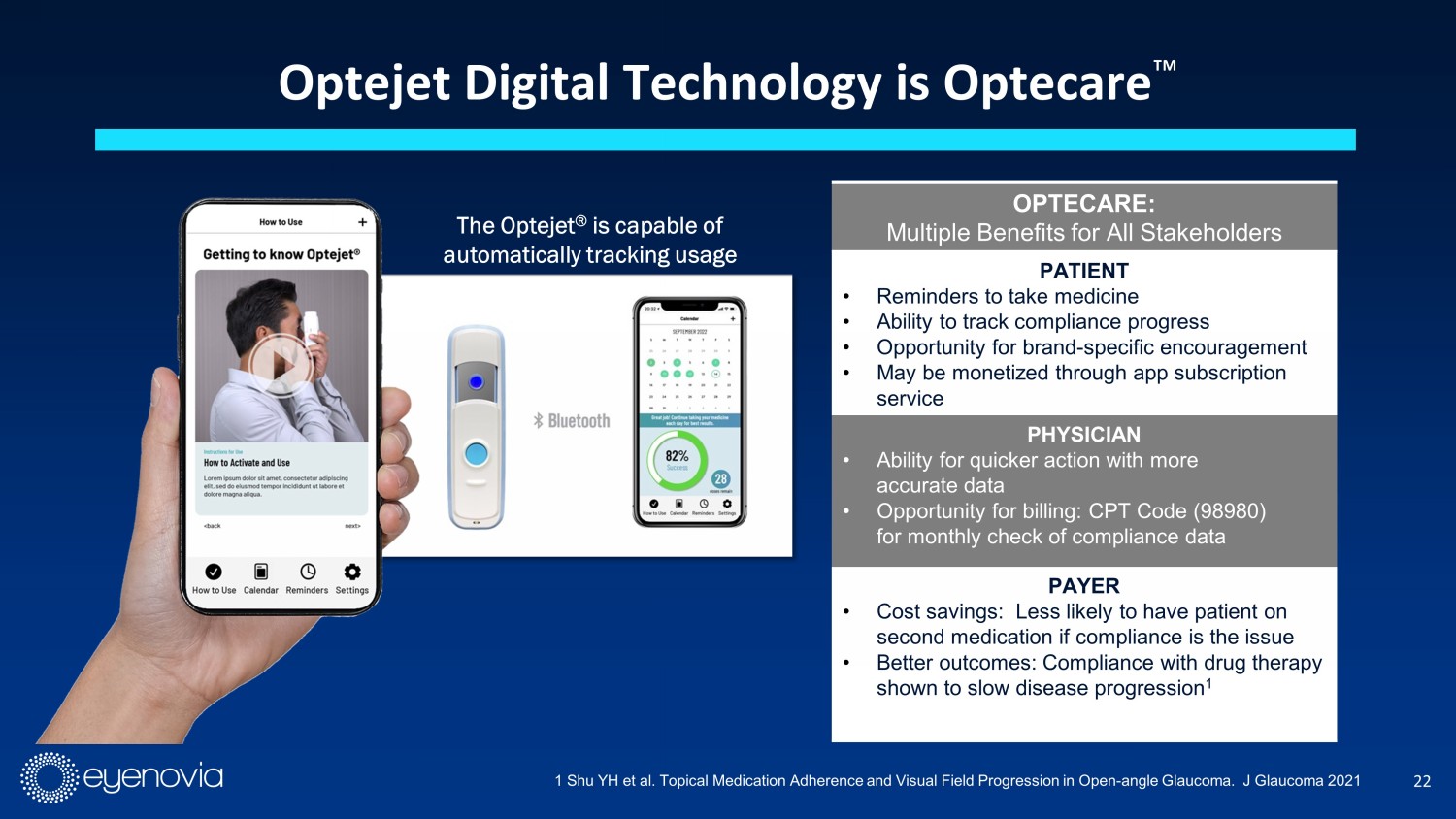
22 Optejet Digital Technology is Optecare Ρ The Optejet ® is capable of automatically tracking usage OPTECARE: Multiple Benefits for All Stakeholders PATIENT • Reminders to take medicine • Ability to track compliance progress • Opportunity for brand - specific encouragement • May be monetized through app subscription service PHYSICIAN • Ability for quicker action with more accurate data • Opportunity for billing: CPT Code (98980) for monthly check of compliance data PAYER • Cost savings: Less likely to have patient on second medication if compliance is the issue • Better outcomes: Compliance with drug therapy shown to slow disease progression 1 1 Shu YH et al. Topical Medication Adherence and Visual Field Progression in Open - angle Glaucoma. J Glaucoma 2021
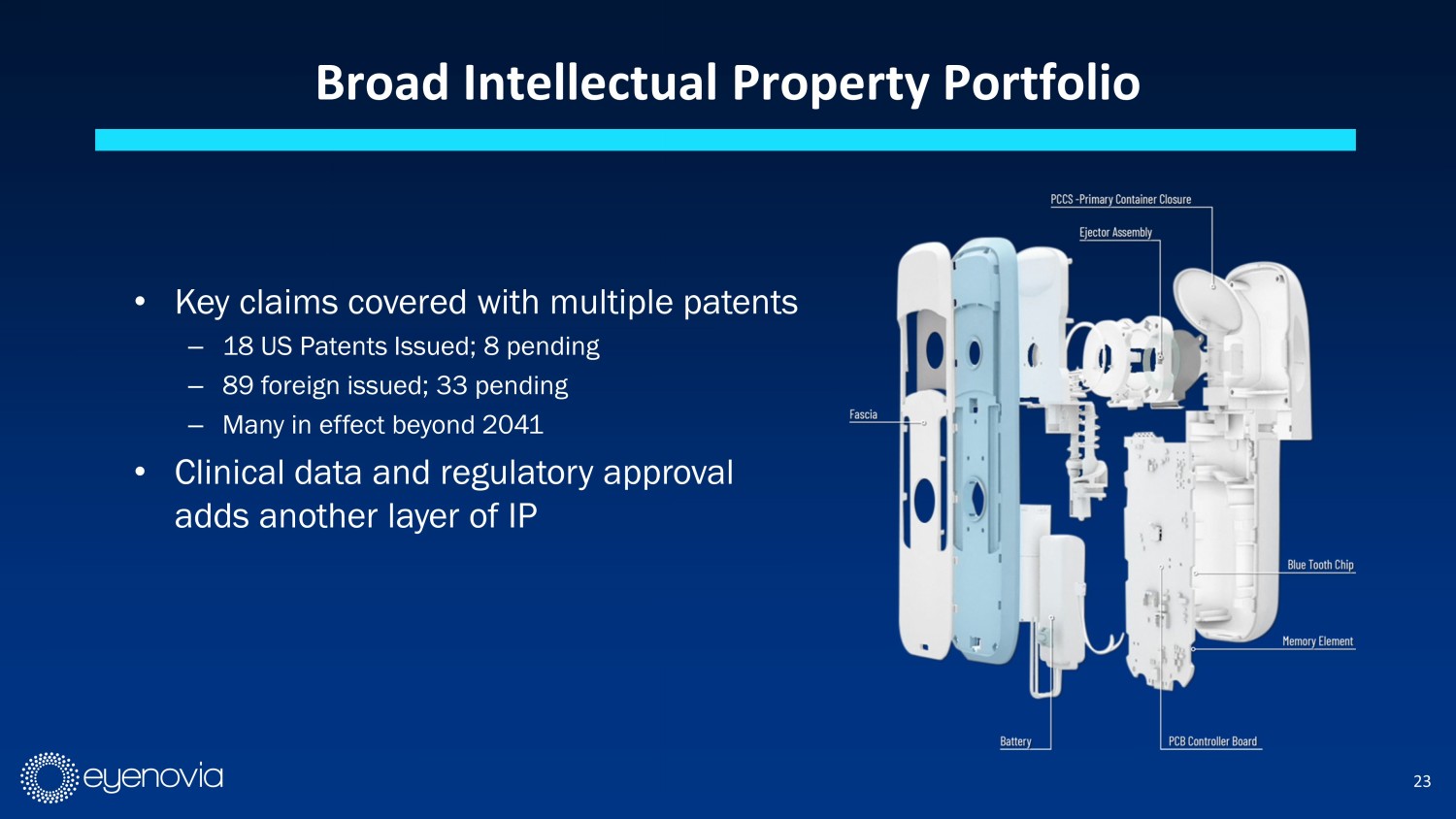
23 Broad Intellectual Property Portfolio • Key claims covered with multiple patents – 18 US Patents Issued; 8 pending – 89 foreign issued; 33 pending – Many in effect beyond 2041 • Clinical data and regulatory approval adds another layer of IP
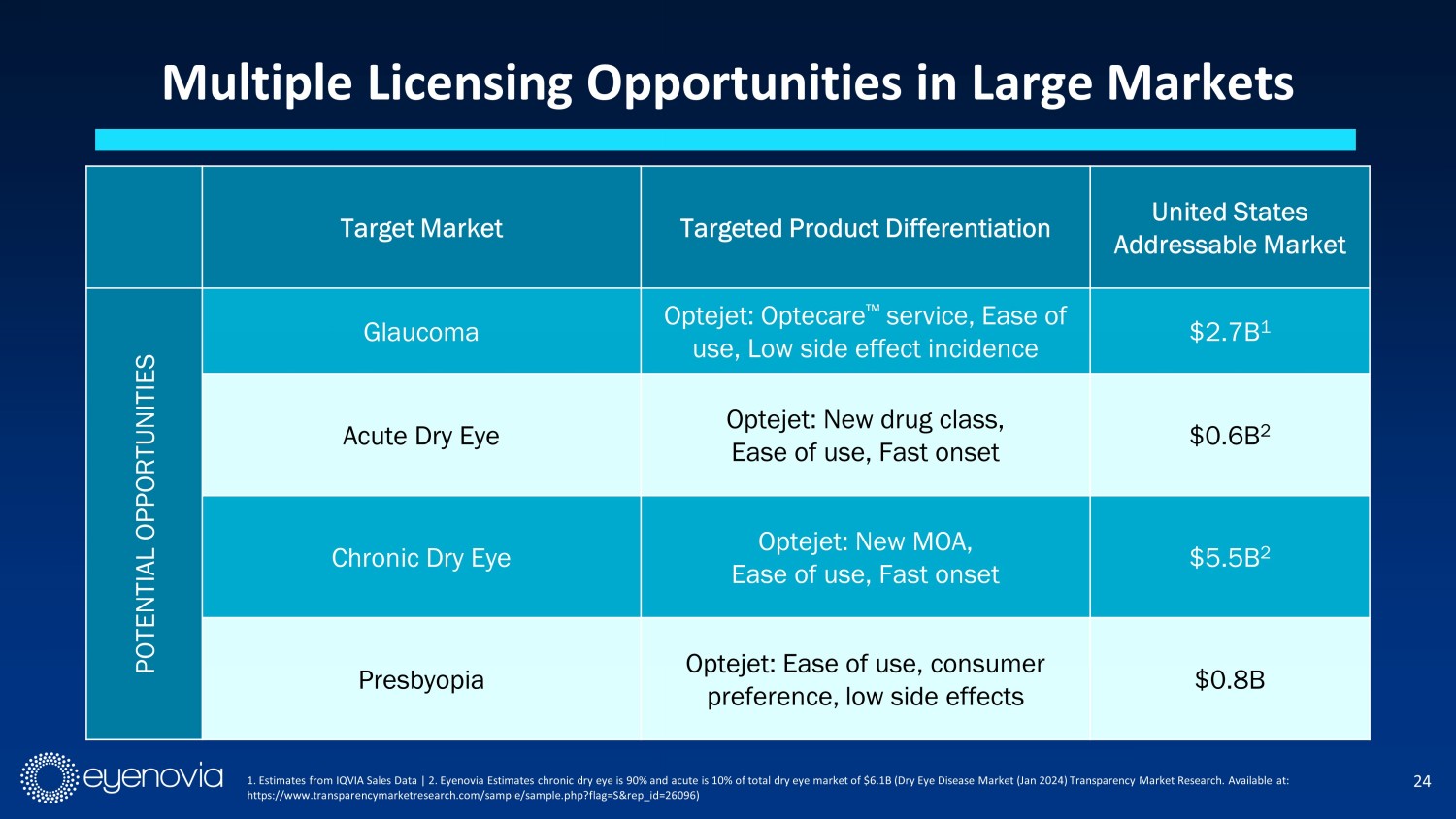
24 Multiple Licensing Opportunities in Large Markets 1. Estimates from IQVIA Sales Data | 2. Eyenovia Estimates chronic dry eye is 90% and acute is 10% of total dry eye market of $6 .1B (Dry Eye Disease Market (Jan 2024) Transparency Market Research. Available at: https://www.transparencymarketresearch.com/sample/sample.php?flag=S&rep_id=26096) Target Market Targeted Product Differentiation United States Addressable Market POTENTIAL OPPORTUNITIES Glaucoma Optejet: Optecare Πservice, Ease of use, Low side effect incidence $2.7B 1 Acute Dry Eye Optejet: New drug class, Ease of use, Fast onset $0.6B 2 Chronic Dry Eye Optejet: New MOA, Ease of use, Fast onset $5.5B 2 Presbyopia Optejet: Ease of use, consumer preference, low side effects $0.8B
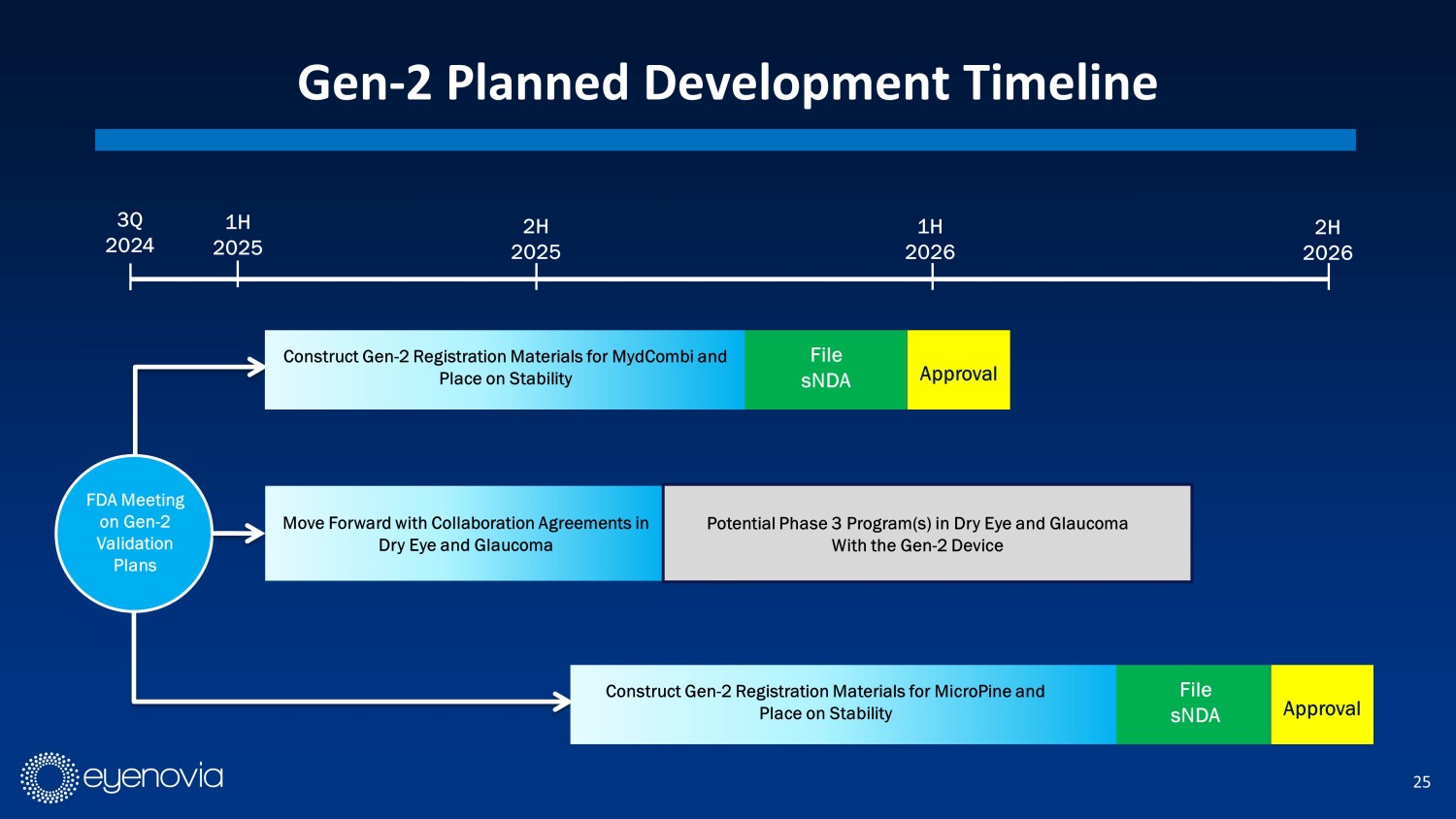
25 Gen - 2 Planned Development Timeline 3Q 2024 2H 2025 1H 2026 2H 2026 1H 2025 Construct Gen - 2 Registration Materials for MydCombi and Place on Stability File sNDA Move Forward with Collaboration Agreements in Dry Eye and Glaucoma FDA Meeting on Gen - 2 Validation Plans Approval Potential Phase 3 Program(s) in Dry Eye and Glaucoma With the Gen - 2 Device Construct Gen - 2 Registration Materials for MicroPine and Place on Stability File sNDA Approval

Clobetasol Propionate Ophthalmic Suspension 0.05% FDA - APPROVED For the treatment of post - operative inflammation and pain following ocular surgery 26 This presentation is not an advertisement for clobetasol propionate.

Safety Information IMPORTANT SAFETY INFORMATION: Clobetasol Propionate Ophthalmic Suspension 0.05% is indicated for the treatment of post - operative inflammation and pain following ocular surgery. CONTRAINDICATIONS: Most active viral diseases of the cornea and conjunctiva, including epithelial herpes simplex keratitis (dendritic keratitis), vaccinia, and varicella, and also in mycobacterial infection of th e e ye and fungal diseases of ocular structures. WARNINGS AND PRECAUTIONS: Intraocular Pressure (IOP) Increase : Prolonged use of corticosteroids may result in glaucoma with damage to the optic nerve, defects in visual acuity and fields of vision. Steroids should be used with caution in the pr ese nce of glaucoma. If this product is used for 10 days or longer, IOP should be monitored. Cataracts : Prolonged use of corticosteroids may result in posterior subcapsular cataract formation. Delayed Healing : The use of steroids after cataract surgery may delay healing and increase the incidence of bleb formation. Corneal and Scleral Melting : In those diseases causing thinning of the cornea or sclera, perforations have been known to occur with the use of topical steroids. The initial prescription and renewal of the medication order should be made by a physician only after exami nat ion of the patient with the aid of magnification, such as slit lamp biomicroscopy, and where appropriate, fluorescein staining. Bacterial Infections : Prolonged use of corticosteroids may suppress the host response and thus increase the hazard of secondary ocular infections. In acute purulent co nditions, steroids may mask infection or enhance existing infection. If signs and symptoms fail to improve after 2 days, the patient should be r eev aluated. Viral Infections : Employment of a corticosteroid medication in the treatment of patients with a history of herpes simplex requires great cau tio n. Use of ocular steroids may prolong the course and may exacerbate the severity of many viral infections of the eye (including herpes sim plex). Fungal Infections : Fungal infections of the cornea are particularly prone to develop coincidentally with long - term local steroid application. Fun gus invasion must be considered in any persistent corneal ulceration where a steroid has been used or is in use. Fungal culture should be tak en when appropriate. ADVERSE REACTIONS: Ocular adverse reactions occurring in ≥ 1% of subjects in clinical studies who received clobetasol propionate ophthalmic suspension 0.05% included eye inflammation (2%), corneal edema (2%), anterior chamber inflammation (2%) , c ystoid macular edema (2%), intraocular pressure elevation (1%), photophobia (1%) and vitreous detachment (1%). Many of these reactio ns may have been the consequence of the surgical procedure . PLEASE GO TO CLOBETASOLBID.COM FOR FULL PRESCRIBING INFORMATION 27
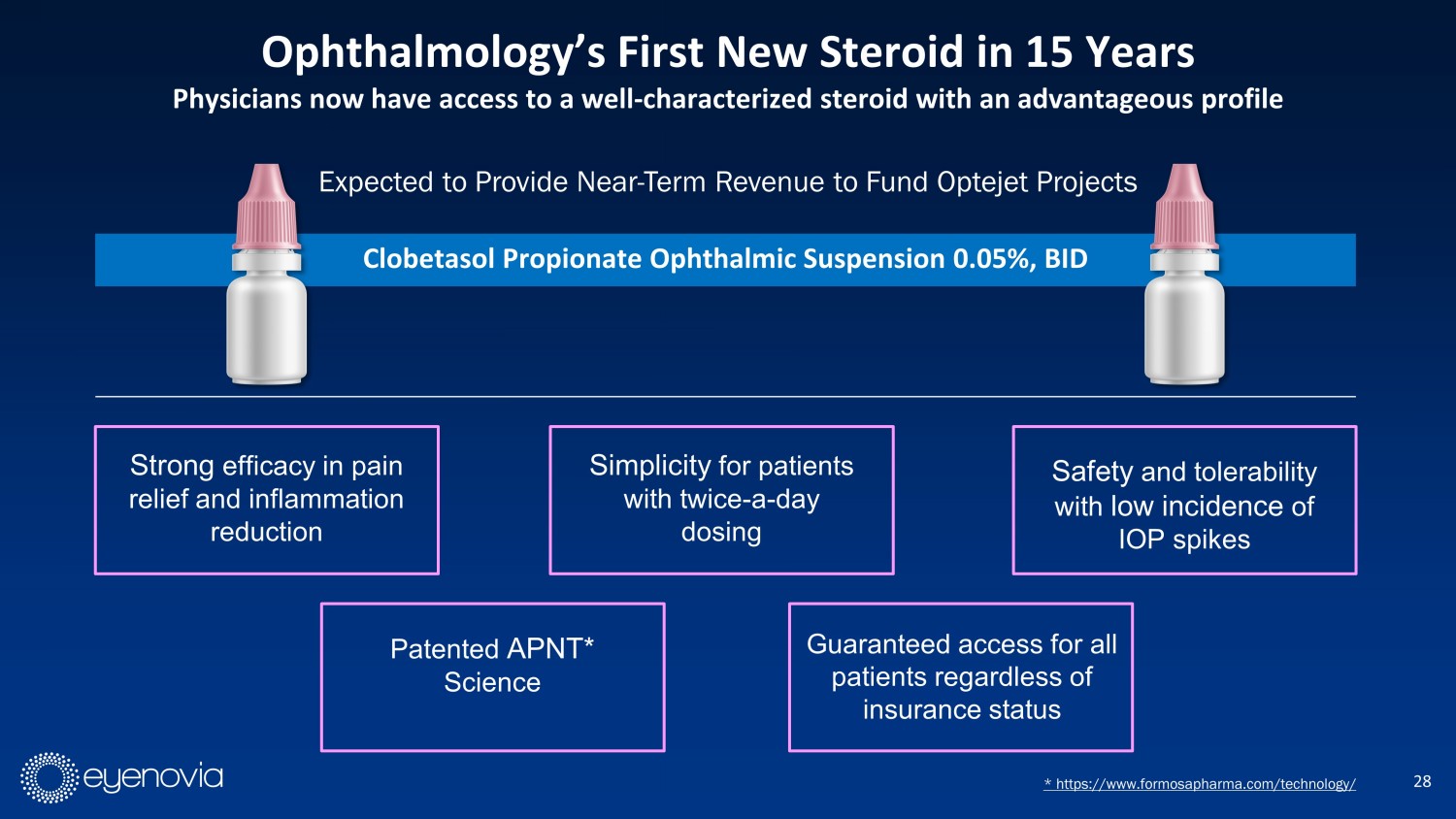
Expected to Provide Near - Term Revenue to Fund Optejet Projects 28 Ophthalmology’s First New Steroid in 15 Years Physicians now have access to a well - characterized steroid with an advantageous profile * https://www.formosapharma.com/technology/ Clobetasol Propionate Ophthalmic Suspension 0.05%, BID Strong efficacy in pain relief and inflammation reduction Safety and tolerability with low incidence of IOP spikes Simplicity for patients with twice - a - day dosing Patented APNT* Science Guaranteed access for all patients regardless of insurance status CD11
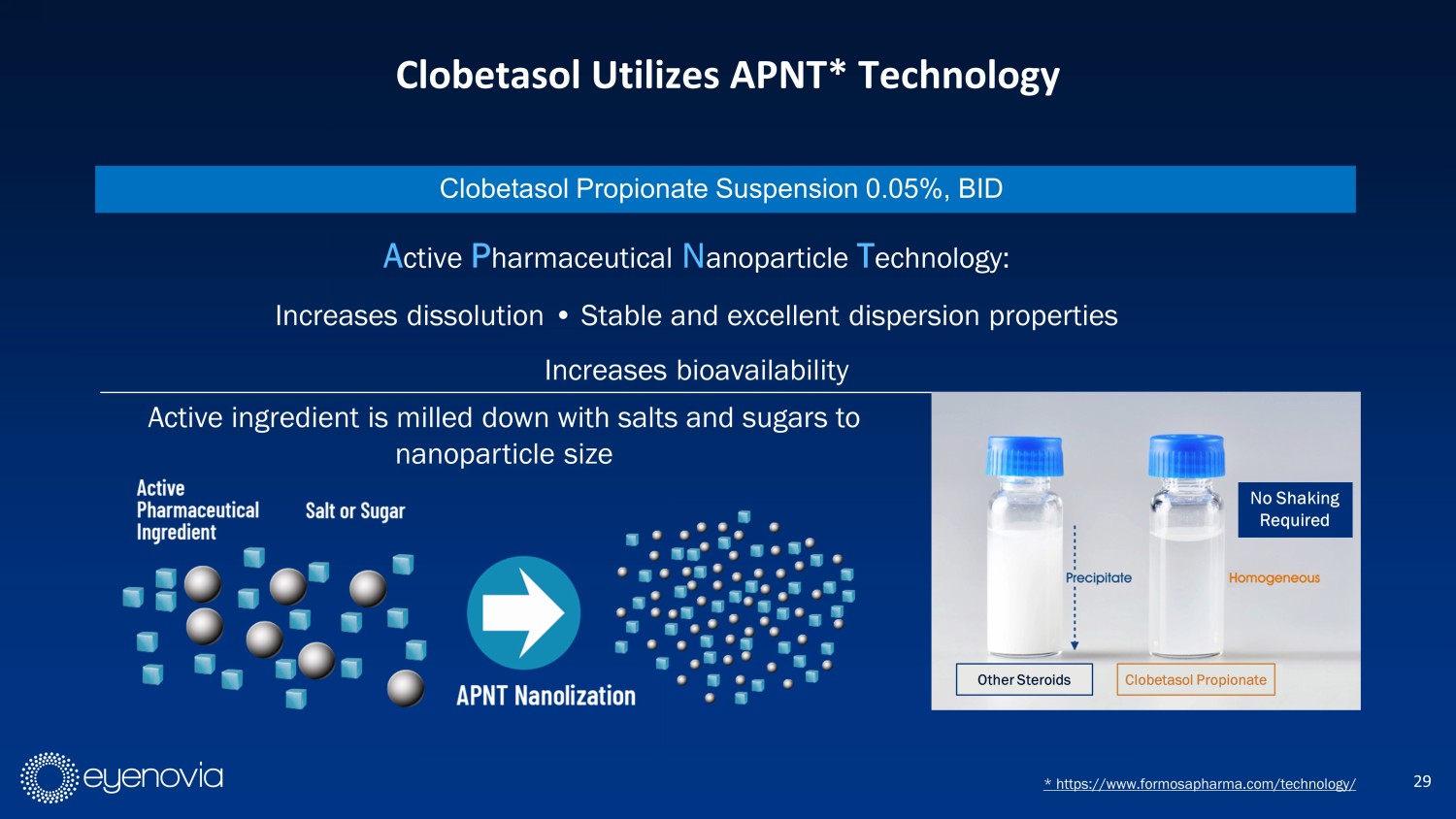
29 Clobetasol Utilizes APNT* Technology * https://www.formosapharma.com/technology/ Clobetasol Propionate Suspension 0.05%, BID Active ingredient is milled down with salts and sugars to nanoparticle size A ctive P harmaceutical N anoparticle T echnology: Increases dissolution • Stable and excellent dispersion properties Increases bioavailability Clobetasol Propionate No Shaking Required Other Steroids
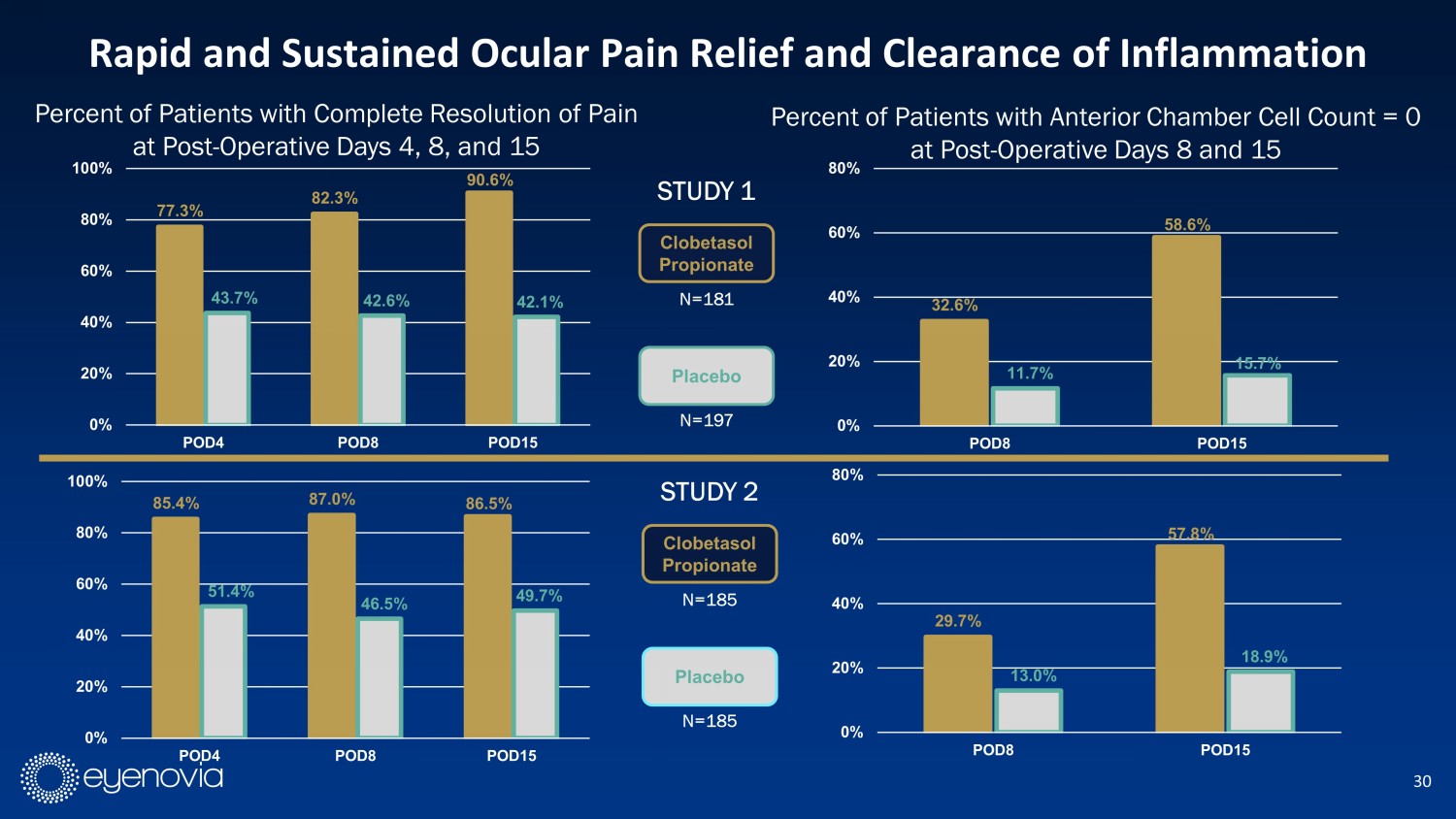
30 77.3% 82.3% 90.6% 43.7% 42.6% 42.1% 0% 20% 40% 60% 80% 100% POD4 POD8 POD15 Rapid and Sustained Ocular Pain Relief and Clearance of Inflammation Percent of Patients with Complete Resolution of Pain at Post - Operative Days 4, 8, and 15 85.4% 87.0% 86.5% 51.4% 46.5% 49.7% 0% 20% 40% 60% 80% 100% POD4 POD8 POD15 Percent of Patients with Anterior Chamber Cell Count = 0 at Post - Operative Days 8 and 15 32.6% 58.6% 11.7% 15.7% 0% 20% 40% 60% 80% POD8 POD15 29.7% 57.8% 13.0% 18.9% 0% 20% 40% 60% 80% POD8 POD15 Clobetasol Propionate Placebo N=181 N=197 STUDY 1 Clobetasol Propionate Placebo N=185 N=185 STUDY 2
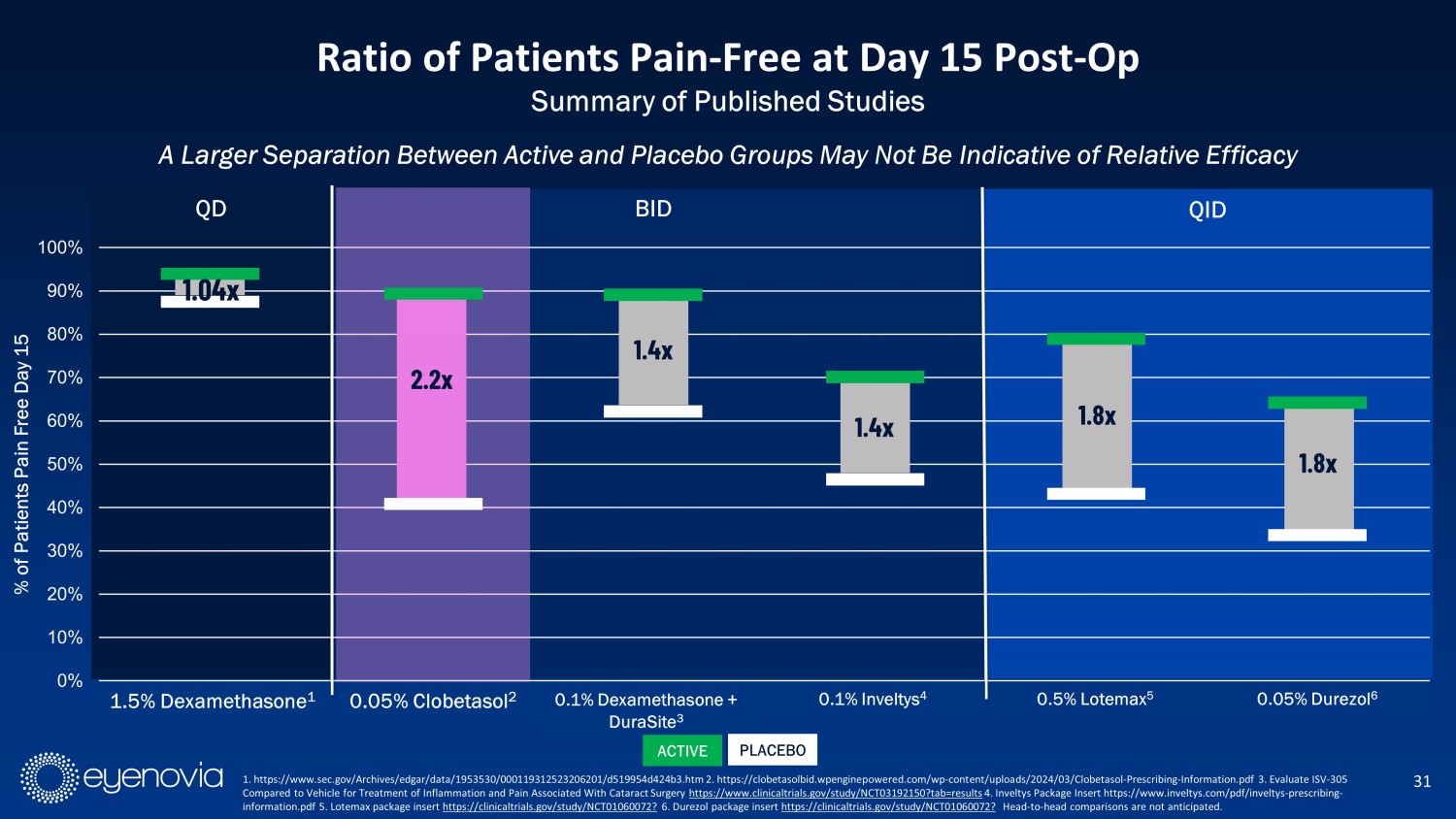
0% 10% 20% 30% 40% 50% 60% 70% 80% 90% 100% 1.5% Dexamethasone 0.05% Clobetasol 0.1% Dexamethasone + DuraSite 0.1 % Inveltys 0.5% Lotemax 0.05% Durezol % of Patients Pain Free Day 15 A Larger Separation Between Active and Placebo Groups May Not Be Indicative of Relative Efficacy 31 Ratio of Patients Pain - Free at Day 15 Post - Op Summary of Published Studies QD QID 2.2 x 1.4x 1.8 x 1.4 x 1.8 x 0.05% Clobetasol 2 0.1% Dexamethasone + DuraSite 3 0.1% Inveltys 4 0.5% Lotemax 5 0.05% Durezol 6 BID 1. https://www.sec.gov/Archives/edgar/data/1953530/000119312523206201/d519954d424b3.htm 2. https://clobetasolbid.wpenginepowe red .com/wp - content/uploads/2024/03/Clobetasol - Prescribing - Information.pdf 3 . Evaluate ISV - 305 Compared to Vehicle for Treatment of Inflammation and Pain Associated With Cataract Surgery https://www.clinicaltrials.gov/study/NCT03192150?tab=results 4. Inveltys Package Insert https://www.inveltys.com/pdf/inveltys - prescribing - information.pdf 5. Lotemax package insert https://clinicaltrials.gov/study/NCT01060072? 6. Durezol package insert https://clinicaltrials.gov/study/NCT01060072? Head - to - head comparisons are not anticipated. 1.04x 1.5% Dexamethasone 1 ACTIVE PLACEBO
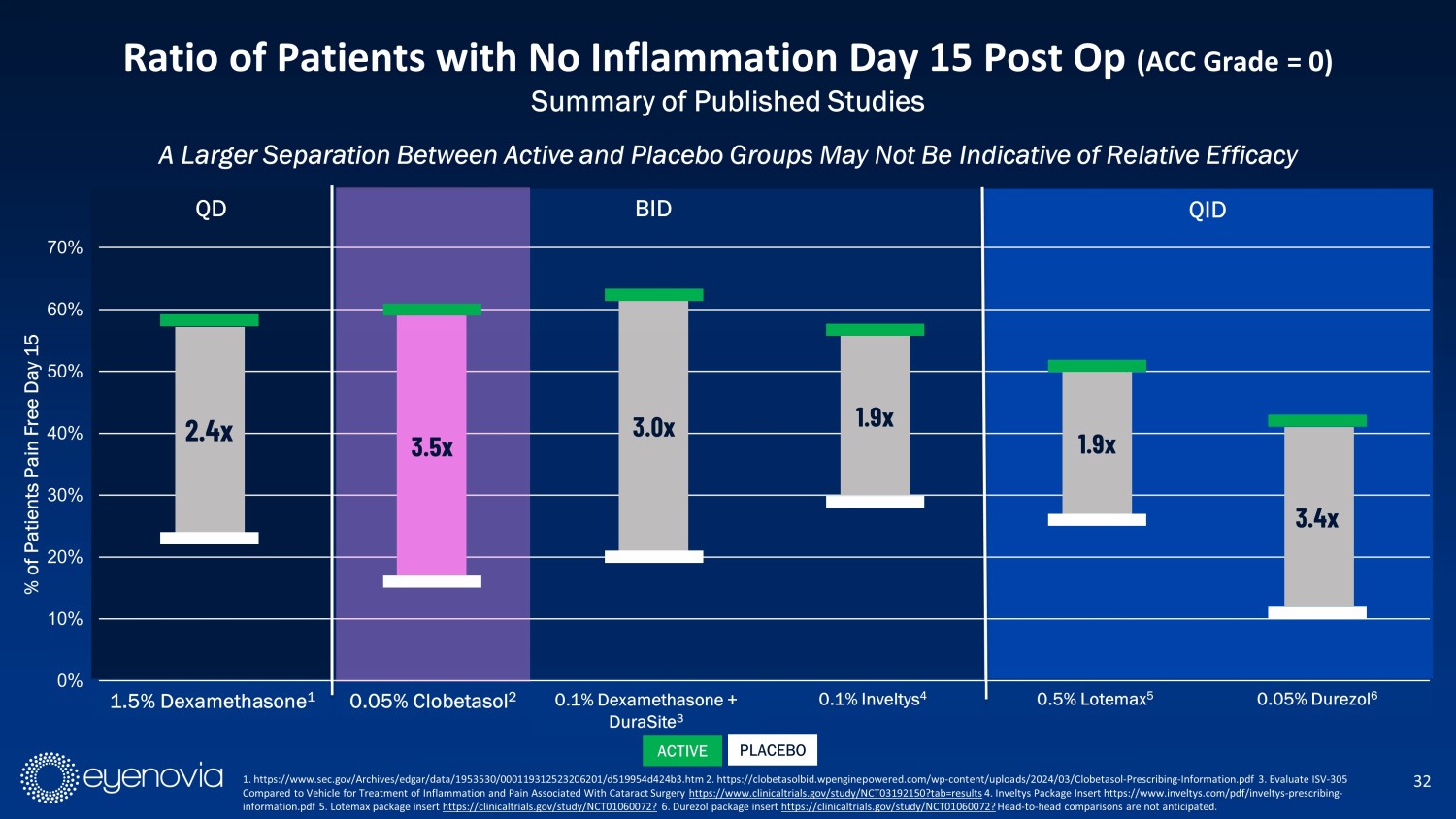
0% 10% 20% 30% 40% 50% 60% 70% 1.5% Dexamethasone 0.05% Clobetasol1 0.1% Dexamethasone + DuraSite2 0.1 % Inveltys3 0.5% Lotemax4 0.05% Durezol5 % of Patients Pain Free Day 15 A Larger Separation Between Active and Placebo Groups May Not Be Indicative of Relative Efficacy 32 Ratio of Patients with No Inflammation Day 15 Post Op (ACC Grade = 0) Summary of Published Studies QD QID 3.5x 3.0x 1.9x 1.9x 3.4x 0.05% Clobetasol 2 0.1% Dexamethasone + DuraSite 3 0.1% Inveltys 4 0.5% Lotemax 5 0.05% Durezol 6 BID 1. https://www.sec.gov/Archives/edgar/data/1953530/000119312523206201/d519954d424b3.htm 2. https://clobetasolbid.wpenginepowe red .com/wp - content/uploads/2024/03/Clobetasol - Prescribing - Information.pdf 3 . Evaluate ISV - 305 Compared to Vehicle for Treatment of Inflammation and Pain Associated With Cataract Surgery https://www.clinicaltrials.gov/study/NCT03192150?tab=results 4. Inveltys Package Insert https://www.inveltys.com/pdf/inveltys - prescribing - information.pdf 5. Lotemax package insert https://clinicaltrials.gov/study/NCT01060072? 6. Durezol package insert https://clinicaltrials.gov/study/NCT01060072? Head - to - head comparisons are not anticipated. 2.4x 1.5% Dexamethasone 1 ACTIVE PLACEBO
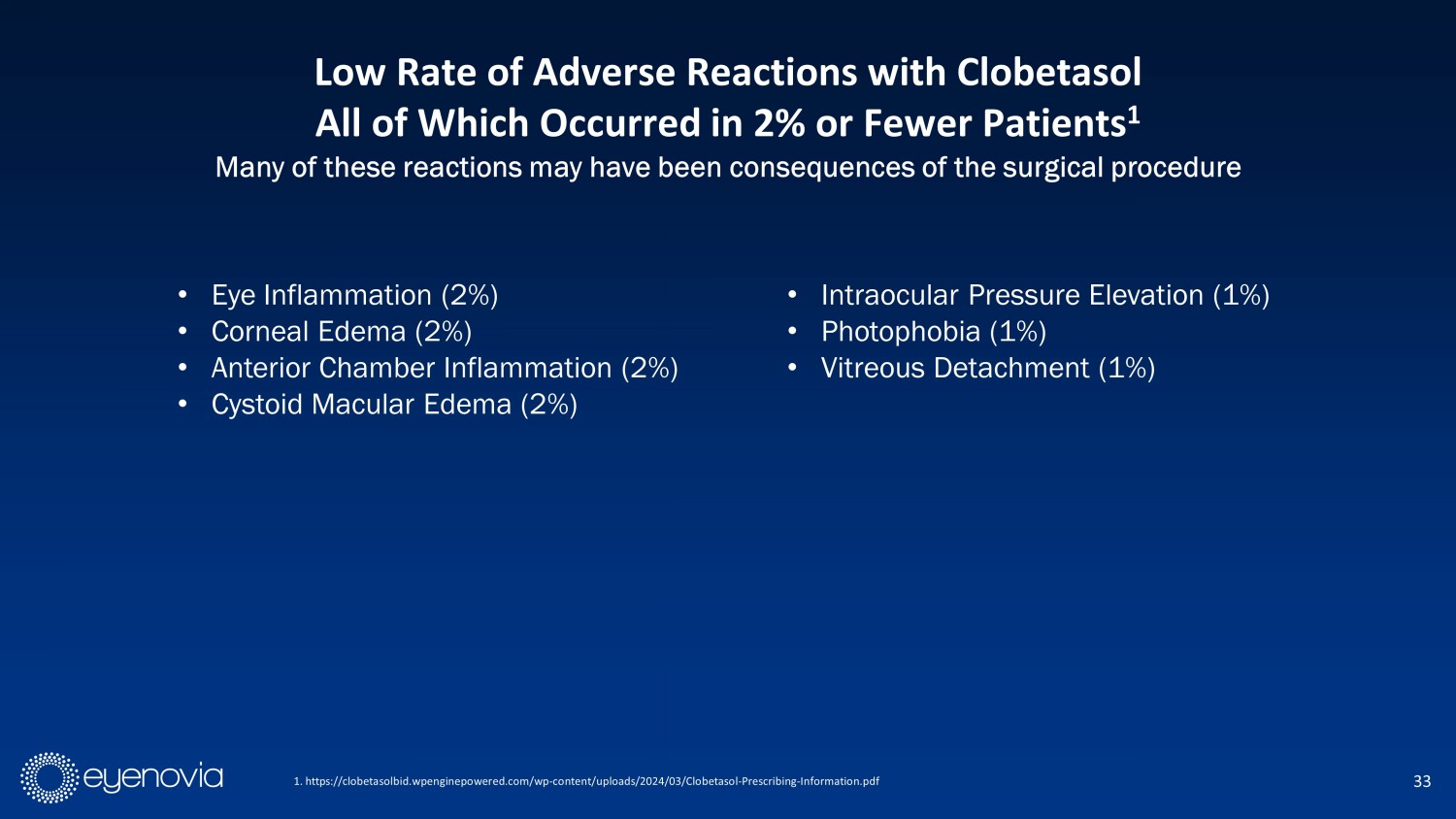
33 1. https://clobetasolbid.wpenginepowered.com/wp - content/uploads/2024/03/Clobetasol - Prescribing - Information.pdf Low Rate of Adverse Reactions with Clobetasol All of Which Occurred in 2% or Fewer Patients 1 Many of these reactions may have been consequences of the surgical procedure • E ye I nflammation (2%) • C orneal E dema (2%) • A nterior C hamber I nflammation (2%) • C ystoid M acular E dema (2%) • I ntraocular P ressure E levation (1%) • P hotophobia (1%) • V itreous D etachment (1%)
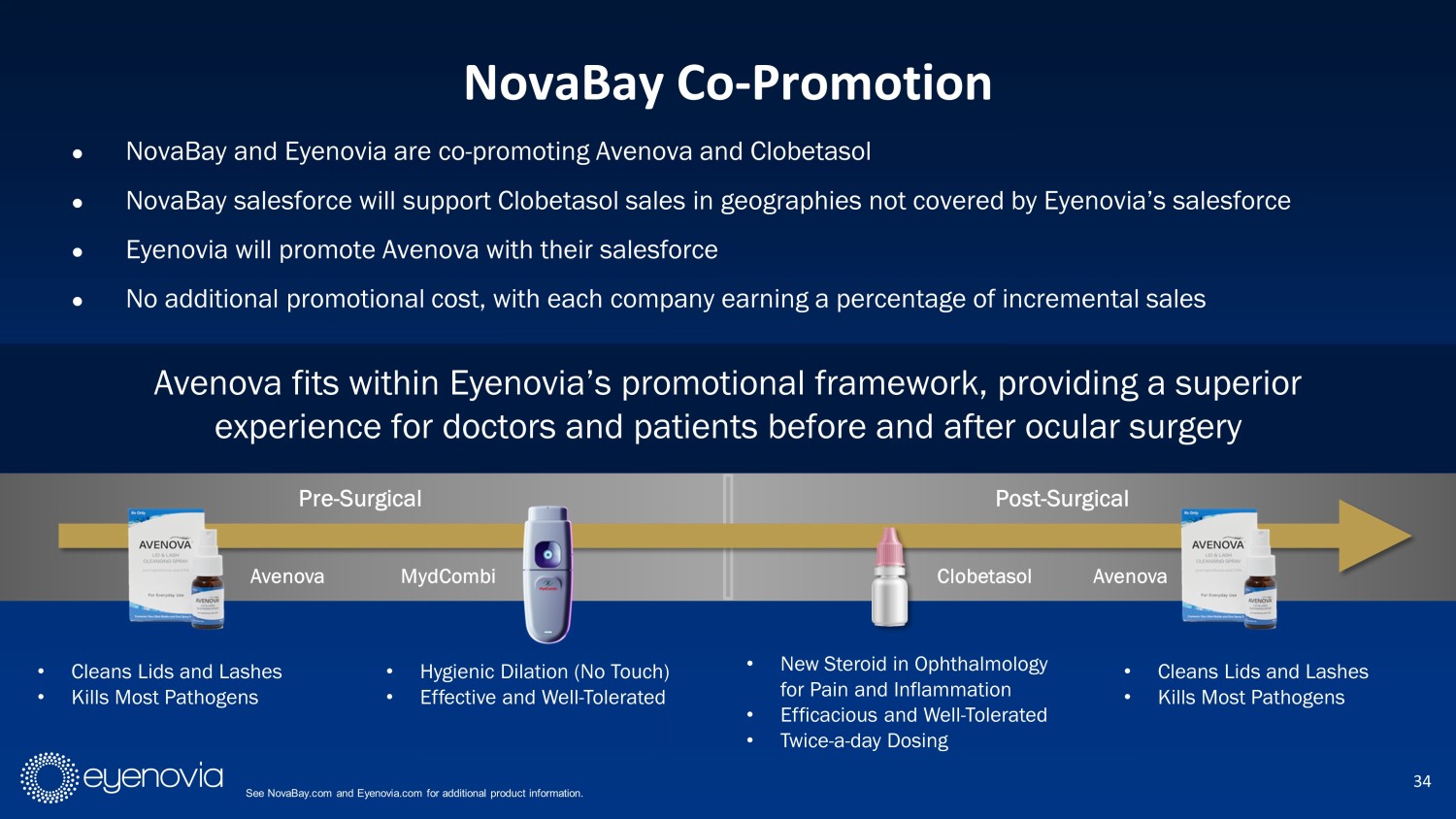
● NovaBay and Eyenovia are co - promoting Avenova and Clobetasol ● NovaBay salesforce will support Clobetasol sales in geographies not covered by Eyenovia’s salesforce ● Eyenovia will promote Avenova with their salesforce ● No additional promotional cost, with each company earning a percentage of incremental sales Avenova fits within Eyenovia’s promotional framework, providing a superior experience for doctors and patients before and after ocular surgery 34 NovaBay Co - Promotion See NovaBay.com and Eyenovia.com for additional product information. Pre - Surgical Post - Surgical • Cleans Lids and Lashes • Kills Most Pathogens • Hygienic Dilation (No Touch) • Effective and Well - Tolerated • New Steroid in Ophthalmology for Pain and Inflammation • Efficacious and Well - Tolerated • Twice - a - day Dosing • Cleans Lids and Lashes • Kills Most Pathogens Avenova Clobetasol MydCombi Avenova
MydCombi Ρ Ophthalmic Spray (1% tropicamide and 2.5% phenylephrine) FDA - APPROVED For short - term in - office or pre - surgical pupil dilation 35 This presentation is not an advertisement for MYDCOMBI.

Safety Information IMPORTANT SAFETY INFORMATION: MYDCOMBI (tropicamide and phenylephrine hydrochloride ophthalmic spray) 1%/2.5% is indicated to induce mydriasis for routine diagnostic procedures and in conditions where short term pupil dilation is desired. CONTRAINDICATIONS: Known hypersensitivity to any component of the formulation. WARNINGS AND PRECAUTIONS: FOR TOPICAL OPHTHALMIC USE. NOT FOR INJECTION. This preparation may cause CNS disturbances which may be dangerous in pediatric patients. The possibility of psychotic reaction and behavioral disturbance due to hypersensitivity to anticholinergic drugs should be considered. Mydriatics may produce a transient elevation of intraocular pressure . Significant elevations in blood pressure have been reported. Caution in patients with elevated blood pressure. Rebound miosis has been reported one day after installation. Remove contact lenses before using . DRUG INTERACTIONS: Atropine - like Drugs : May exaggerate the adrenergic pressor response. Cholinergic Agonists and Ophthalmic Cholinesterase Inhibitors : May interfere with the antihypertensive action of carbachol, pilocarpine, or ophthalmic cholinesterase inhibitors. Potent Inhalation Anesthetic Agents : May potentiate cardiovascular depressant effects of some inhalation anesthetic agents. ADVERSE REACTIONS: Most common ocular adverse reactions include transient blurred vision, reduced visual acuity, photophobia, superficial punctate keratitis, and mild eye discomfort. Increased intraocular pressure has been reported following the use of mydriatics. Systemic adverse reactions including dryness of the mouth, tachycardia, headache, allergic reactions, nausea, vomiting, pallor, central nervous system disturbances and muscle rigidity have been reported with the use of tropicamide. PLEASE GO TO MYDCOMBI.COM FOR FULL PRESCRIBING INFORMATION 36

37 MydCombi Ρ Modern Mydriasis: An Easy Way to Dilate 1 Indication: MYDCOMBI (tropicamide 1% and phenylephrine HCl 2.5%) ophthalmic spray is indicated to induce mydriasis for routin e d iagnostic procedures and in conditions where short term pupil dilation is desired. IMPORTANT SAFETY INFORMATION. CONTRAINDICATIONS: Known hypersensitivity to any component of the formulation. WARNINGS AND PRECAUTIONS. FOR PRECAUTIONS. FOR TOPICAL OPHTHALMIC USE. NOT FOR INJECTION. This preparation may cause CNS disturbances which may be dangerous in pediatri c p atients. The possibility of psychotic pediatric patients. The possibility of psychotic reaction and behavioral disturbance due to hypersensitivity to anticholinerg ic drugs should be considered. Mydriatics may produce a transient considered. Mydriatics may produce a transient elevation of intraocular pressure. Significant elevations in blood pressure ha ve been reported. Caution in patients with elevated blood pressure. in patients with elevated blood pressure. Rebound miosis has been reported one day after installation. Remove contact lenses bef ore using. DRUG INTERACTIONS. Atropine - like Drugs: May INTERACTIONS. Atropine - like Drugs: May exaggerate the adrenergic pressor response. Cholinergic Agonists and Ophthalmic Cholinesterase Inhibito rs: May interfere with the antihypertensive action of carbachol, pilocarpine, or ophthalmic cholinesterase inhibitors. Potent Inhalation Anesthetic Agents: May potentiat e c ardiovascular depressant effects of some inhalation anesthetic agents. ADVERSE REACTIONS. Most common ocular adverse reactions include transient blurred vision, reduced visual acuity, photophobia, superficial puncta te keratitis, and mild eye discomfort. Increased intraocular pressure has been reported following the use of mydriatics. Systemic adverse reactions including drynes s o f the mouth, tachycardia, headache, allergic reactions, nausea, vomiting, pallor, central nervous system disturbances and muscle rigidity have been reported with the use of tropicamide. To rep ort SUSPECTED ADVERSE REACTIONS, contact Eyenovia, Inc. At 1 - 833 - 393 - 6684 or FDA at 1 - 800 - FDA - 1088 (www.fda.gov/medwatch) www.mydcombi.com for FULL PRESCRIBING INFORMATION The only FDA - approved fixed - dose combination of the leading pupil dilating drugs and validation of the Optejet Œ delivery platform Quickly achieves clinically necessary dilation and reliable time to resolution. 1 Well tolerated. In clinical studies 97% of patients reported zero side effects 1 Transitioned Eyenovia to a commercial stage company along with the creation of an internal sales force and distribution channels Introduced easy drug delivery and the Optejet Œ experience to patients, physicians, and technicians in a $250MM addressable market 2 1. Wirta DL, Walters TR, Flynn WJ, Rathi S, Ianchulev T. Mydriasis with micro - array print touch - free tropicamide - phenylephrine fixed combination MIST: pooled randomized Phase III tr ials. Ther Deliv . 2021 Mar;12(3):201 - 214. 2. $200M annual sales of pharmaceutical mydriatic products used during 108M office - based exams ($2 * 100M) + $50M of single bottle mydriatic agents used cataract replacement surgery ($12.5 x 4M)
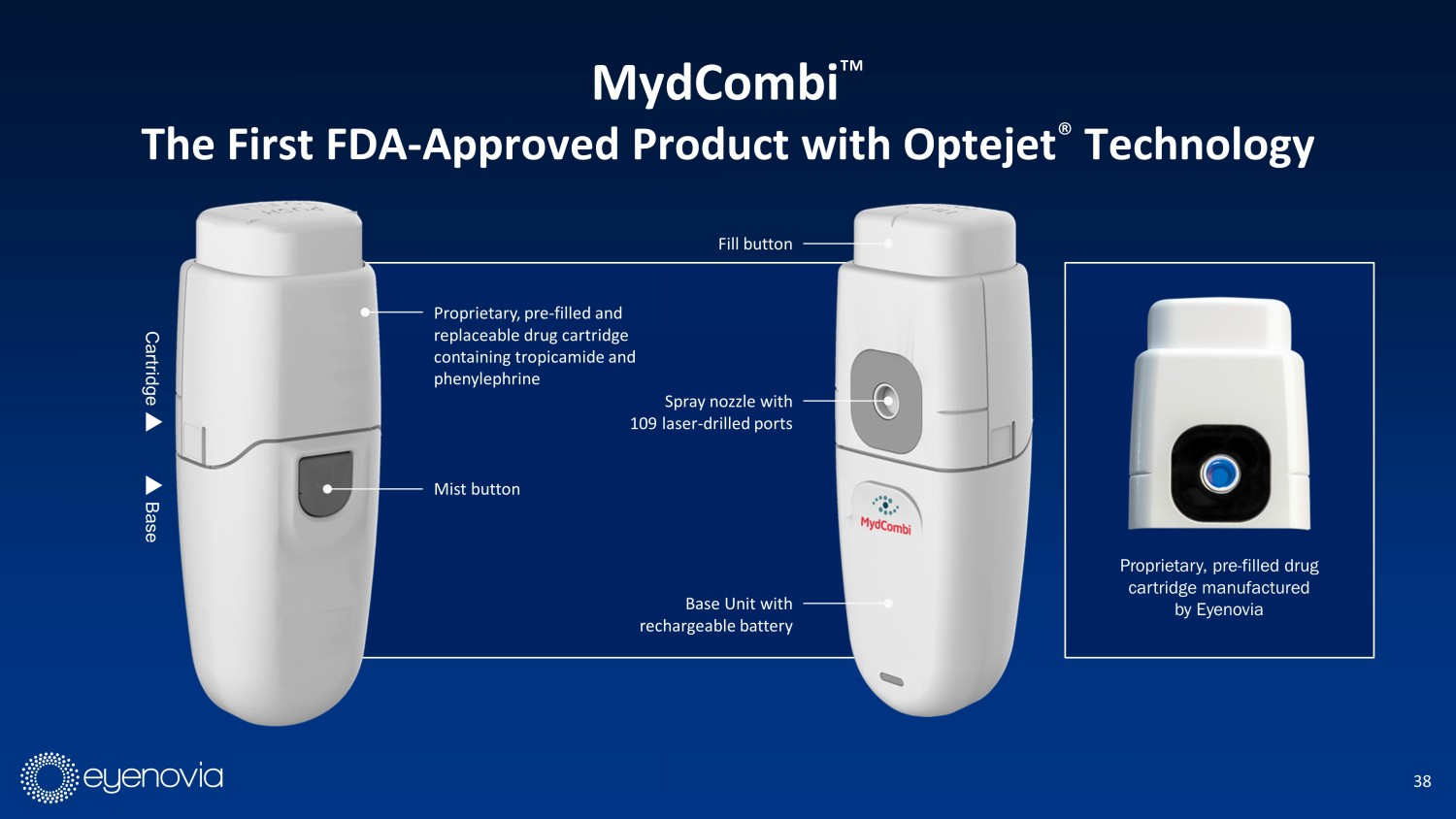
38 Spray nozzle with 109 laser - drilled ports Mist button Proprietary, pre - filled and replaceable drug cartridge containing tropicamide and phenylephrine Fill button Base Unit with rechargeable battery Cartridge Base Proprietary, pre - filled drug cartridge manufactured by Eyenovia MydCombi Ρ The First FDA - Approved Product with Optejet ® Technology
39 The Office - Based and Surgical Pupil Dilation Market $250 Million Opportunity 1 in the United States 1. $200M annual sales of pharmaceutical mydriatic products used during 108M office - based exams ($2 * 100M) + $50M of single bott le mydriatic agents used cataract replacement surgery ($12.5 x 4M) ● The leading pupil dilation drugs are tropicamide and phenylephrine, both used individually and together and delivered as eye drops ● There are approximately 108 million office - based dilations performed annually in the United States ● The current process suffers from a number of shortfalls: ○ - Multiple eyedrops are usually needed ○ - Patient discomfort and avoidance ○ - Time - consuming administration and slow recovery to “normal” ○ - Cross - contamination risk
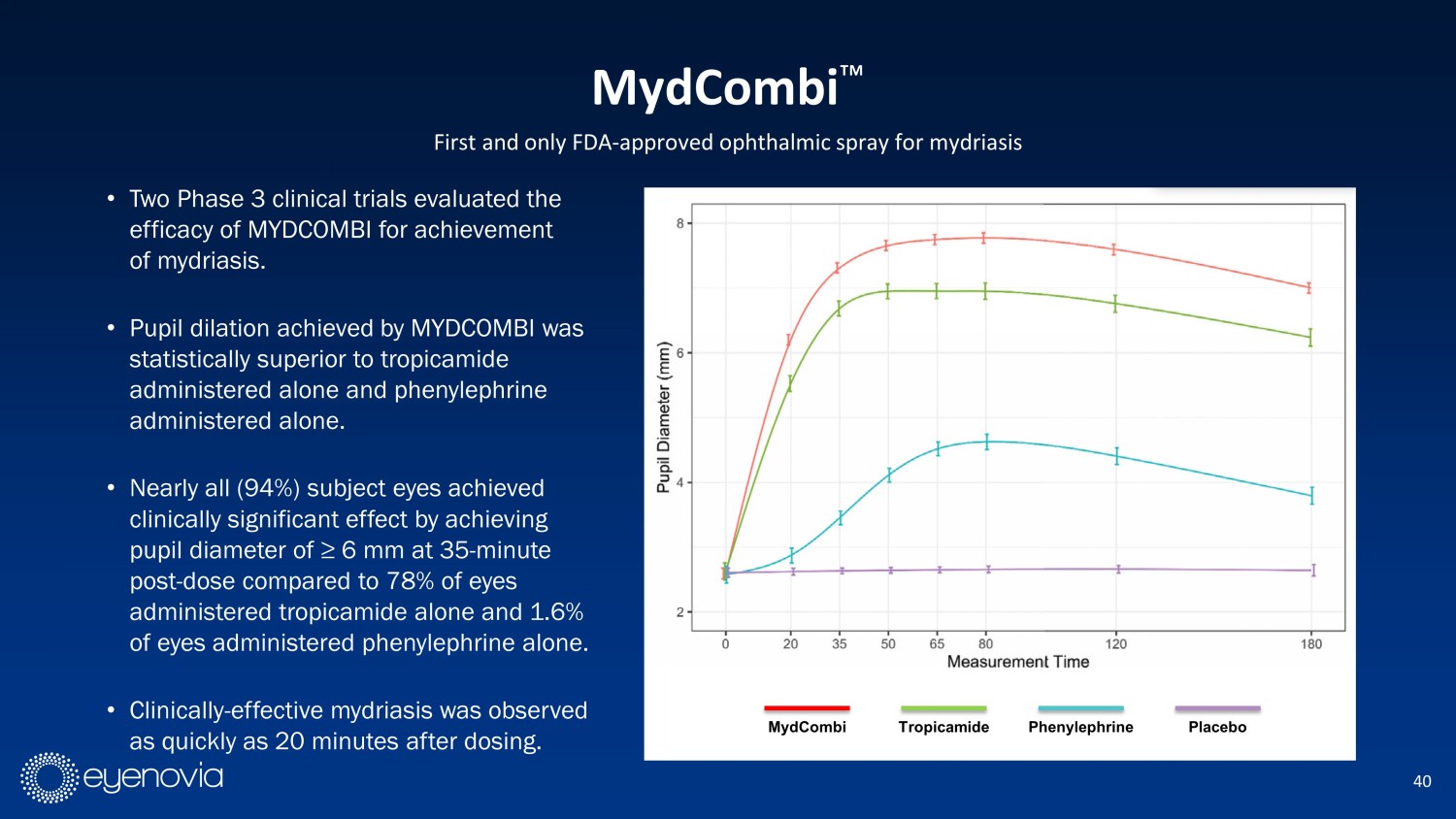
40 First and only FDA - approved ophthalmic spray for mydriasis • Two Phase 3 clinical trials evaluated the efficacy of MYDCOMBI for achievement of mydriasis. • Pupil dilation achieved by MYDCOMBI was statistically superior to tropicamide administered alone and phenylephrine administered alone. • Nearly all (94%) subject eyes achieved clinically significant effect by achieving pupil diameter of ≥ 6 mm at 35 - minute post - dose compared to 78% of eyes administered tropicamide alone and 1.6% of eyes administered phenylephrine alone. • Clinically - effective mydriasis was observed as quickly as 20 minutes after dosing. MydCombi Ρ MydCombi Tropicamide Phenylephrine Placebo
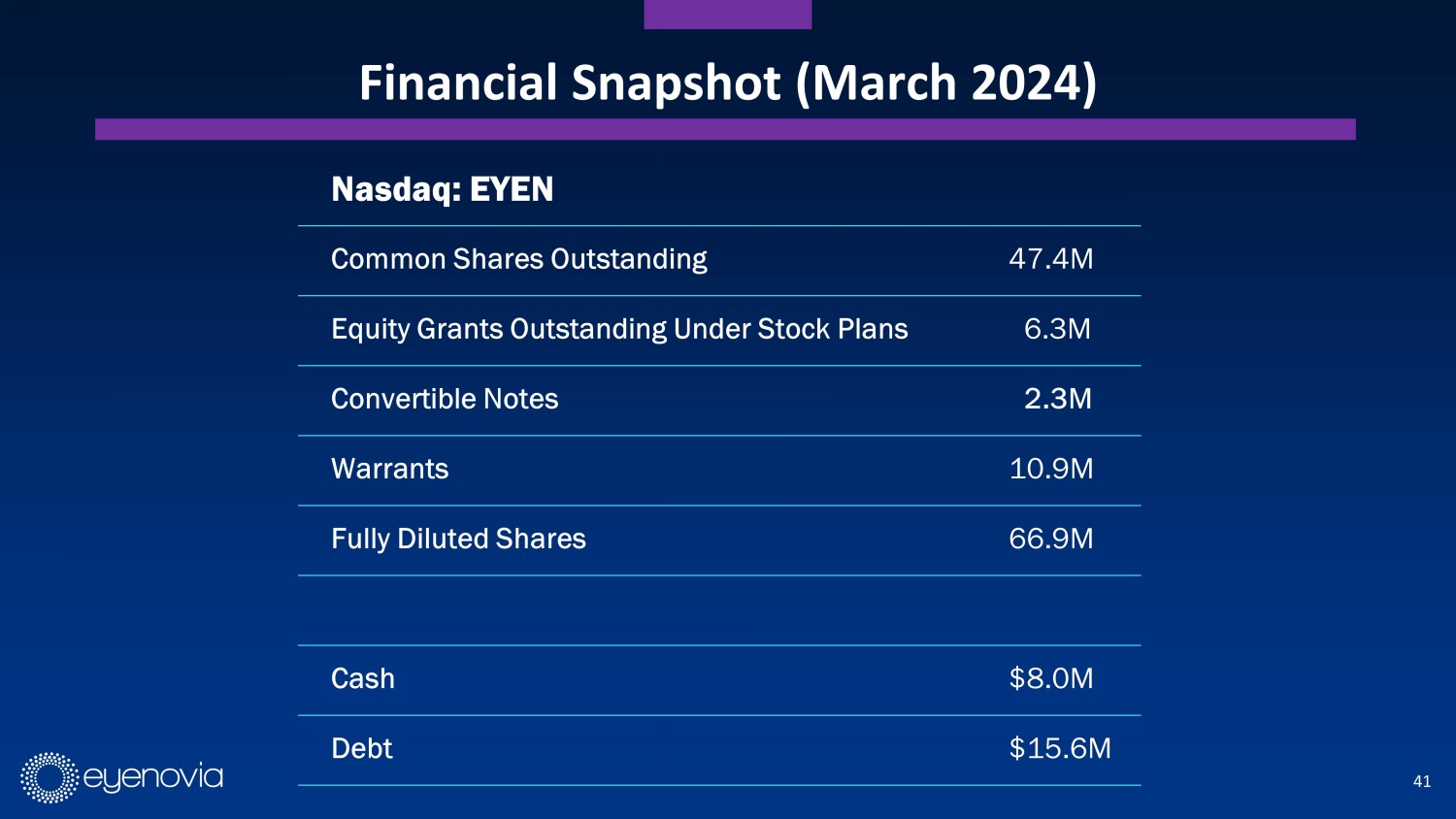
41 Financial Snapshot (March 2024) Nasdaq: EYEN Common Shares Outstanding 47.4M Equity Grants Outstanding Under Stock Plans 6.3M Convertible Notes 2.3M Warrants 10.9M Fully Diluted Shares 66.9M Cash $8.0M Debt $15.6M

42 Experienced Leadership Team Bren Kern Chief Operating Officer Michael Rowe Chief Executive Officer John Gandolfo Chief Financial Officer Norbert Lowe VP, Commercial Operations Greg Bennett VP, Clinical Program Strategy and Development Malini Batheja , PhD VP, Pharm R&D and CMC Regulatory Enrico Brambilla VP, Device R&D and Engineering Lauren Gidden VP, Quality and Regulatory Affairs Rob Richardson VP, Manufacturing
Cover |
Jun. 04, 2024 |
|---|---|
| Cover [Abstract] | |
| Document Type | 8-K |
| Amendment Flag | false |
| Document Period End Date | Jun. 04, 2024 |
| Entity File Number | 001-38365 |
| Entity Registrant Name | EYENOVIA, INC. |
| Entity Central Index Key | 0001682639 |
| Entity Tax Identification Number | 47-1178401 |
| Entity Incorporation, State or Country Code | DE |
| Entity Address, Address Line One | 295 Madison Avenue |
| Entity Address, Address Line Two | Suite 2400 |
| Entity Address, City or Town | New York |
| Entity Address, State or Province | NY |
| Entity Address, Postal Zip Code | 10017 |
| City Area Code | 833 |
| Local Phone Number | 393-6684 |
| Written Communications | false |
| Soliciting Material | false |
| Pre-commencement Tender Offer | false |
| Pre-commencement Issuer Tender Offer | false |
| Title of 12(b) Security | Common stock, par value $0.0001 per share |
| Trading Symbol | EYEN |
| Security Exchange Name | NASDAQ |
| Entity Emerging Growth Company | false |
1 Year Eyenovia Chart |
1 Month Eyenovia Chart |

It looks like you are not logged in. Click the button below to log in and keep track of your recent history.
Support: +44 (0) 203 8794 460 | support@advfn.com
By accessing the services available at ADVFN you are agreeing to be bound by ADVFN's Terms & Conditions