
We could not find any results for:
Make sure your spelling is correct or try broadening your search.
| Share Name | Share Symbol | Market | Type |
|---|---|---|---|
| Erasca Inc | NASDAQ:ERAS | NASDAQ | Common Stock |
| Price Change | % Change | Share Price | Bid Price | Offer Price | High Price | Low Price | Open Price | Shares Traded | Last Trade | |
|---|---|---|---|---|---|---|---|---|---|---|
| 0.04 | 1.61% | 2.52 | 2.52 | 2.59 | 2.56 | 2.47 | 2.47 | 1,822,228 | 01:00:00 |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported):
(Exact name of Registrant as Specified in Its Charter)
| (State or Other Jurisdiction of Incorporation) |
(Commission File Number) |
(IRS Employer Identification No.) |
| |
||||
| (Address of Principal Executive Offices) | (Zip Code) | |||
Registrant’s Telephone Number, Including Area Code:
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class |
Trading Symbol(s) |
Name of each exchange on which registered | ||
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act.
| Item 1.01 | Entry into a Material Definitive Agreement. |
Joyo License Agreement
On May 14, 2024, Erasca, Inc. (the “Company”) entered into an exclusive license agreement (the “Joyo License Agreement”) with Guangzhou Joyo Pharmatech Co., Ltd. (“Joyo”) under which the Company was granted an exclusive, worldwide (except mainland China, Hong Kong and Macau), royalty-bearing license to certain patent and other intellectual property rights owned or controlled by Joyo to develop, manufacture, and commercialize certain pan-RAS inhibitors in all fields of use. The Company has an option to expand the territory of the license to include mainland China, Hong Kong and Macau by making a $50.0 million payment to Joyo on or prior to the first dosing of the first patient in a Phase 2 clinical trial by either the Company or Joyo, or a payment of $150.0 million after the first dosing of the first patient in a Phase 2 clinical trial by either the Company or Joyo, and before filing a new drug application (or the foreign equivalent) by either the Company or Joyo. The Company has the right to sublicense (through multiple tiers) its rights under the Joyo License Agreement, subject to certain limitations and conditions, and is required to use commercially reasonable efforts to commercialize licensed products in certain geographical markets.
The license granted under the Joyo License Agreement is subject to Joyo’ reserved right to develop, manufacture, use, and commercialize licensed products in mainland China, Hong Kong and Macau, unless the Company exercises its option to expand the license to include mainland China, Hong Kong and Macau.
Under the Joyo License Agreement, the Company will make an upfront cash payment to Joyo of $12.5 million within 30 days of the effective date of the Joyo License Agreement.
In addition, the Company is obligated to make development and regulatory milestone payments of up to $51.5 million (or up to $57.5 million if the Company’s territory is expanded to include mainland China, Hong Kong, and Macau) and commercial milestone payments of up to $125.0 million upon the achievement of the corresponding milestones. The Company is also obligated to pay tiered royalties on net sales of all licensed products, in the low- to mid-single digit percentages, subject to certain reductions.
The Joyo License Agreement will expire upon the last to expire royalty term, which is determined on a licensed product-by-licensed product and country-by-country basis, and is the later of: (i) ten years from the date of first commercial sale for the licensed product in such country, (ii) the last to expire valid claim within the licensed patent rights covering such licensed product, or (iii) the expiration of all regulatory exclusivity for the licensed product in such country. Upon expiration of the Joyo License Agreement, on a licensed product-by-licensed product and country-by-country basis, the license granted to the Company with respect to such product in such countries shall be deemed to be fully paid-up, royalty-free, non-terminable, irrevocable and perpetual.
The Joyo License Agreement may be terminated in its entirety by either party in the event of an uncured material breach by the other party or in the event the other party becomes subject to specified bankruptcy, insolvency, or similar circumstances. Joyo may terminate the Joyo License Agreement in the event that the Company or its affiliates or any of its or their sublicensees institutes, prosecutes or otherwise participates in any challenge to the licensed patents. The Company may terminate the Joyo License Agreement in its entirety at any time upon the provision of prior written notice to Joyo.
Upon termination of the Joyo License Agreement for any reason, all rights and licenses granted to the Company will terminate. In addition, the licenses granted to Joyo under certain patent and other intellectual property rights owned or controlled by the Company to develop, manufacture, use, and commercialize the licensed products in mainland China, Hong Kong and Macau will survive the termination of the Joyo License Agreement for any reason, unless the Company exercises its option to expand the license to include mainland China, Hong Kong and Macau, in which case such licenses to Joyo will terminate automatically; and Joyo has an option to negotiate a license under any patent rights, know-how, or other intellectual property rights relating to the licensed products that are owned or controlled by the Company for the purpose of developing, manufacturing and commercializing the licensed products in the Company’s territory on terms to be negotiated between the parties.
The foregoing description of the Joyo License Agreement is not complete and is qualified in its entirety by reference to the full text of the Joyo License Agreement, a copy of which will be filed as an exhibit to the Company’s Quarterly Report on Form 10-Q to be filed with respect to the quarter ending June 30, 2024.
Medshine License Agreement
On May 14, 2024, the Company entered into an exclusive license agreement (the “Medshine License Agreement”) with Medshine Discovery Inc. (“Medshine”) under which the Company was granted an exclusive, worldwide, royalty-bearing license to certain patent and other intellectual property rights owned or controlled by Medshine to develop, manufacture and commercialize certain pan-KRAS inhibitors in all fields of use. The Company has the right to sublicense (through multiple tiers) its rights under the Medshine License Agreement, subject to certain limitations and conditions, and is required to use commercially reasonable efforts to commercialize licensed products in certain geographical markets.
Under the Medshine License Agreement, the Company will make an upfront cash payment to Medshine of $10.0 million within 30 days of the effective date of the Medshine License Agreement.
In addition, the Company is obligated to make development and regulatory milestone payments of up to $30.0 million and commercial milestone payments of up to $130.0 million upon the achievement of the corresponding milestones. The Company is also obligated to pay a low-single digit percentage royalty on net sales of all licensed products, subject to certain reductions.
The Medshine License Agreement will expire upon the last to expire royalty term, which is determined on a licensed product-by-licensed product and country-by-country basis, and is the later of: (i) ten years from the date of first commercial sale for the licensed product in such country, (ii) the last to expire valid claim within the licensed patent rights covering such licensed product, or (iii) the expiration of all regulatory exclusivity for the licensed product in such country. Upon expiration of the Medshine License Agreement, on a licensed product-by-licensed product and country-by-country basis, the license granted to the Company with respect to such product in such countries shall be deemed to be fully paid-up, royalty-free, non-terminable, irrevocable and perpetual.
The Medshine License Agreement may be terminated in its entirety by either party in the event of an uncured material breach by the other party or in the event the other party becomes subject to specified bankruptcy, insolvency, or similar circumstances. Medshine may terminate the Medshine License Agreement in the event that the Company or its affiliates or any of its or their sublicensees commences or actively and voluntarily participates in any challenge to the licensed patents. The Company may terminate the Medshine License Agreement in its entirety at any time upon the provision of prior written notice to Medshine.
Upon termination of the Medshine License Agreement for any reason, all rights and licenses granted to the Company will terminate. In addition, upon termination of the Medshine License Agreement by Medshine for cause, Medshine has an option to negotiate a license under any patent rights, know-how, or other intellectual property rights relating to the licensed products that are owned or controlled by the Company for the purpose of developing, manufacturing and commercializing the licensed products on terms to be negotiated between the parties.
The foregoing description of the Medshine License Agreement is not complete and is qualified in its entirety by reference to the full text of the Medshine License Agreement, a copy of which will be filed as an exhibit to the Company’s Quarterly Report on Form 10-Q to be filed with respect to the quarter ending June 30, 2024.
| Item 2.05 | Costs Associated with Exit or Disposal Activities. |
On May 15, 2024, in connection with entering into the Joyo License Agreement and Medshine License Agreement, a review of the Company’s strategic priorities, and the Company’s decision to deemphasize certain drug discovery activities, the Company approved a strategic reprioritization to focus the Company’s resources on its naporafenib program, its product candidate ERAS-0015, which is the lead candidate from the Joyo License Agreement, and its product candidate ERAS-4001, which is the lead candidate from the Medshine License Agreement. The Company will deprioritize the ERAS-007 program as the Company believes the clinical efficacy data from the HERKULES-3 clinical trial evaluating ERAS-007 in combination with encorafenib and cetuximab (“EC”) in patients with EC-naïve BRAFm colorectal cancer do not support continued evaluation. The Company will also deprioritize its ERAS-801 program and explore further advancement of the program via select investigator-sponsored trials. Finally, the Company will discontinue its existing internal ERAS-4 pan-KRAS program, although certain of the existing ERAS-4 molecules may serve as potential backup compounds for ERAS-4001.
In connection with this strategic reprioritization, the Company also approved a reduction in the Company’s workforce by approximately 18%, primarily affecting employees working in drug discovery functions or on the deprioritized programs. The Company anticipates recognizing approximately $2.2 million in total charges in the second quarter of 2024 in connection with the reduction in force. These charges will consist primarily of one-time cash charges for termination benefits.
| Item 8.01 | Other Events. |
An updated Company presentation, including an overview of the Joyo License Agreement and the Medshine License Agreement, is attached as Exhibit 99.1 to this report and is incorporated herein by reference.
| Item 9.01 | Financial Statements and Exhibits. |
(d) Exhibits
| Exhibit |
Description | |
| 99.1 | Erasca, Inc. Corporate Presentation, dated May 2024 | |
| 104 | Cover Page Interactive Data File (embedded within the Inline XBRL document) | |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
| Erasca, Inc. | ||||||
| Date: May 16, 2024 | By: | /s/ Ebun Garner | ||||
| Ebun Garner, General Counsel | ||||||

Exhibit 99.1 On a Journey to Erase Cancer Erasca Investor Update May 2024

Disclaimer: Forward Looking Statements & Market Data We caution you that this presentation contains forward-looking statements. All statements other than statements of historical facts contained in this presentation, including statements regarding our future results of operations and financial position, business strategy, research and development plans, the anticipated timing (including the timing of initiation and the timing of data readouts), costs, design and conduct of our ongoing and planned preclinical studies and clinical trials for our product candidates, the potential therapeutic benefits of our product candidates, the potential benefits from our current or future arrangements with third parties, including the anticipated benefits of the license agreements with Medshine Discovery Inc. and Joyo Pharmatech Co., Ltd., the timing and likelihood of success of our plans and objectives, the impact of the deprioritization of certain programs, and future results of anticipated product development efforts, are forward-looking statements. In some cases, you can identify forward-looking statements by terms such as “may,” “will,” “should,” “expect,” “plan,” “anticipate,” “could,” “intend,” “target,” “project,” “contemplates,” “believes,” “estimates,” “predicts,” “potential” or “continue” or the negative of these terms or other similar expressions. The inclusion of forward-looking statements should not be regarded as a representation by us that any of our plans will be achieved. Actual results may differ from those set forth in this presentation due to the risks and uncertainties inherent in our business, including, without limitation: our approach to the discovery and development of product candidates based on our singular focus on shutting down the RAS/MAPK pathway, a novel and unproven approach; our assumptions about ERAS-0015 or ERAS-4001 development potential are based in large part on the preclinical data generated by the licensors and we may observe materially and adversely different results as we conduct our planned studies; we only have one product candidate in clinical development and all of our other development efforts are in the preclinical or development stage; our assumptions around which programs may have a higher probability of success may not be accurate, and we may expend our limited resources to pursue a particular product candidate and/or indication and fail to capitalize on product candidates or indications with greater development or commercial potential; potential delays in the commencement, enrollment, data readout, and completion of clinical trials and preclinical studies; our dependence on third parties in connection with manufacturing, research, and preclinical and clinical testing; unexpected adverse side effects or inadequate efficacy of our product candidates that may limit their development, regulatory approval, and/or commercialization, or may result in recalls or product liability claims; unfavorable results from preclinical studies or clinical trials; results from preclinical studies or early clinical trials not necessarily being predictive of future results; our planned SEACRAFT trials may not support the registration of naporafenib; the inability to realize any benefits from our current licenses, acquisitions, or collaborations, and any future licenses, acquisitions, or collaborations, and our ability to fulfill our obligations under such arrangements; regulatory developments in the United States and foreign countries; later developments with the FDA or EU health authorities may be inconsistent with the feedback received to date regarding our development plans and trial designs; our ability to obtain and maintain intellectual property protection for our product candidates and maintain our rights under intellectual property licenses; our ability to fund our operating plans with our current cash, cash equivalents, and marketable securities; and other risks described in our prior filings with the Securities and Exchange Commission (SEC), including under the heading “Risk Factors” in our annual report on Form 10-K for the year ended December 31, 2023, and any subsequent filings with the SEC. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof, and we undertake no obligation to update such statements to reflect events that occur or circumstances that exist after the date hereof. All forward-looking statements are qualified in their entirety by this cautionary statement, which is made under the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. This presentation also contains estimates and other statistical data made by independent parties and by us relating to market size and growth and other data about our industry. This data involves a number of assumptions, and you are cautioned not to give undue weight to such estimates. In addition, projections, assumptions, and estimates of our future performance and the future performance of the markets in which we operate are necessarily subject to a high degree of uncertainty and risk. These and other factors could cause results to differ materially from those expressed in the estimates made by the independent parties and by us. 2
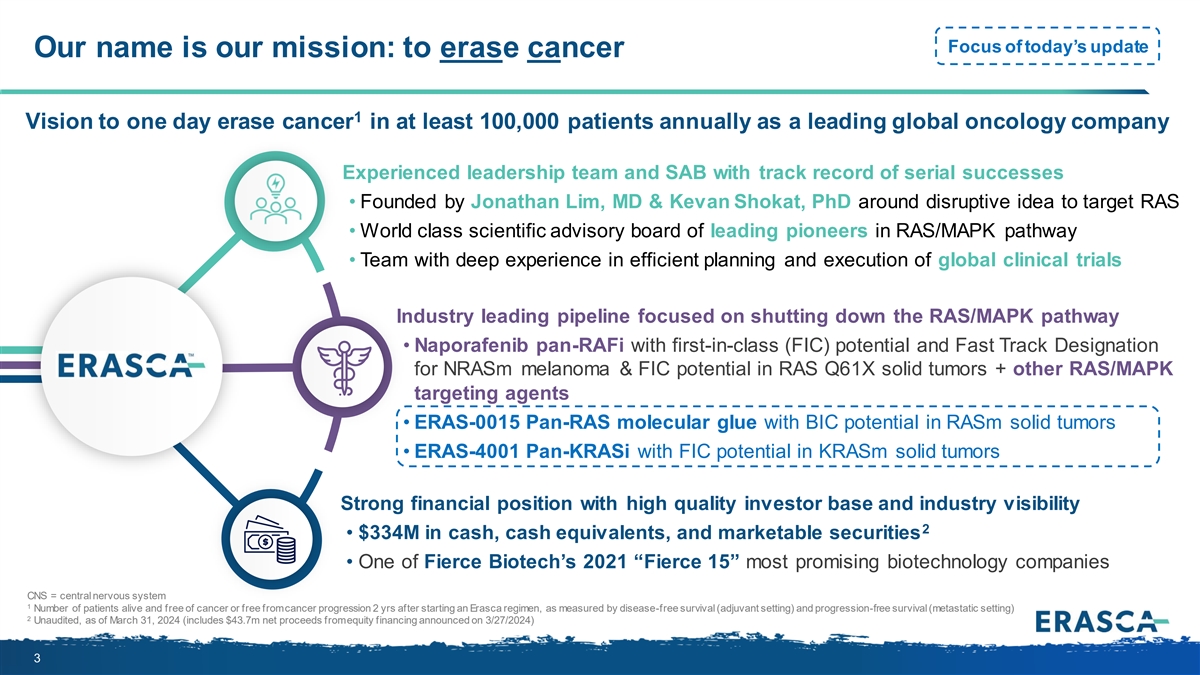
Focus of today’s update Our name is our mission: to erase cancer 1 Vision to one day erase cancer in at least 100,000 patients annually as a leading global oncology company Experienced leadership team and SAB with track record of serial successes • Founded by Jonathan Lim, MD & Kevan Shokat, PhD around disruptive idea to target RAS • World class scientific advisory board of leading pioneers in RAS/MAPK pathway • Team with deep experience in efficient planning and execution of global clinical trials Industry leading pipeline focused on shutting down the RAS/MAPK pathway • Naporafenib pan-RAFi with first-in-class (FIC) potential and Fast Track Designation for NRASm melanoma & FIC potential in RAS Q61X solid tumors + other RAS/MAPK targeting agents • ERAS-0015 Pan-RAS molecular glue with BIC potential in RASm solid tumors • ERAS-4001 Pan-KRASi with FIC potential in KRASm solid tumors Strong financial position with high quality investor base and industry visibility 2 • $334M in cash, cash equivalents, and marketable securities • One of Fierce Biotech’s 2021 “Fierce 15” most promising biotechnology companies CNS = central nervous system 1 Number of patients alive and free of cancer or free from cancer progression 2 yrs after starting an Erasca regimen, as measured by disease-free survival (adjuvant setting) and progression-free survival (metastatic setting) 2 Unaudited, as of March 31, 2024 (includes $43.7m net proceeds from equity financing announced on 3/27/2024) 3
Erasca Investor Update Agenda ERAS-0015: Pan-RAS molecular glue RAS-Targeting Franchise ERAS-4001: Pan-KRAS inhibitor Pipeline and resource prioritization Corporate Update Key milestones and clinical trial readouts Q&A Session 4

RAS-Targeting Franchise
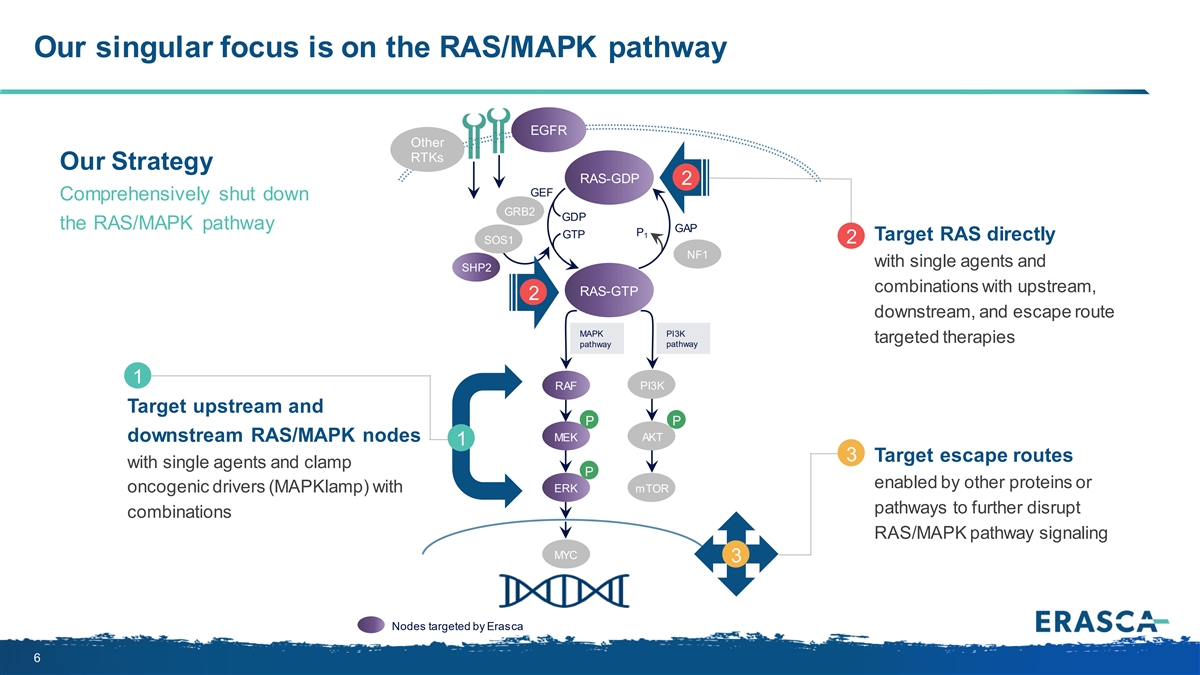
Our singular focus is on the RAS/MAPK pathway EGFR Other RTKs Our Strategy RAS-GDP 2 GEF Comprehensively shut down GRB2 GDP the RAS/MAPK pathway GAP P GTP 1 Target RAS directly SOS1 2 NF1 with single agents and SHP2 combinations with upstream, RAS-GTP 2 downstream, and escape route PI3K MAPK targeted therapies pathway pathway 1 RAF PI3K Target upstream and P P MEK AKT downstream RAS/MAPK nodes 1 3 Target escape routes with single agents and clamp P enabled by other proteins or oncogenic drivers (MAPKlamp) with ERK mTOR pathways to further disrupt combinations RAS/MAPK pathway signaling MYC 3 Nodes targeted by Erasca 6
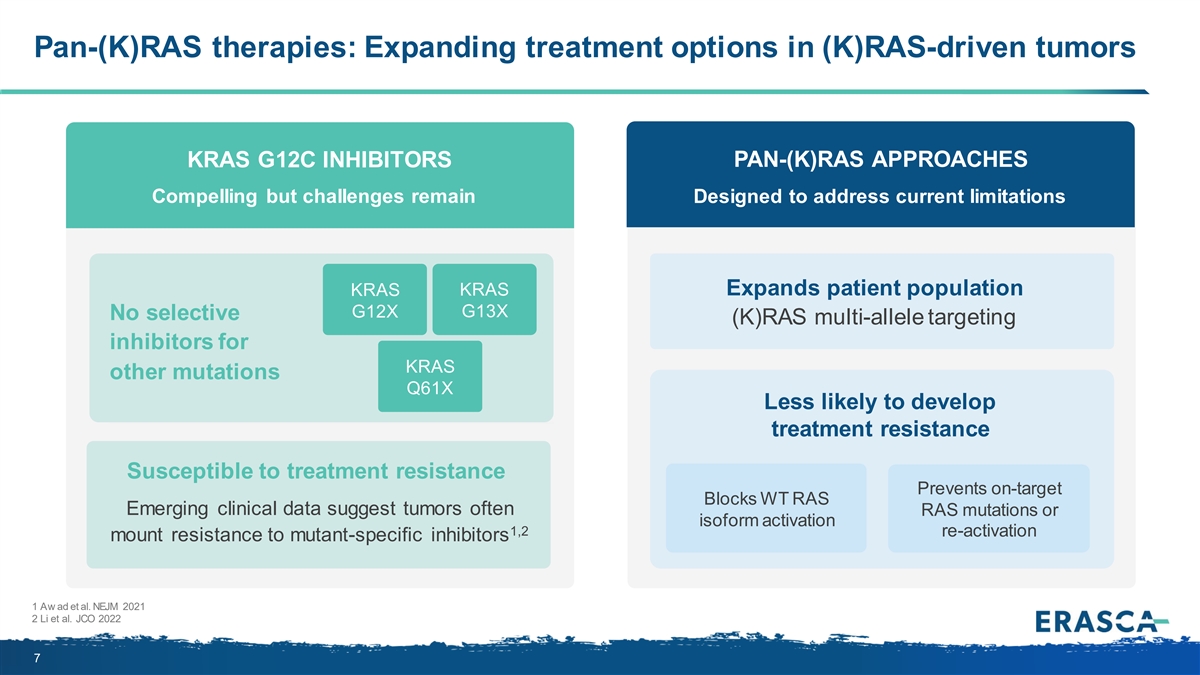
Pan-(K)RAS therapies: Expanding treatment options in (K)RAS-driven tumors KRAS G12C INHIBITORS PAN-(K)RAS APPROACHES Compelling but challenges remain Designed to address current limitations Expands patient population KRAS KRAS G12X G13X No selective (K)RAS multi-allele targeting inhibitors for KRAS other mutations Q61X Less likely to develop treatment resistance Susceptible to treatment resistance Prevents on-target Blocks WT RAS Emerging clinical data suggest tumors often RAS mutations or isoform activation 1,2 re-activation mount resistance to mutant-specific inhibitors 1 Aw ad et al. NEJM 2021 2 Li et al. JCO 2022 7
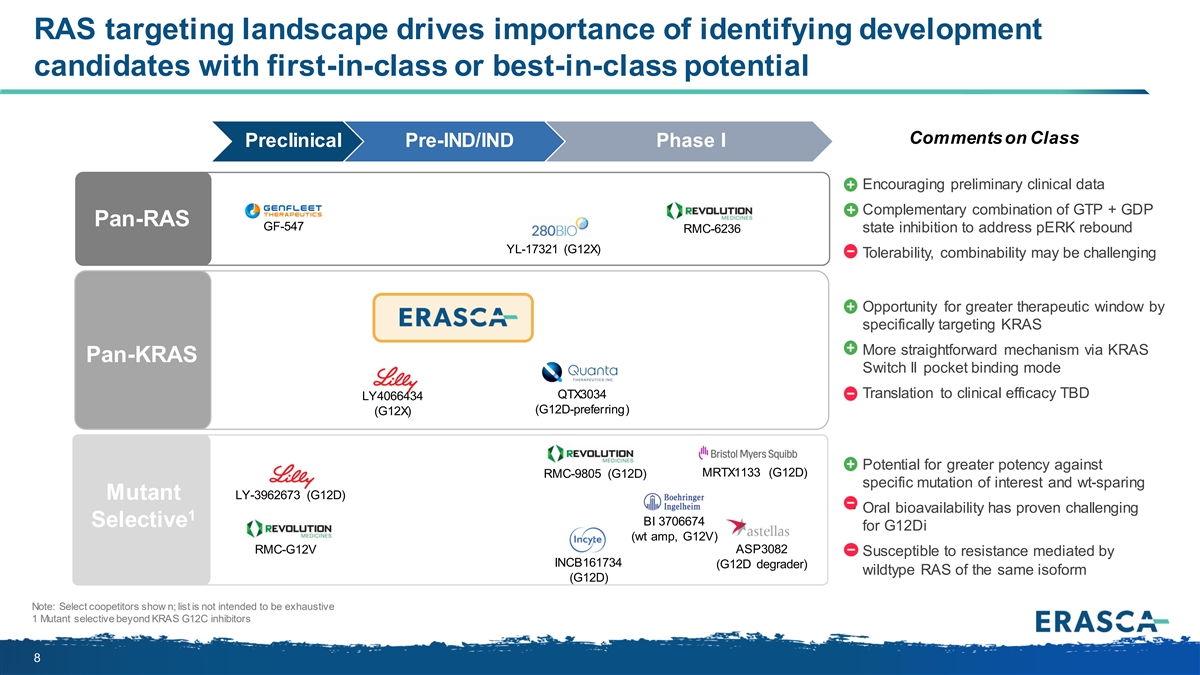
RAS targeting landscape drives importance of identifying development candidates with first-in-class or best-in-class potential Comments on Class Preclinical Pre-IND/IND Phase I • Encouraging preliminary clinical data • Complementary combination of GTP + GDP Pan-RAS GF-547 RMC-6236 state inhibition to address pERK rebound YL-17321 (G12X) • Tolerability, combinability may be challenging • Opportunity for greater therapeutic window by specifically targeting KRAS • More straightforward mechanism via KRAS Pan-KRAS Switch II pocket binding mode QTX3034 • Translation to clinical efficacy TBD LY4066434 (G12D-preferring) (G12X) • Potential for greater potency against MRTX1133 (G12D) RMC-9805 (G12D) specific mutation of interest and wt-sparing Mutant LY-3962673 (G12D) • Oral bioavailability has proven challenging 1 BI 3706674 Selective for G12Di (wt amp, G12V) RMC-G12V ASP3082 • Susceptible to resistance mediated by INCB161734 (G12D degrader) wildtype RAS of the same isoform (G12D) Note: Select coopetitors show n; list is not intended to be exhaustive 1 Mutant selective beyond KRAS G12C inhibitors 8
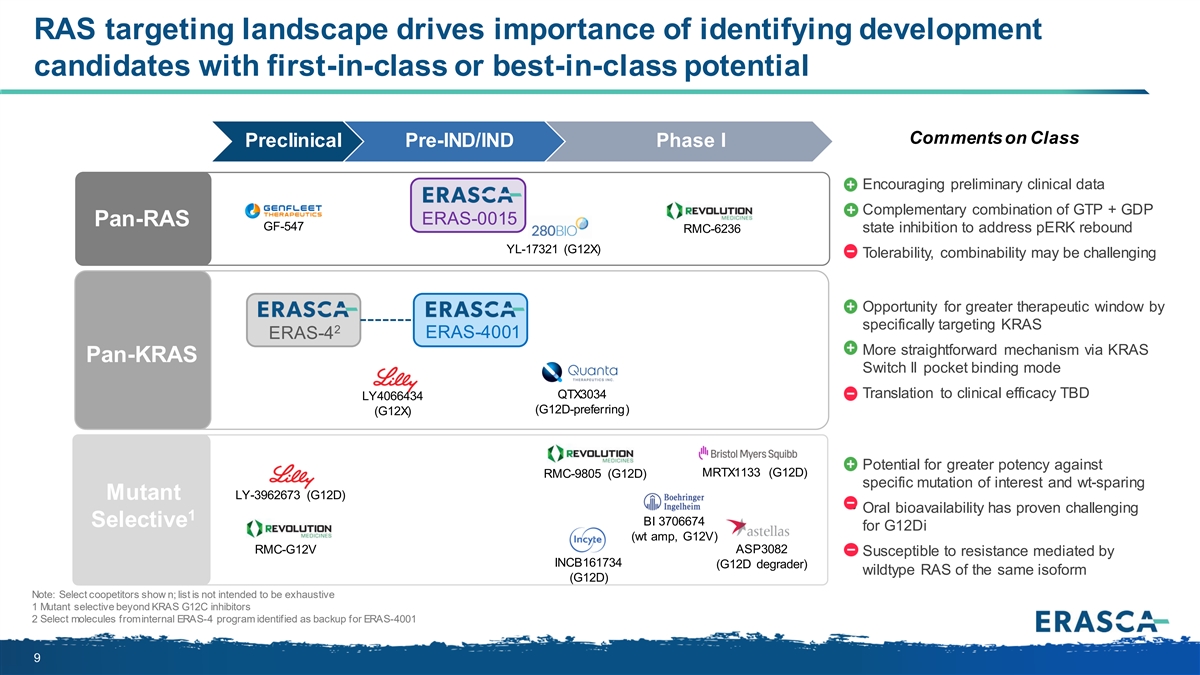
RAS targeting landscape drives importance of identifying development candidates with first-in-class or best-in-class potential Comments on Class Preclinical Pre-IND/IND Phase I • Encouraging preliminary clinical data • Complementary combination of GTP + GDP ERAS-0015 Pan-RAS GF-547 RMC-6236 state inhibition to address pERK rebound YL-17321 (G12X) • Tolerability, combinability may be challenging • Opportunity for greater therapeutic window by specifically targeting KRAS 2 ERAS-4 ERAS-4001 • More straightforward mechanism via KRAS Pan-KRAS Switch II pocket binding mode QTX3034 • Translation to clinical efficacy TBD LY4066434 (G12D-preferring) (G12X) • Potential for greater potency against MRTX1133 (G12D) RMC-9805 (G12D) specific mutation of interest and wt-sparing Mutant LY-3962673 (G12D) • Oral bioavailability has proven challenging 1 BI 3706674 Selective for G12Di (wt amp, G12V) RMC-G12V ASP3082 • Susceptible to resistance mediated by INCB161734 (G12D degrader) wildtype RAS of the same isoform (G12D) Note: Select coopetitors show n; list is not intended to be exhaustive 1 Mutant selective beyond KRAS G12C inhibitors 2 Select molecules from internal ERAS-4 program identified as backup for ERAS-4001 9
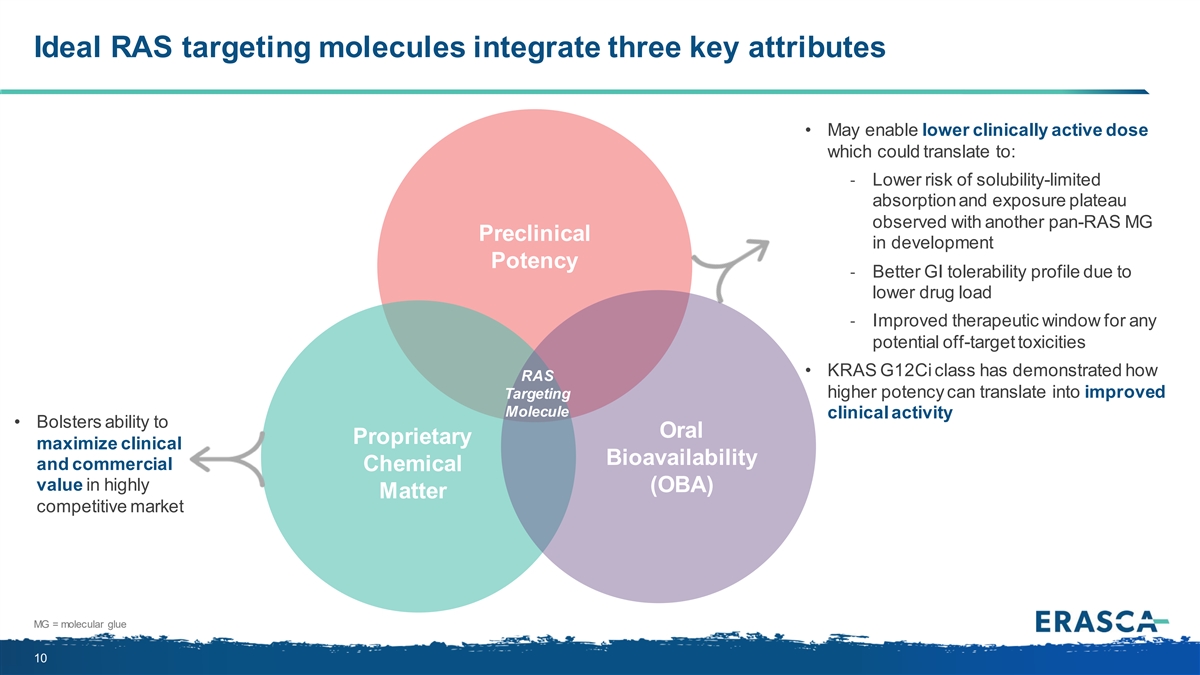
Ideal RAS targeting molecules integrate three key attributes • May enable lower clinically active dose which could translate to: - Lower risk of solubility-limited absorption and exposure plateau observed with another pan-RAS MG Preclinical in development Potency - Better GI tolerability profile due to lower drug load - Improved therapeutic window for any potential off-target toxicities • KRAS G12Ci class has demonstrated how RAS higher potency can translate into improved Targeting Molecule clinical activity • Bolsters ability to Oral Proprietary maximize clinical Bioavailability and commercial Chemical value in highly (OBA) Matter competitive market MG = molecular glue 10

ERAS-0015 and ERAS-4001 exhibit competitive profiles that exceed our TPP 1 2 OBA IP Preclinical (in vitro and in vivo) Potency TPP: target product profile; OBA: oral bioavailability; IP: intellectual property; FIC: first-in-class; BIC: best-in-class; WT: w ildtype; SMi: small molecule inhibitor; MG: molecular glue; DC: development candidate 1 2 3 4 in vitro potency assessed by CTG 2D and 3D-cell proliferation assay IC s; OBA or oral bioavailability assessed by %F; Predicted based on molecular profile; absent any patent term adjustments or extensions 50 11
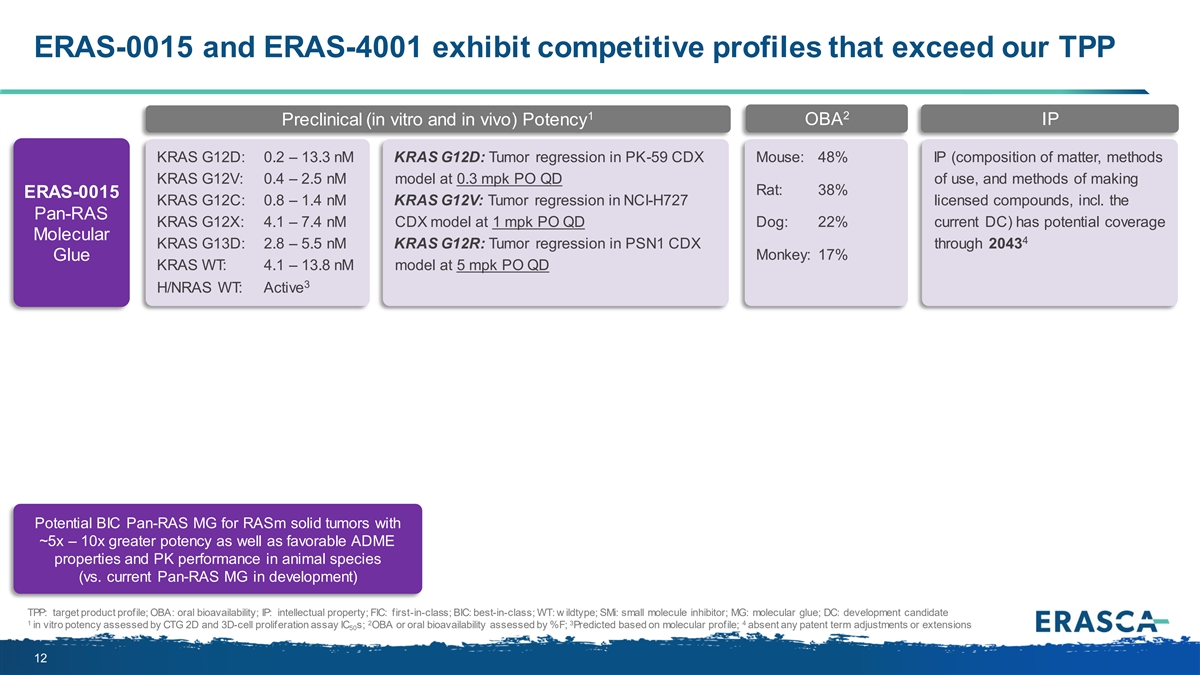
ERAS-0015 and ERAS-4001 exhibit competitive profiles that exceed our TPP 1 2 OBA IP Preclinical (in vitro and in vivo) Potency KRAS G12D: 0.2 – 13.3 nM KRAS G12D: Tumor regression in PK-59 CDX Mouse: 48% IP (composition of matter, methods KRAS G12V: 0.4 – 2.5 nM model at 0.3 mpk PO QD of use, and methods of making Rat: 38% ERAS-0015 KRAS G12C: 0.8 – 1.4 nM KRAS G12V: Tumor regression in NCI-H727 licensed compounds, incl. the Pan-RAS KRAS G12X: 4.1 – 7.4 nM CDX model at 1 mpk PO QD Dog: 22% current DC) has potential coverage Molecular 4 KRAS G13D: 2.8 – 5.5 nM KRAS G12R: Tumor regression in PSN1 CDX through 2043 Monkey: 17% Glue KRAS WT: 4.1 – 13.8 nM model at 5 mpk PO QD 3 H/NRAS WT: Active Potential BIC Pan-RAS MG for RASm solid tumors with ~5x – 10x greater potency as well as favorable ADME properties and PK performance in animal species (vs. current Pan-RAS MG in development) TPP: target product profile; OBA: oral bioavailability; IP: intellectual property; FIC: first-in-class; BIC: best-in-class; WT: w ildtype; SMi: small molecule inhibitor; MG: molecular glue; DC: development candidate 1 2 3 4 in vitro potency assessed by CTG 2D and 3D-cell proliferation assay IC s; OBA or oral bioavailability assessed by %F; Predicted based on molecular profile; absent any patent term adjustments or extensions 50 12
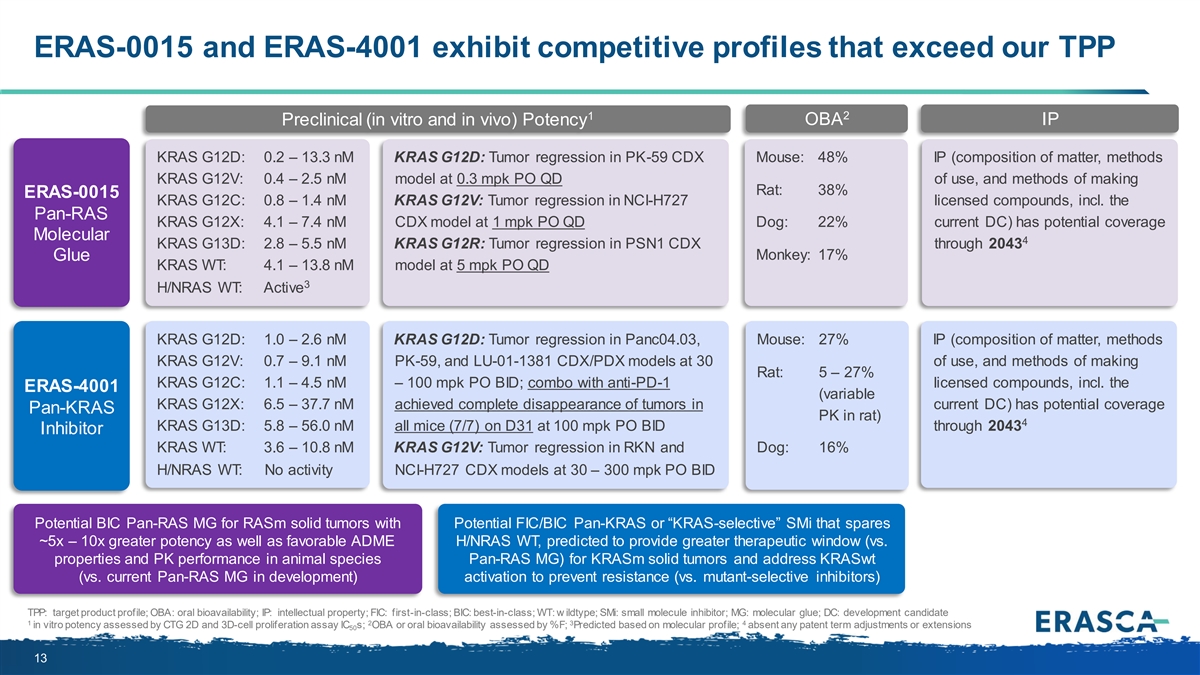
ERAS-0015 and ERAS-4001 exhibit competitive profiles that exceed our TPP 1 2 OBA IP Preclinical (in vitro and in vivo) Potency KRAS G12D: 0.2 – 13.3 nM KRAS G12D: Tumor regression in PK-59 CDX Mouse: 48% IP (composition of matter, methods KRAS G12V: 0.4 – 2.5 nM model at 0.3 mpk PO QD of use, and methods of making Rat: 38% ERAS-0015 KRAS G12C: 0.8 – 1.4 nM KRAS G12V: Tumor regression in NCI-H727 licensed compounds, incl. the Pan-RAS KRAS G12X: 4.1 – 7.4 nM CDX model at 1 mpk PO QD Dog: 22% current DC) has potential coverage Molecular 4 KRAS G13D: 2.8 – 5.5 nM KRAS G12R: Tumor regression in PSN1 CDX through 2043 Monkey: 17% Glue KRAS WT: 4.1 – 13.8 nM model at 5 mpk PO QD 3 H/NRAS WT: Active KRAS G12D: 1.0 – 2.6 nM KRAS G12D: Tumor regression in Panc04.03, Mouse: 27% IP (composition of matter, methods KRAS G12V: 0.7 – 9.1 nM PK-59, and LU-01-1381 CDX/PDX models at 30 of use, and methods of making Rat: 5 – 27% KRAS G12C: 1.1 – 4.5 nM – 100 mpk PO BID; combo with anti-PD-1 licensed compounds, incl. the ERAS-4001 (variable KRAS G12X: 6.5 – 37.7 nM achieved complete disappearance of tumors in current DC) has potential coverage Pan-KRAS PK in rat) 4 KRAS G13D: 5.8 – 56.0 nM all mice (7/7) on D31 at 100 mpk PO BID through 2043 Inhibitor KRAS WT: 3.6 – 10.8 nM KRAS G12V: Tumor regression in RKN and Dog: 16% H/NRAS WT: No activity NCI-H727 CDX models at 30 – 300 mpk PO BID Potential BIC Pan-RAS MG for RASm solid tumors with Potential FIC/BIC Pan-KRAS or “KRAS-selective” SMi that spares ~5x – 10x greater potency as well as favorable ADME H/NRAS WT, predicted to provide greater therapeutic window (vs. properties and PK performance in animal species Pan-RAS MG) for KRASm solid tumors and address KRASwt (vs. current Pan-RAS MG in development) activation to prevent resistance (vs. mutant-selective inhibitors) TPP: target product profile; OBA: oral bioavailability; IP: intellectual property; FIC: first-in-class; BIC: best-in-class; WT: w ildtype; SMi: small molecule inhibitor; MG: molecular glue; DC: development candidate 1 2 3 4 in vitro potency assessed by CTG 2D and 3D-cell proliferation assay IC s; OBA or oral bioavailability assessed by %F; Predicted based on molecular profile; absent any patent term adjustments or extensions 50 13
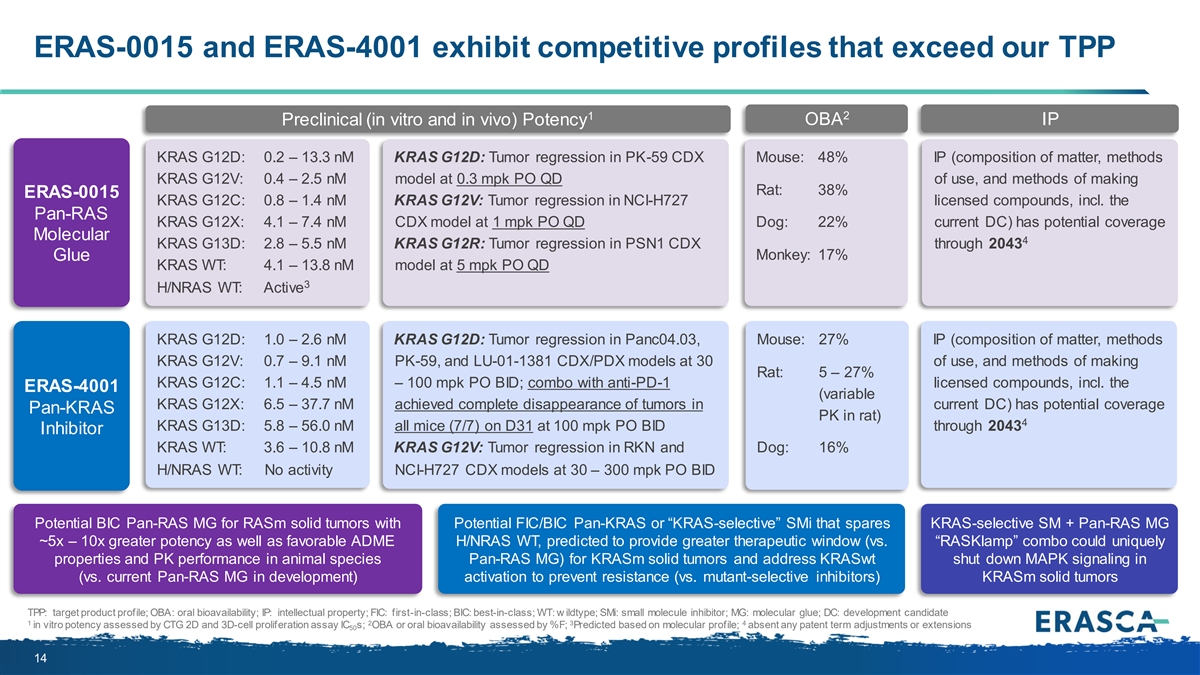
ERAS-0015 and ERAS-4001 exhibit competitive profiles that exceed our TPP 1 2 OBA IP Preclinical (in vitro and in vivo) Potency KRAS G12D: 0.2 – 13.3 nM KRAS G12D: Tumor regression in PK-59 CDX Mouse: 48% IP (composition of matter, methods KRAS G12V: 0.4 – 2.5 nM model at 0.3 mpk PO QD of use, and methods of making Rat: 38% ERAS-0015 KRAS G12C: 0.8 – 1.4 nM KRAS G12V: Tumor regression in NCI-H727 licensed compounds, incl. the Pan-RAS KRAS G12X: 4.1 – 7.4 nM CDX model at 1 mpk PO QD Dog: 22% current DC) has potential coverage Molecular 4 KRAS G13D: 2.8 – 5.5 nM KRAS G12R: Tumor regression in PSN1 CDX through 2043 Monkey: 17% Glue KRAS WT: 4.1 – 13.8 nM model at 5 mpk PO QD 3 H/NRAS WT: Active KRAS G12D: 1.0 – 2.6 nM KRAS G12D: Tumor regression in Panc04.03, Mouse: 27% IP (composition of matter, methods KRAS G12V: 0.7 – 9.1 nM PK-59, and LU-01-1381 CDX/PDX models at 30 of use, and methods of making Rat: 5 – 27% KRAS G12C: 1.1 – 4.5 nM – 100 mpk PO BID; combo with anti-PD-1 licensed compounds, incl. the ERAS-4001 (variable KRAS G12X: 6.5 – 37.7 nM achieved complete disappearance of tumors in current DC) has potential coverage Pan-KRAS PK in rat) 4 KRAS G13D: 5.8 – 56.0 nM all mice (7/7) on D31 at 100 mpk PO BID through 2043 Inhibitor KRAS WT: 3.6 – 10.8 nM KRAS G12V: Tumor regression in RKN and Dog: 16% H/NRAS WT: No activity NCI-H727 CDX models at 30 – 300 mpk PO BID Potential BIC Pan-RAS MG for RASm solid tumors with Potential FIC/BIC Pan-KRAS or “KRAS-selective” SMi that spares KRAS-selective SM + Pan-RAS MG ~5x – 10x greater potency as well as favorable ADME H/NRAS WT, predicted to provide greater therapeutic window (vs. “RASKlamp” combo could uniquely properties and PK performance in animal species Pan-RAS MG) for KRASm solid tumors and address KRASwt shut down MAPK signaling in (vs. current Pan-RAS MG in development) activation to prevent resistance (vs. mutant-selective inhibitors) KRASm solid tumors TPP: target product profile; OBA: oral bioavailability; IP: intellectual property; FIC: first-in-class; BIC: best-in-class; WT: w ildtype; SMi: small molecule inhibitor; MG: molecular glue; DC: development candidate 1 2 3 4 in vitro potency assessed by CTG 2D and 3D-cell proliferation assay IC s; OBA or oral bioavailability assessed by %F; Predicted based on molecular profile; absent any patent term adjustments or extensions 50 14
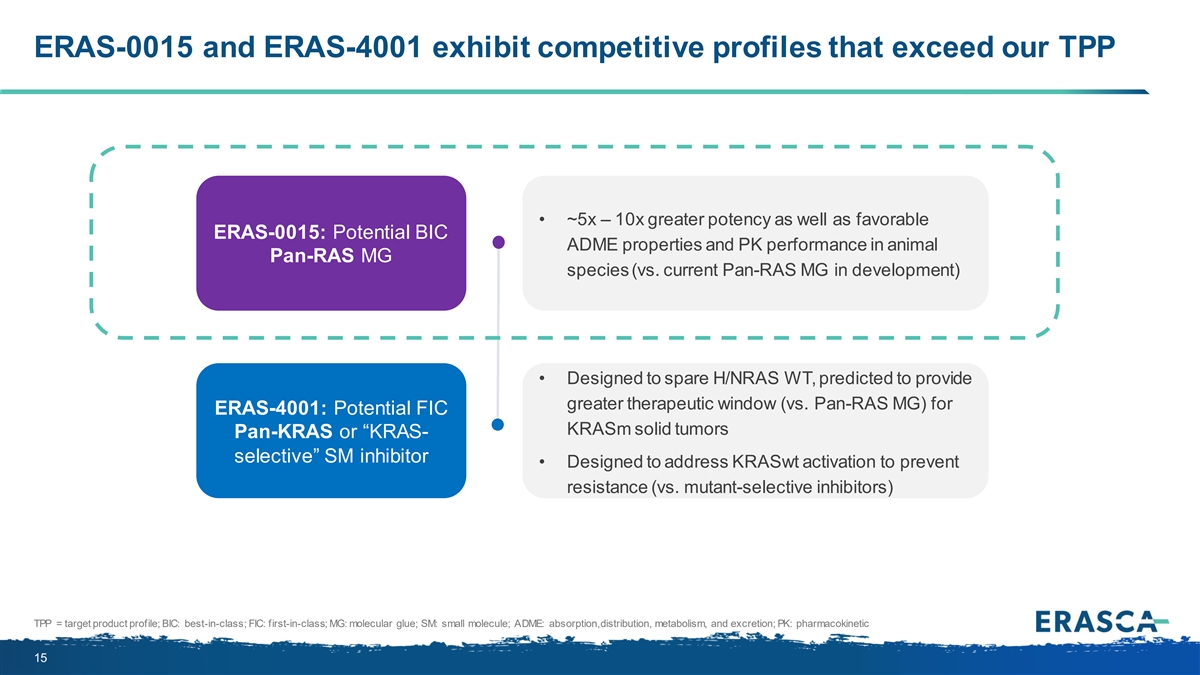
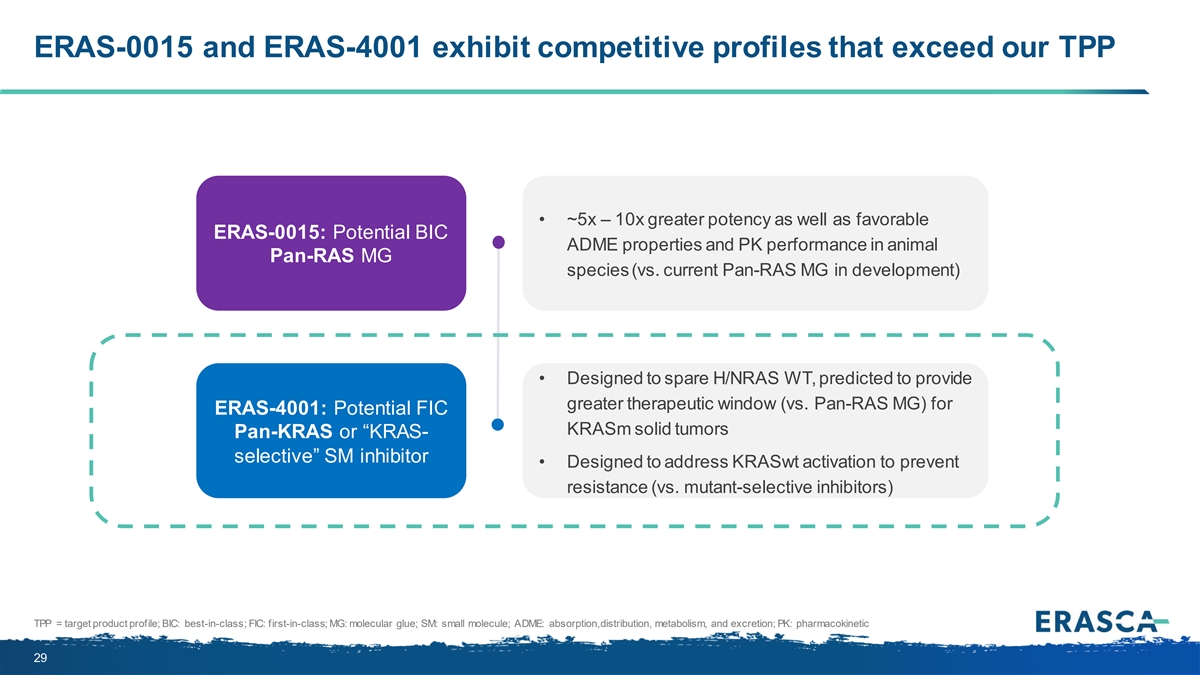
ERAS-0015 and ERAS-4001 exhibit competitive profiles that exceed our TPP • ~5x – 10x greater potency as well as favorable ERAS-0015: Potential BIC ADME properties and PK performance in animal Pan-RAS MG species (vs. current Pan-RAS MG in development) • Designed to spare H/NRAS WT, predicted to provide greater therapeutic window (vs. Pan-RAS MG) for ERAS-4001: Potential FIC KRASm solid tumors Pan-KRAS or “KRAS- selective” SM inhibitor • Designed to address KRASwt activation to prevent resistance (vs. mutant-selective inhibitors) TPP = target product profile; BIC: best-in-class; FIC: first-in-class; MG: molecular glue; SM: small molecule; ADME: absorption, distribution, metabolism, and excretion; PK: pharmacokinetic 15
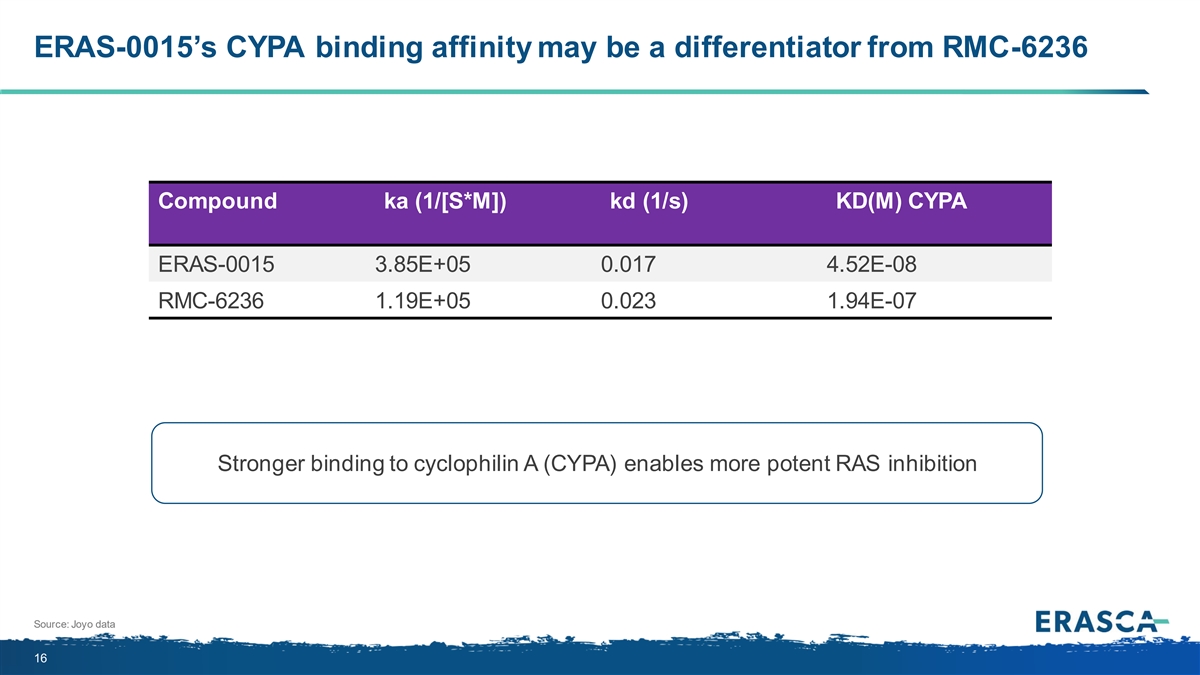
ERAS-0015’s CYPA binding affinity may be a differentiator from RMC-6236 Compound ka (1/[S*M]) kd (1/s) KD(M) CYPA ERAS-0015 3.85E+05 0.017 4.52E-08 RMC-6236 1.19E+05 0.023 1.94E-07 Stronger binding to cyclophilin A (CYPA) enables more potent RAS inhibition Source: Joyo data 16
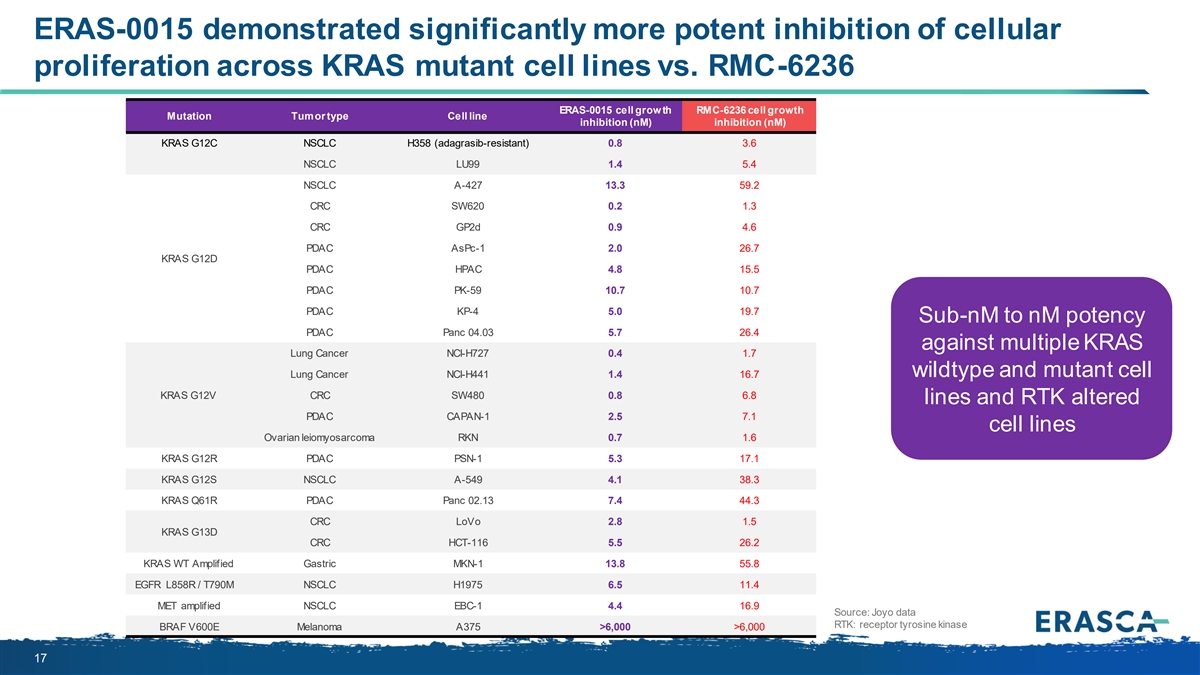
ERAS-0015 demonstrated significantly more potent inhibition of cellular proliferation across KRAS mutant cell lines vs. RMC-6236 ERAS-0015 cell growth RMC-6236 cell growth Mutation Tumor type Cell line inhibition (nM) inhibition (nM) KRAS G12C NSCLC H358 (adagrasib-resistant) 0.8 3.6 NSCLC LU99 1.4 5.4 NSCLC A-427 13.3 59.2 CRC SW620 0.2 1.3 CRC GP2d 0.9 4.6 PDAC AsPc-1 2.0 26.7 KRAS G12D PDAC HPAC 4.8 15.5 PDAC PK-59 10.7 10.7 PDAC KP-4 5.0 19.7 Sub-nM to nM potency PDAC Panc 04.03 5.7 26.4 against multiple KRAS Lung Cancer NCI-H727 0.4 1.7 wildtype and mutant cell Lung Cancer NCI-H441 1.4 16.7 KRAS G12V CRC SW480 0.8 6.8 lines and RTK altered PDAC CAPAN-1 2.5 7.1 cell lines Ovarian leiomyosarcoma RKN 0.7 1.6 KRAS G12R PDAC PSN-1 5.3 17.1 KRAS G12S NSCLC A-549 4.1 38.3 KRAS Q61R PDAC Panc 02.13 7.4 44.3 CRC LoVo 2.8 1.5 KRAS G13D CRC HCT-116 5.5 26.2 KRAS WT Amplified Gastric MKN-1 13.8 55.8 EGFR L858R / T790M NSCLC H1975 6.5 11.4 MET amplified NSCLC EBC-1 4.4 16.9 Source: Joyo data RTK: receptor tyrosine kinase BRAF V600E Melanoma A375 >6,000 >6,000 17
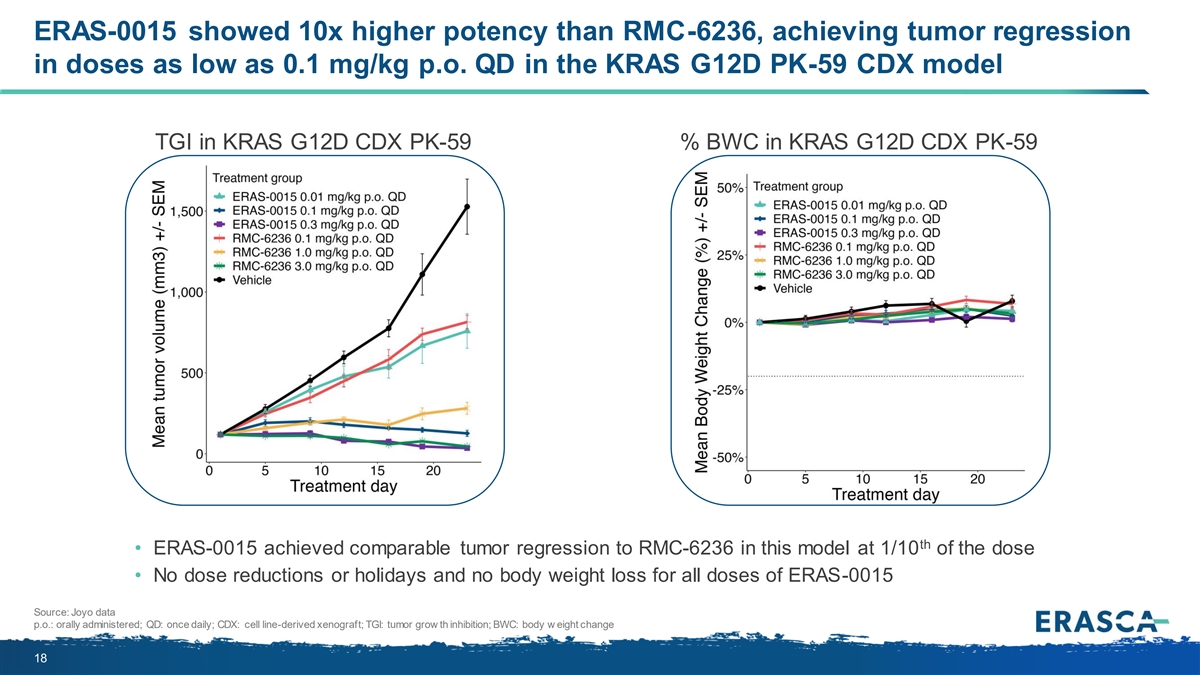
ERAS-0015 showed 10x higher potency than RMC-6236, achieving tumor regression in doses as low as 0.1 mg/kg p.o. QD in the KRAS G12D PK-59 CDX model TGI in KRAS G12D CDX PK-59 % BWC in KRAS G12D CDX PK-59 th • ERAS-0015 achieved comparable tumor regression to RMC-6236 in this model at 1/10 of the dose • No dose reductions or holidays and no body weight loss for all doses of ERAS-0015 Source: Joyo data p.o.: orally administered; QD: once daily; CDX: cell line-derived xenograft; TGI: tumor grow th inhibition; BWC: body w eight change 18
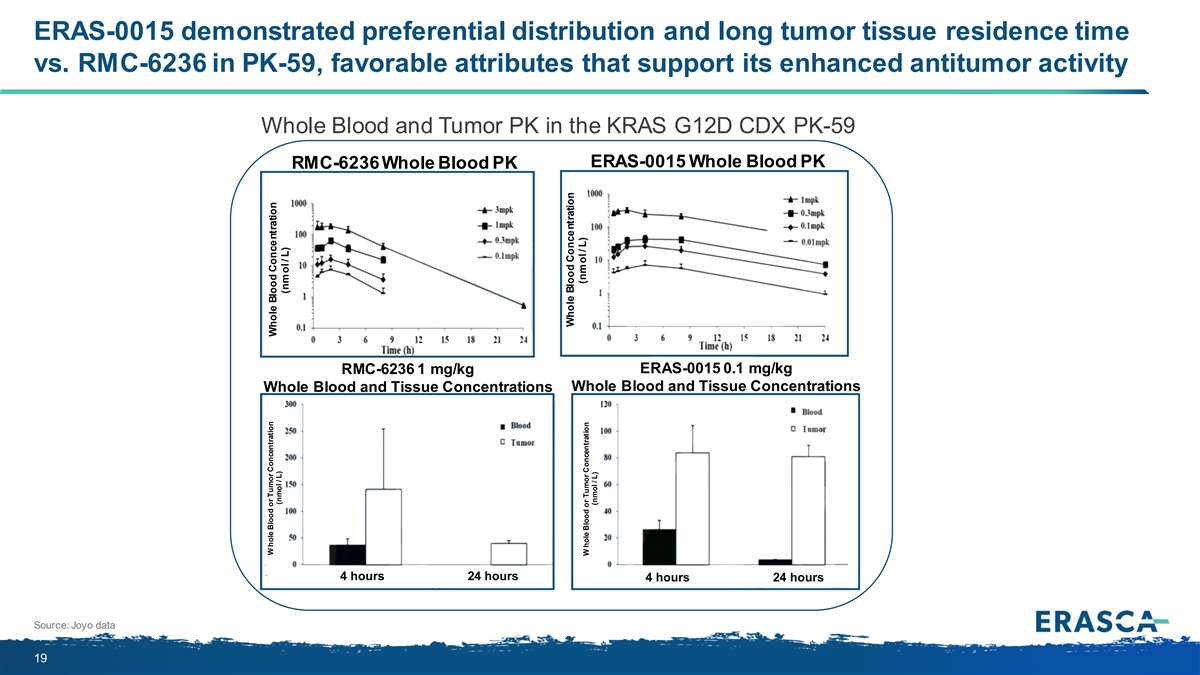
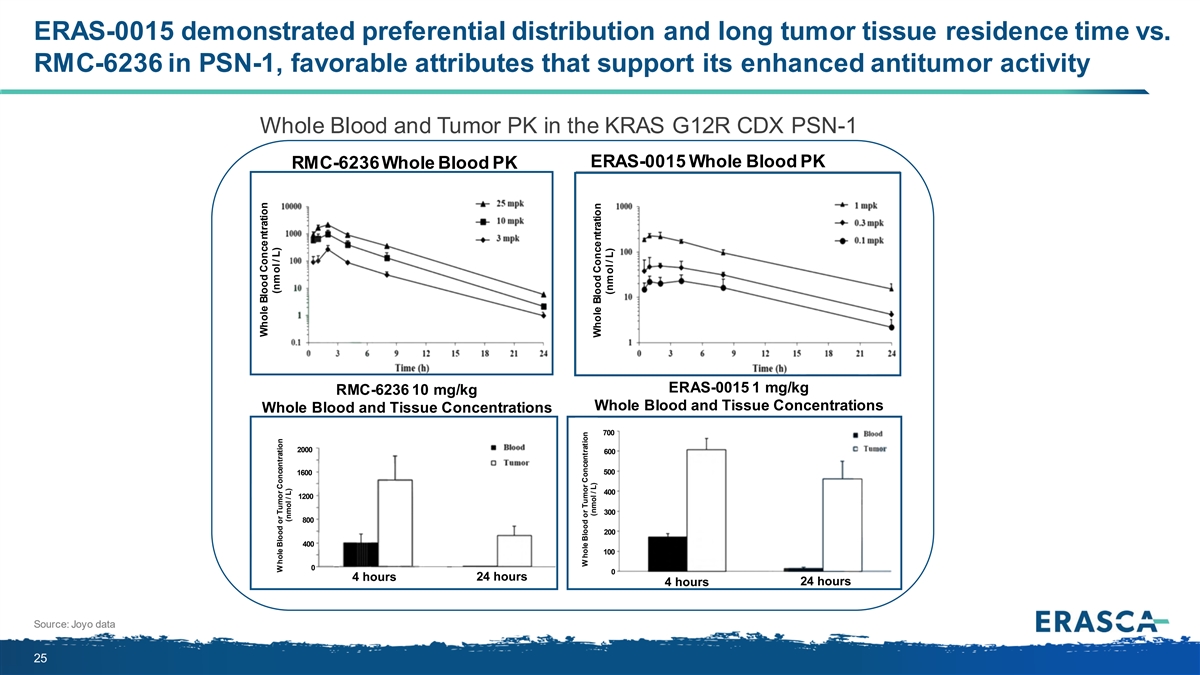
ERAS-0015 demonstrated preferential distribution and long tumor tissue residence time vs. RMC-6236 in PK-59, favorable attributes that support its enhanced antitumor activity Whole Blood and Tumor PK in the KRAS G12D CDX PK-59 ERAS-0015 Whole Blood PK RMC-6236 Whole Blood PK RMC-6236 1 mg/kg ERAS-0015 0.1 mg/kg Whole Blood and Tissue Concentrations Whole Blood and Tissue Concentrations 4 hours 24 hours 4 hours 24 hours Source: Joyo data 19 W hole Blood or Tumor Concentration Whole Blood Concentration (nmol / L) (nmol / L) Whole Blood Concentration (nmol / L) W hole Blood or Tumor Concentration (nmol / L)
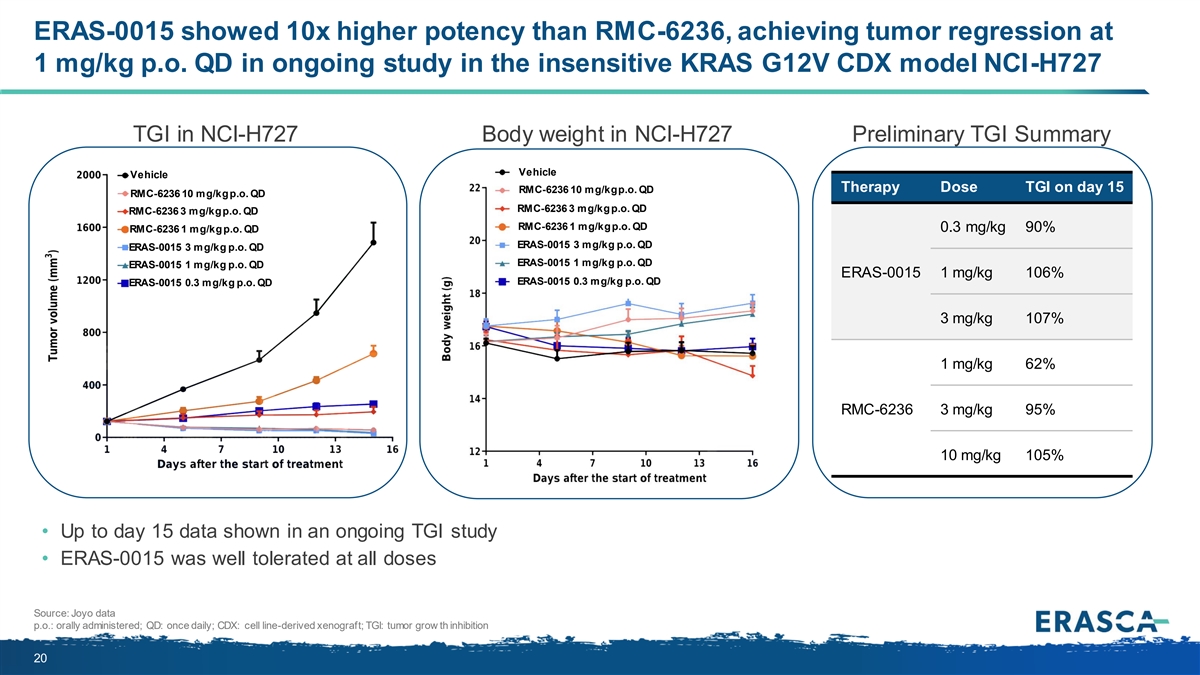
ERAS-0015 showed 10x higher potency than RMC-6236, achieving tumor regression at 1 mg/kg p.o. QD in ongoing study in the insensitive KRAS G12V CDX model NCI-H727 TGI in NCI-H727 Body weight in NCI-H727 Preliminary TGI Summary Vehicle Vehicle Therapy Dose TGI on day 15 RMC-6236 10 mg/kg p.o. QD RMC-6236 10 mg/kg p.o. QD RMC-6236 3 mg/kg p.o. QD RMC-6236 3 mg/kg p.o. QD RMC-6236 1 mg/kg p.o. QD RMC-6236 1 mg/kg p.o. QD 0.3 mg/kg 90% ERAS-0015 3 mg/kg p.o. QD ERAS-0015 3 mg/kg p.o. QD ERAS-0015 1 mg/kg p.o. QD ERAS-0015 1 mg/kg p.o. QD ERAS-0015 1 mg/kg 106% ERAS-0015 0.3 mg/kg p.o. QD ERAS-0015 0.3 mg/kg p.o. QD 3 mg/kg 107% 1 mg/kg 62% RMC-6236 3 mg/kg 95% 10 mg/kg 105% • Up to day 15 data shown in an ongoing TGI study • ERAS-0015 was well tolerated at all doses Source: Joyo data p.o.: orally administered; QD: once daily; CDX: cell line-derived xenograft; TGI: tumor grow th inhibition 20
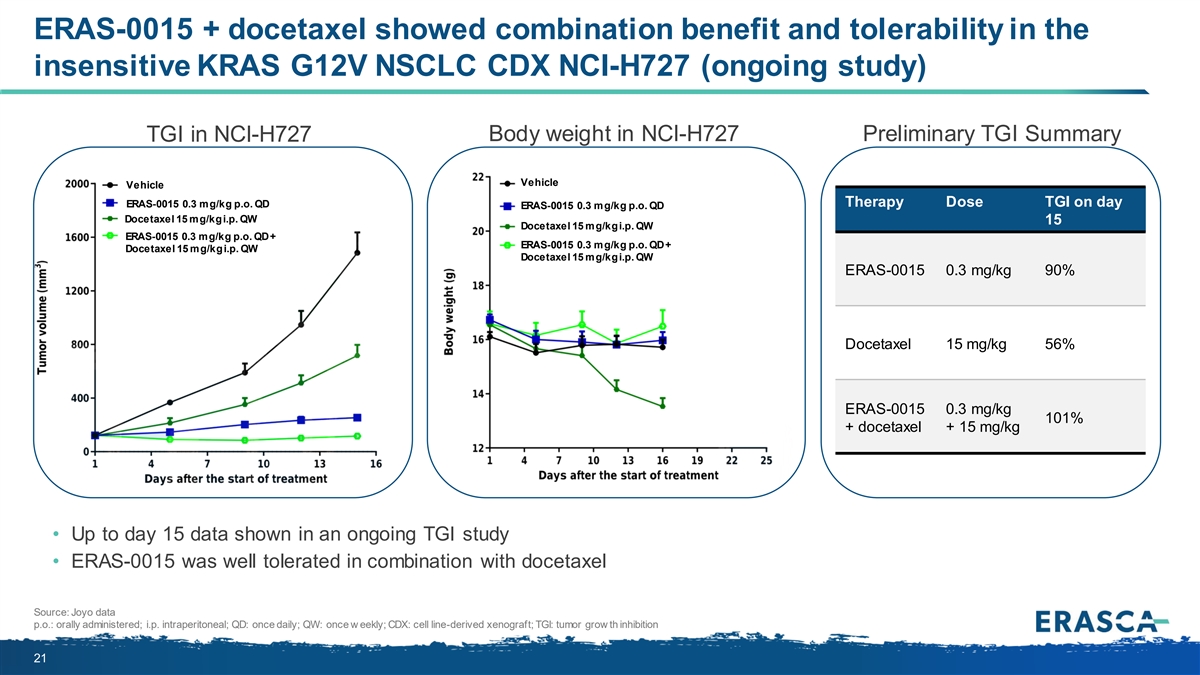
ERAS-0015 + docetaxel showed combination benefit and tolerability in the insensitive KRAS G12V NSCLC CDX NCI-H727 (ongoing study) Body weight in NCI-H727 Preliminary TGI Summary TGI in NCI-H727 Vehicle Vehicle ERAS-0015 0.3 mg/kg p.o. QD Therapy Dose TGI on day ERAS-0015 0.3 mg/kg p.o. QD Docetaxel 15 mg/kg i.p. QW 15 Docetaxel 15 mg/kg i.p. QW ERAS-0015 0.3 mg/kg p.o. QD + ERAS-0015 0.3 mg/kg p.o. QD + Docetaxel 15 mg/kg i.p. QW Docetaxel 15 mg/kg i.p. QW ERAS-0015 0.3 mg/kg 90% Docetaxel 15 mg/kg 56% ERAS-0015 0.3 mg/kg 101% + docetaxel + 15 mg/kg • Up to day 15 data shown in an ongoing TGI study • ERAS-0015 was well tolerated in combination with docetaxel Source: Joyo data p.o.: orally administered; i.p. intraperitoneal; QD: once daily; QW: once w eekly; CDX: cell line-derived xenograft; TGI: tumor grow th inhibition 21
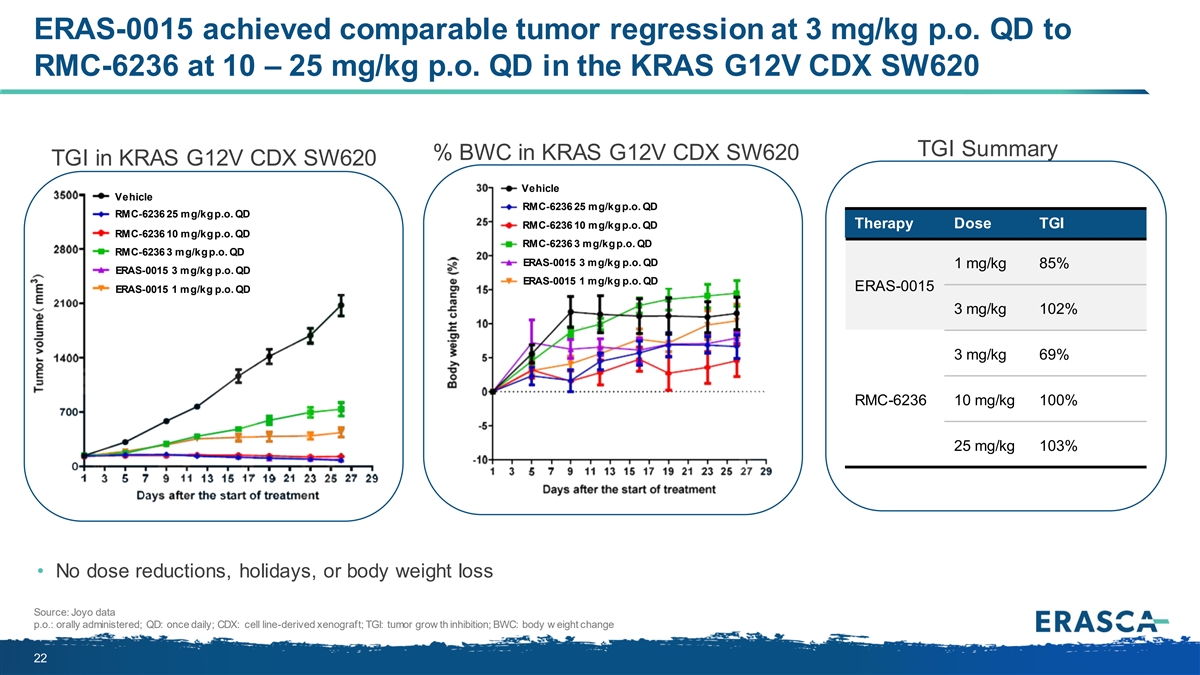
ERAS-0015 achieved comparable tumor regression at 3 mg/kg p.o. QD to RMC-6236 at 10 – 25 mg/kg p.o. QD in the KRAS G12V CDX SW620 TGI Summary % BWC in KRAS G12V CDX SW620 TGI in KRAS G12V CDX SW620 Vehicle Vehicle RMC-6236 25 mg/kg p.o. QD RMC-6236 25 mg/kg p.o. QD RMC-6236 10 mg/kg p.o. QD Therapy Dose TGI RMC-6236 10 mg/kg p.o. QD RMC-6236 3 mg/kg p.o. QD RMC-6236 3 mg/kg p.o. QD ERAS-0015 3 mg/kg p.o. QD 1 mg/kg 85% ERAS-0015 3 mg/kg p.o. QD ERAS-0015 1 mg/kg p.o. QD ERAS-0015 ERAS-0015 1 mg/kg p.o. QD 3 mg/kg 102% 3 mg/kg 69% RMC-6236 10 mg/kg 100% 25 mg/kg 103% • No dose reductions, holidays, or body weight loss Source: Joyo data p.o.: orally administered; QD: once daily; CDX: cell line-derived xenograft; TGI: tumor grow th inhibition; BWC: body w eight change 22
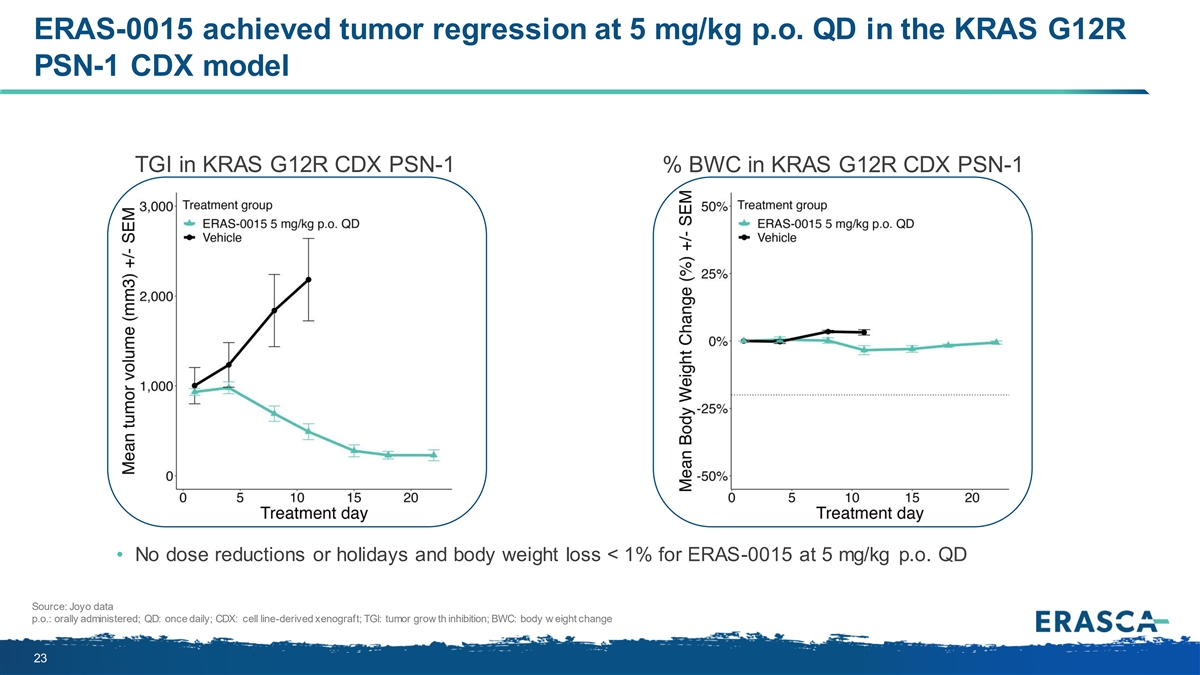
ERAS-0015 achieved tumor regression at 5 mg/kg p.o. QD in the KRAS G12R PSN-1 CDX model TGI in KRAS G12R CDX PSN-1 % BWC in KRAS G12R CDX PSN-1 • No dose reductions or holidays and body weight loss < 1% for ERAS-0015 at 5 mg/kg p.o. QD Source: Joyo data p.o.: orally administered; QD: once daily; CDX: cell line-derived xenograft; TGI: tumor grow th inhibition; BWC: body w eight change 23
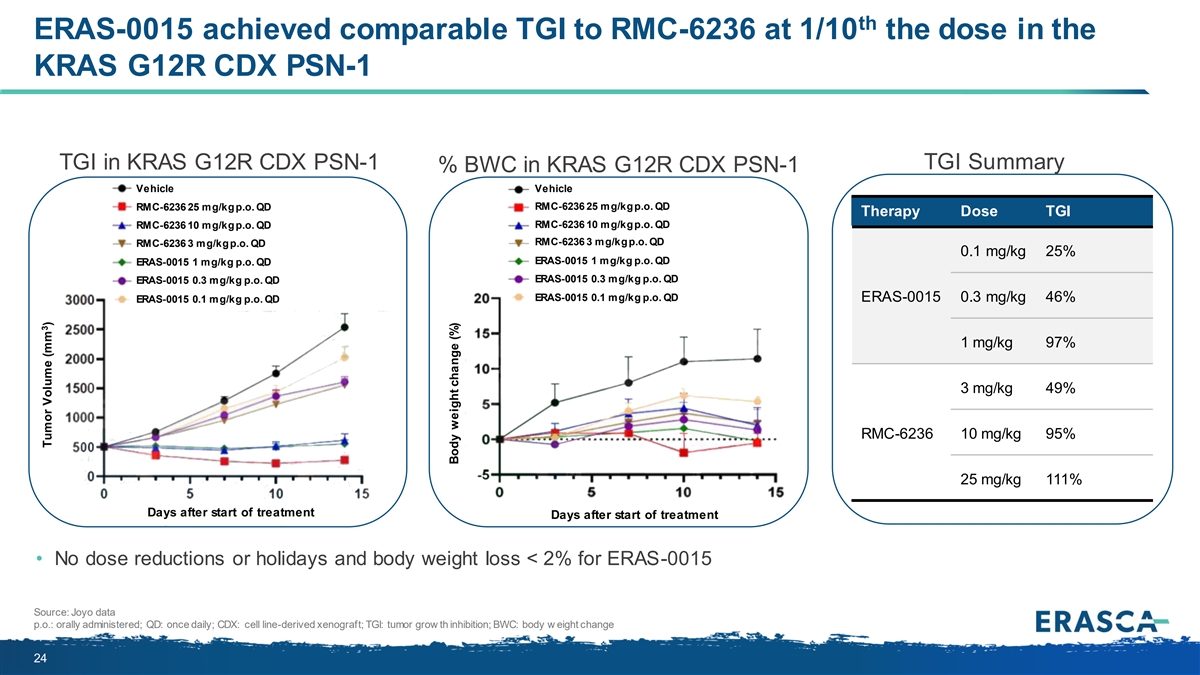
th ERAS-0015 achieved comparable TGI to RMC-6236 at 1/10 the dose in the KRAS G12R CDX PSN-1 TGI in KRAS G12R CDX PSN-1 TGI Summary % BWC in KRAS G12R CDX PSN-1 Vehicle Vehicle RMC-6236 25 mg/kg p.o. QD RMC-6236 25 mg/kg p.o. QD Therapy Dose TGI RMC-6236 10 mg/kg p.o. QD RMC-6236 10 mg/kg p.o. QD RMC-6236 3 mg/kg p.o. QD RMC-6236 3 mg/kg p.o. QD 0.1 mg/kg 25% ERAS-0015 1 mg/kg p.o. QD ERAS-0015 1 mg/kg p.o. QD ERAS-0015 0.3 mg/kg p.o. QD ERAS-0015 0.3 mg/kg p.o. QD ERAS-0015 0.1 mg/kg p.o. QD ERAS-0015 0.1 mg/kg p.o. QD ERAS-0015 0.3 mg/kg 46% 1 mg/kg 97% 3 mg/kg 49% RMC-6236 10 mg/kg 95% 25 mg/kg 111% Days after start of treatment Days after start of treatment • No dose reductions or holidays and body weight loss < 2% for ERAS-0015 Source: Joyo data p.o.: orally administered; QD: once daily; CDX: cell line-derived xenograft; TGI: tumor grow th inhibition; BWC: body w eight change 24 3 Tumor Volume (mm ) Body weight change (%)
ERAS-0015 demonstrated preferential distribution and long tumor tissue residence time vs. RMC-6236 in PSN-1, favorable attributes that support its enhanced antitumor activity Whole Blood and Tumor PK in the KRAS G12R CDX PSN-1 ERAS-0015 Whole Blood PK RMC-6236 Whole Blood PK ERAS-0015 1 mg/kg RMC-6236 10 mg/kg Whole Blood and Tissue Concentrations Whole Blood and Tissue Concentrations 700 2000 600 1600 500 400 1200 300 800 200 400 100 0 0 4 hours 24 hours 24 hours 4 hours Source: Joyo data 25 Whole Blood Concentration (nmol / L) W hole Blood or Tumor Concentration (nmol / L) W hole Blood or Tumor Concentration W hole Blood or Tumor Concentration (nmol / L) (nmol / L) Whole Blood Concentration (nmol / L)
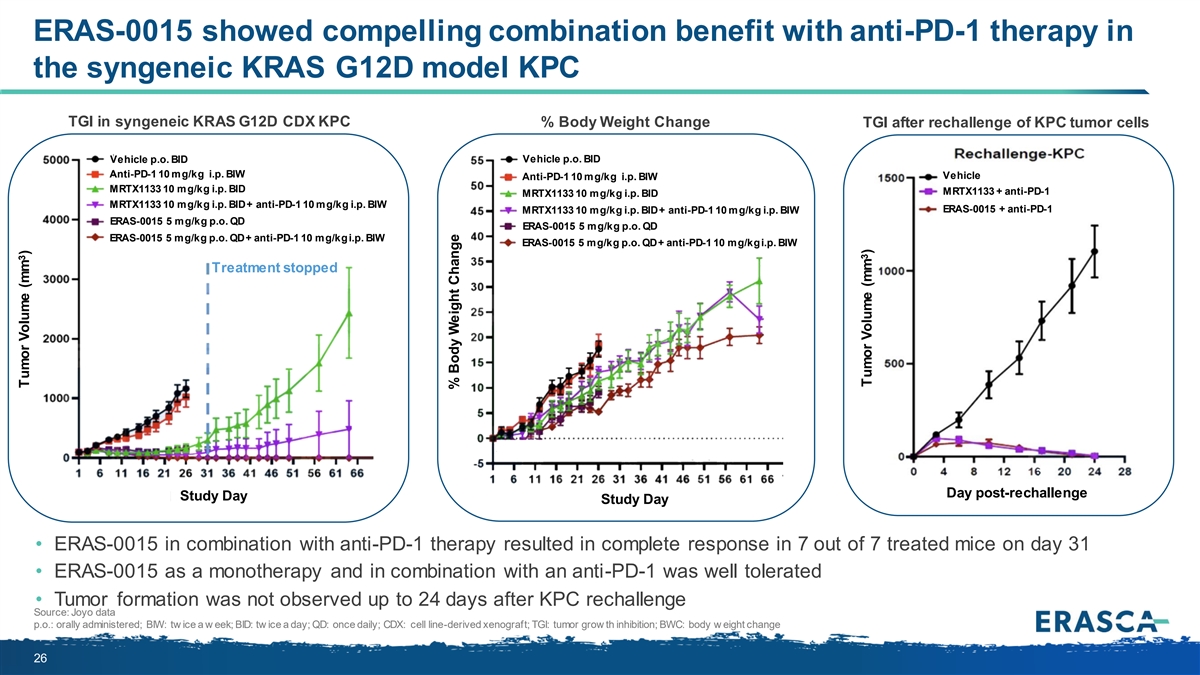
ERAS-0015 showed compelling combination benefit with anti-PD-1 therapy in the syngeneic KRAS G12D model KPC TGI in syngeneic KRAS G12D CDX KPC % Body Weight Change TGI after rechallenge of KPC tumor cells Vehicle p.o. BID Vehicle p.o. BID Anti-PD-1 10 mg/kg i.p. BIW Vehicle Anti-PD-1 10 mg/kg i.p. BIW MRTX1133 10 mg/kg i.p. BID MRTX1133 + anti-PD-1 MRTX1133 10 mg/kg i.p. BID MRTX1133 10 mg/kg i.p. BID + anti-PD-1 10 mg/kg i.p. BIW ERAS-0015 + anti-PD-1 MRTX1133 10 mg/kg i.p. BID + anti-PD-1 10 mg/kg i.p. BIW ERAS-0015 5 mg/kg p.o. QD ERAS-0015 5 mg/kg p.o. QD ERAS-0015 5 mg/kg p.o. QD + anti-PD-1 10 mg/kg i.p. BIW ERAS-0015 5 mg/kg p.o. QD + anti-PD-1 10 mg/kg i.p. BIW Treatment stopped Day post-rechallenge Study Day Study Day • ERAS-0015 in combination with anti-PD-1 therapy resulted in complete response in 7 out of 7 treated mice on day 31 • ERAS-0015 as a monotherapy and in combination with an anti-PD-1 was well tolerated • Tumor formation was not observed up to 24 days after KPC rechallenge Source: Joyo data p.o.: orally administered; BIW: tw ice a w eek; BID: tw ice a day; QD: once daily; CDX: cell line-derived xenograft; TGI: tumor grow th inhibition; BWC: body w eight change 26 3 Tumor Volume (mm ) % Body Weight Change 3 Tumor Volume (mm )
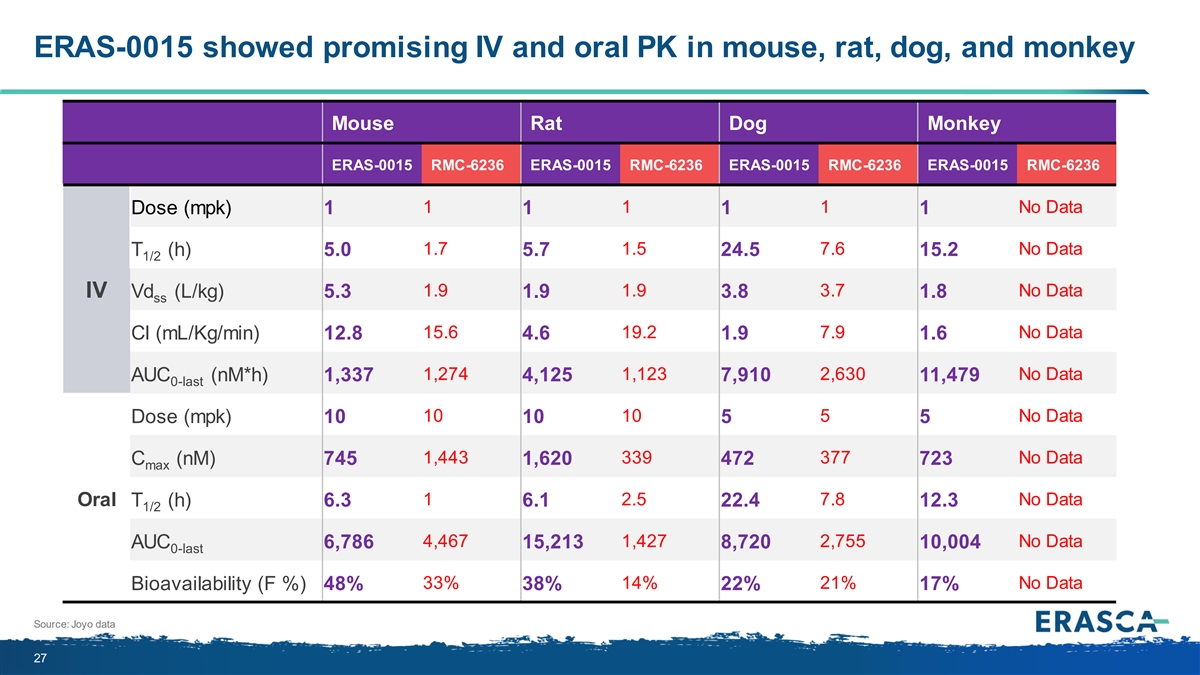
ERAS-0015 showed promising IV and oral PK in mouse, rat, dog, and monkey Mouse Rat Dog Monkey ERAS-0015 RMC-6236 ERAS-0015 RMC-6236 ERAS-0015 RMC-6236 ERAS-0015 RMC-6236 1 1 1 No Data Dose (mpk) 1 1 1 1 1.7 1.5 7.6 No Data T (h) 5.0 5.7 24.5 15.2 1/2 1.9 1.9 3.7 No Data IV Vd (L/kg) 5.3 1.9 3.8 1.8 ss 15.6 19.2 7.9 No Data Cl (mL/Kg/min) 12.8 4.6 1.9 1.6 1,274 1,123 2,630 No Data AUC (nM*h) 1,337 4,125 7,910 11,479 0-last 10 10 5 No Data Dose (mpk) 10 10 5 5 1,443 339 377 No Data C (nM) 745 1,620 472 723 max 1 2.5 7.8 No Data Oral T (h) 6.3 6.1 22.4 12.3 1/2 4,467 1,427 2,755 No Data AUC 6,786 15,213 8,720 10,004 0-last 33% 14% 21% No Data Bioavailability (F %) 48% 38% 22% 17% Source: Joyo data 27
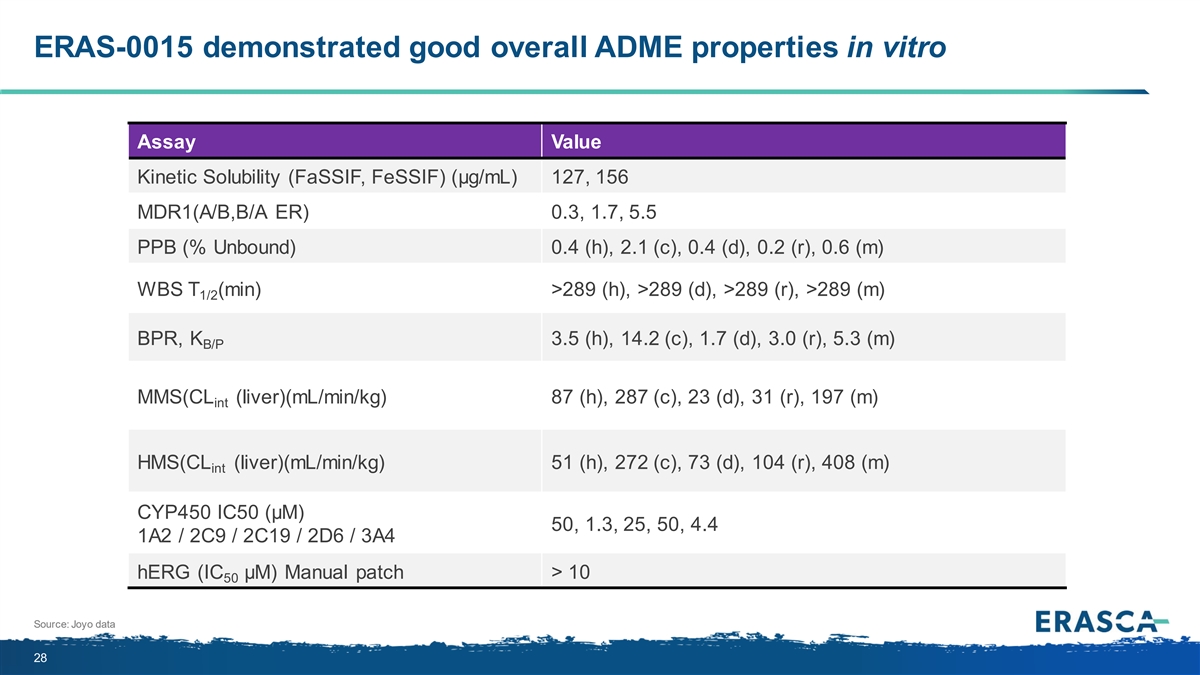
ERAS-0015 demonstrated good overall ADME properties in vitro Assay Value Kinetic Solubility (FaSSIF, FeSSIF) (μg/mL) 127, 156 MDR1(A/B,B/A ER) 0.3, 1.7, 5.5 PPB (% Unbound) 0.4 (h), 2.1 (c), 0.4 (d), 0.2 (r), 0.6 (m) WBS T (min) >289 (h), >289 (d), >289 (r), >289 (m) 1/2 BPR, K 3.5 (h), 14.2 (c), 1.7 (d), 3.0 (r), 5.3 (m) B/P MMS(CL (liver)(mL/min/kg) 87 (h), 287 (c), 23 (d), 31 (r), 197 (m) int HMS(CL (liver)(mL/min/kg) 51 (h), 272 (c), 73 (d), 104 (r), 408 (m) int CYP450 IC50 (µM) 50, 1.3, 25, 50, 4.4 1A2 / 2C9 / 2C19 / 2D6 / 3A4 hERG (IC μM) Manual patch > 10 50 Source: Joyo data 28
ERAS-0015 and ERAS-4001 exhibit competitive profiles that exceed our TPP • ~5x – 10x greater potency as well as favorable ERAS-0015: Potential BIC ADME properties and PK performance in animal Pan-RAS MG species (vs. current Pan-RAS MG in development) • Designed to spare H/NRAS WT, predicted to provide greater therapeutic window (vs. Pan-RAS MG) for ERAS-4001: Potential FIC KRASm solid tumors Pan-KRAS or “KRAS- selective” SM inhibitor • Designed to address KRASwt activation to prevent resistance (vs. mutant-selective inhibitors) TPP = target product profile; BIC: best-in-class; FIC: first-in-class; MG: molecular glue; SM: small molecule; ADME: absorption, distribution, metabolism, and excretion; PK: pharmacokinetic 29
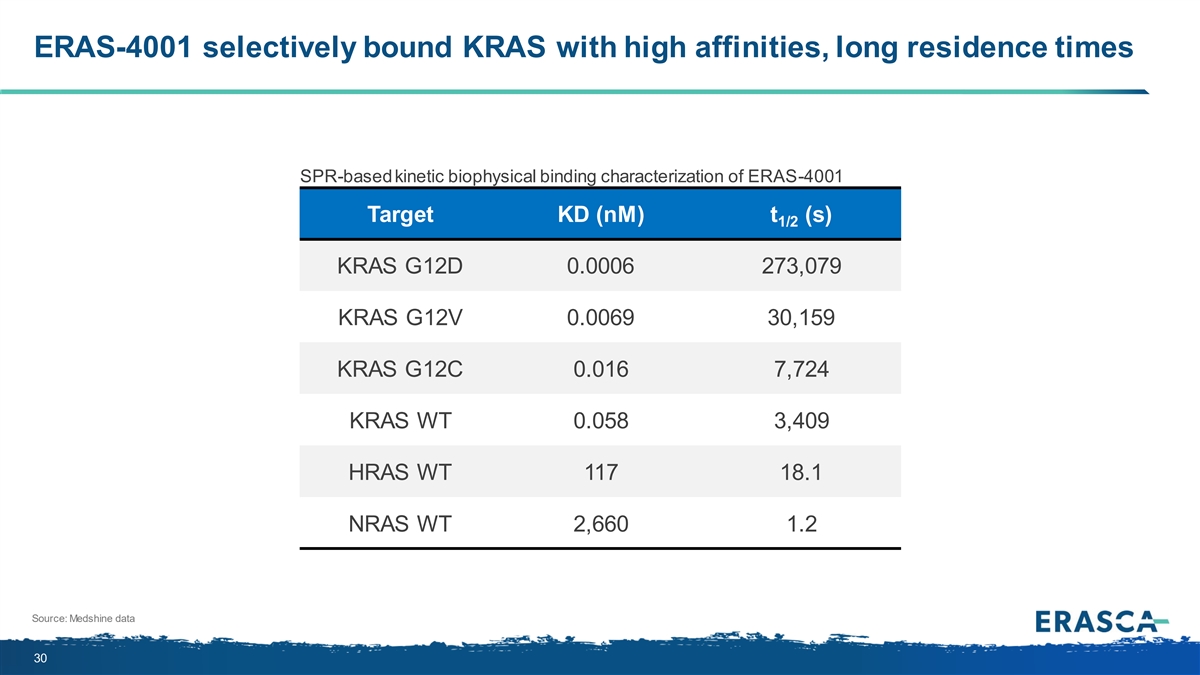
ERAS-4001 selectively bound KRAS with high affinities, long residence times SPR-based kinetic biophysical binding characterization of ERAS-4001 Target KD (nM) t (s) 1/2 KRAS G12D 0.0006 273,079 KRAS G12V 0.0069 30,159 KRAS G12C 0.016 7,724 KRAS WT 0.058 3,409 HRAS WT 117 18.1 NRAS WT 2,660 1.2 Source: Medshine data 30
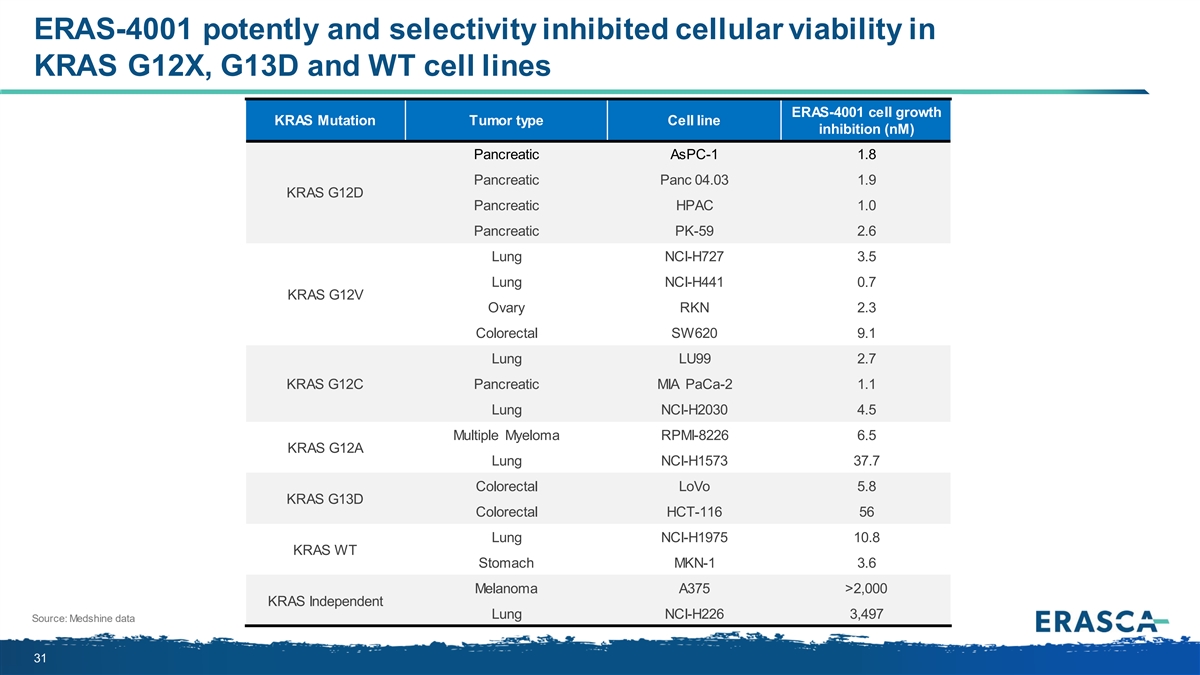
ERAS-4001 potently and selectivity inhibited cellular viability in KRAS G12X, G13D and WT cell lines ERAS-4001 cell growth KRAS Mutation Tumor type Cell line inhibition (nM) Pancreatic AsPC-1 1.8 Pancreatic Panc 04.03 1.9 KRAS G12D Pancreatic HPAC 1.0 Pancreatic PK-59 2.6 Lung NCI-H727 3.5 Lung NCI-H441 0.7 KRAS G12V Ovary RKN 2.3 Colorectal SW620 9.1 Lung LU99 2.7 KRAS G12C Pancreatic MIA PaCa-2 1.1 Lung NCI-H2030 4.5 Multiple Myeloma RPMI-8226 6.5 KRAS G12A Lung NCI-H1573 37.7 Colorectal LoVo 5.8 KRAS G13D Colorectal HCT-116 56 Lung NCI-H1975 10.8 KRAS WT Stomach MKN-1 3.6 Melanoma A375 >2,000 KRAS Independent Lung NCI-H226 3,497 Source: Medshine data 31
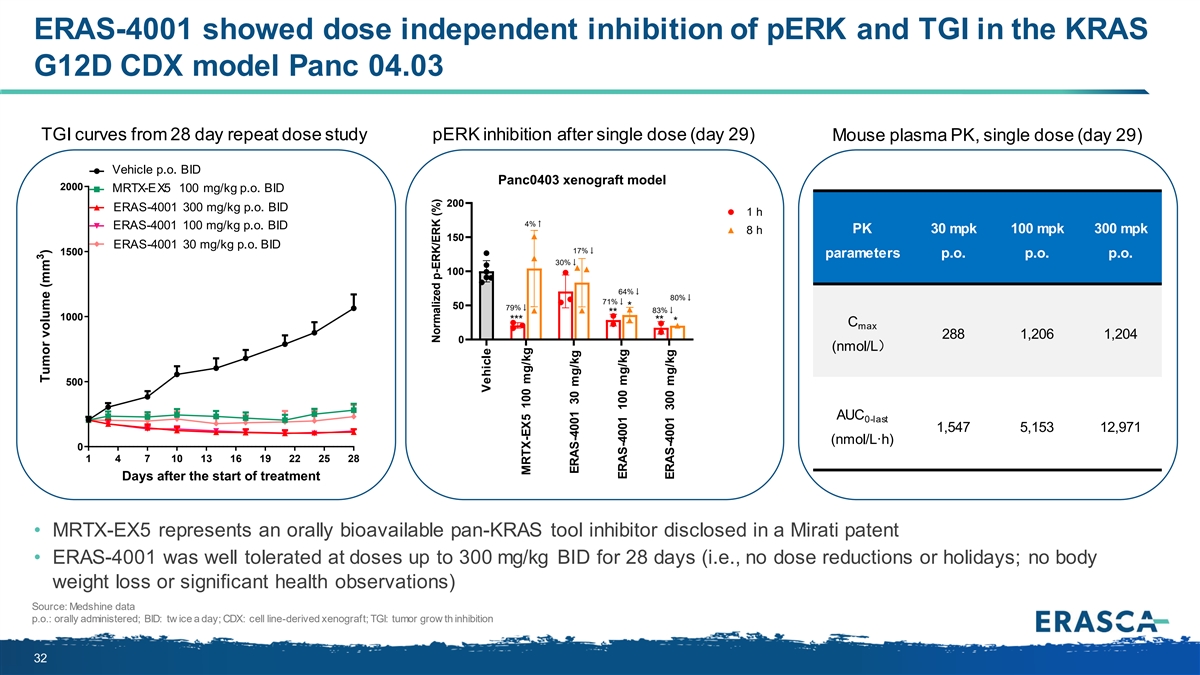
ERAS-4001 showed dose independent inhibition of pERK and TGI in the KRAS G12D CDX model Panc 04.03 TGI curves from 28 day repeat dose study pERK inhibition after single dose (day 29) Mouse plasma PK, single dose (day 29) Vehicle p.o. BID Vehicle,p.o.BID Panc0403 xenograft model 2000 MRTX-EX5 100 mg/kg p.o. BID MRTX-EX5,100 mg/kg,p.o.,BID 200 ERAS-4001 300 mg/kg p.o. BID WXSHA015,300 mg/kg,p.o.,BID 1 h 4%↑ ERAS-4001 100 mg/kg p.o. BID WXSHA015,100 mg/kg,p.o.,BID 8 h PK 30 mpk 100 mpk 300 mpk 150 W ER XA S S H -4 A 00 01 15 3 ,3 00 m m g/g k/g k g p.,o p.. o B.I,D BID 17%↓ 1500 parameters p.o. p.o. p.o. 30%↓ 100 64%↓ 80%↓ 71%↓ 50 * 79%↓ 83%↓ ** 1000 *** ** * C max 288 1,206 1,204 0 (nmol/L) 500 AUC 0-last 1,547 5,153 12,971 (nmol/L·h) 0 1 4 7 10 13 16 19 22 25 28 Days after the start of treatment • MRTX-EX5 represents an orally bioavailable pan-KRAS tool inhibitor disclosed in a Mirati patent • ERAS-4001 was well tolerated at doses up to 300 mg/kg BID for 28 days (i.e., no dose reductions or holidays; no body weight loss or significant health observations) Source: Medshine data p.o.: orally administered; BID: tw ice a day; CDX: cell line-derived xenograft; TGI: tumor grow th inhibition 32 Vehicle Ex.5 100 mpk WX-A015 30 mpk WX-A015 100 mpk WX-A015 300 mpk 3 Tumor volume (mm ) Normalized p-ERK/ERK (%) Vehicle MRTX-EX5 100 mg/kg ERAS-4001 30 mg/kg ERAS-4001 100 mg/kg ERAS-4001 300 mg/kg
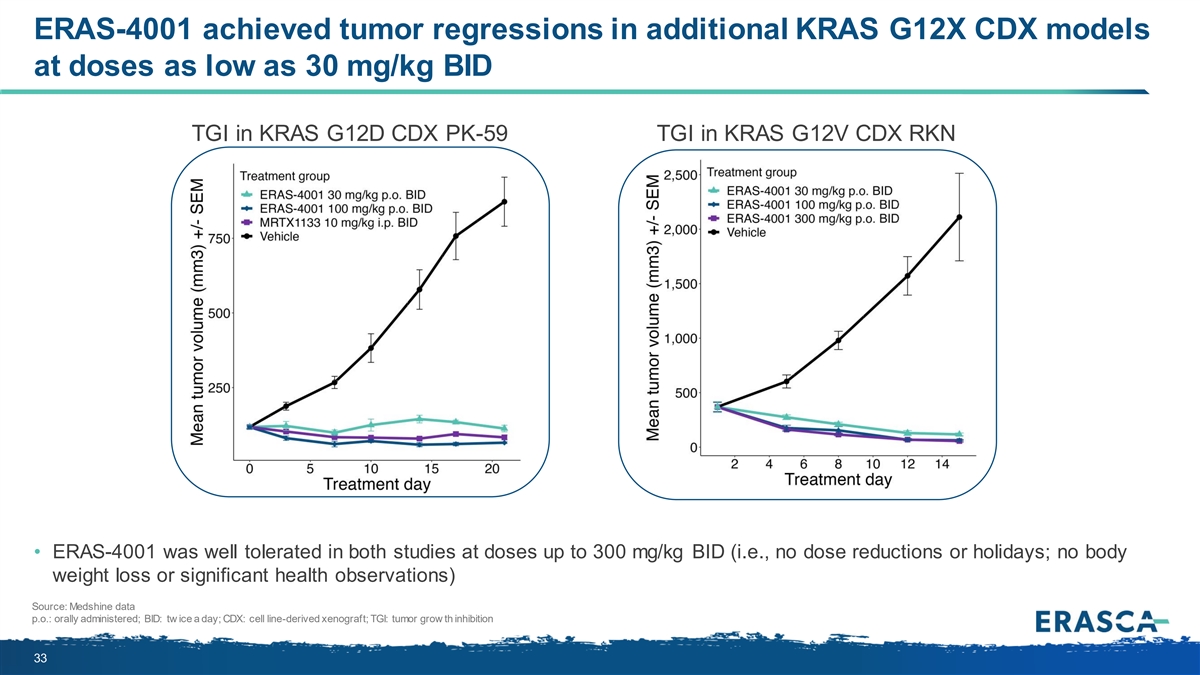
ERAS-4001 achieved tumor regressions in additional KRAS G12X CDX models at doses as low as 30 mg/kg BID TGI in KRAS G12D CDX PK-59 TGI in KRAS G12V CDX RKN • ERAS-4001 was well tolerated in both studies at doses up to 300 mg/kg BID (i.e., no dose reductions or holidays; no body weight loss or significant health observations) Source: Medshine data p.o.: orally administered; BID: tw ice a day; CDX: cell line-derived xenograft; TGI: tumor grow th inhibition 33

ERAS-4001 achieved tumor regression in a pan-KRASi insensitive KRAS G12V CDX model NCI-H727 TGI in KRAS G12V CDX NCI-H727 % BWC in KRAS G12V CDX NCI-H727 • ERAS-4001 was well tolerated at doses ranging from 10 mg/kg p.o. BID to 100 mg/kg p.o. BID (i.e., no dose holidays or mortality) • ERAS-4001 at 300 mg/kg p.o. BID showed borderline tolerability with 4 out of 6 mice receiving continuous treatment, one mouse receiving a dose holiday due to body weight loss on days 16-21, and one mouse death on day 13 • Observed borderline tolerability may be model and/or study specific; ERAS-4001 at 300 mg/kg p.o. BID was well tolerated in the Panc 04.03 CDX TGI study (no dose holidays or mortality) Source: Medshine data p.o.: orally administered; BID: tw ice a day; CDX: cell line-derived xenograft; TGI: tumor grow th inhibition 34
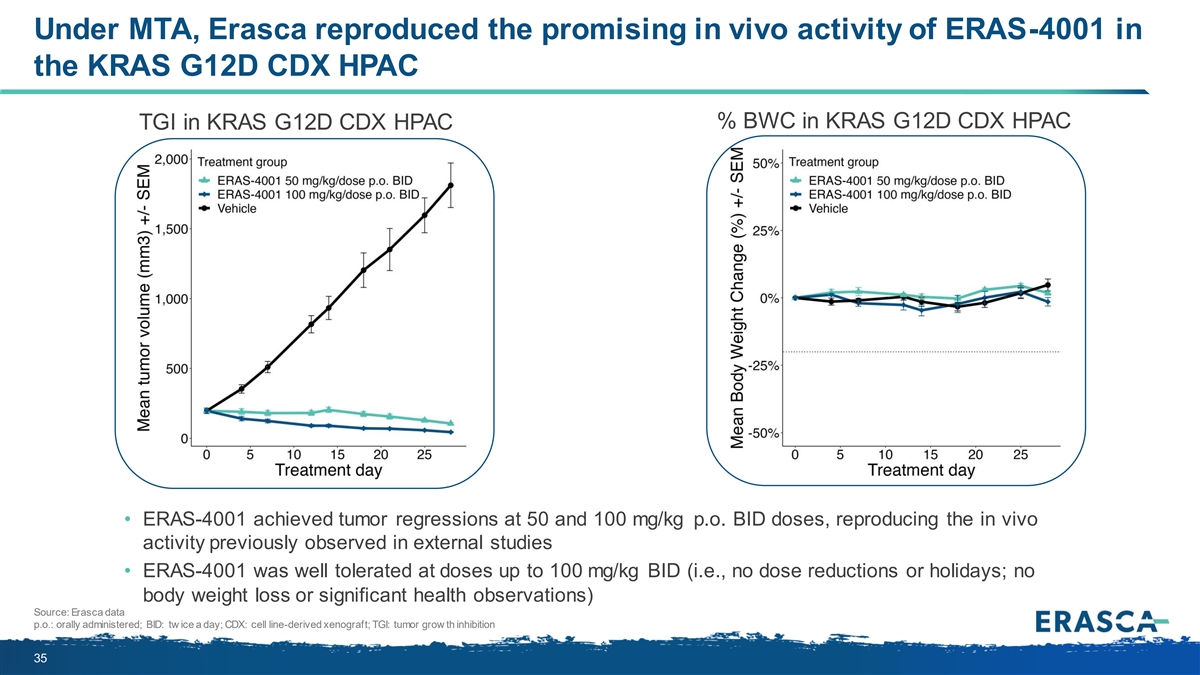
Under MTA, Erasca reproduced the promising in vivo activity of ERAS-4001 in the KRAS G12D CDX HPAC % BWC in KRAS G12D CDX HPAC TGI in KRAS G12D CDX HPAC • ERAS-4001 achieved tumor regressions at 50 and 100 mg/kg p.o. BID doses, reproducing the in vivo activity previously observed in external studies • ERAS-4001 was well tolerated at doses up to 100 mg/kg BID (i.e., no dose reductions or holidays; no body weight loss or significant health observations) Source: Erasca data p.o.: orally administered; BID: tw ice a day; CDX: cell line-derived xenograft; TGI: tumor grow th inhibition 35
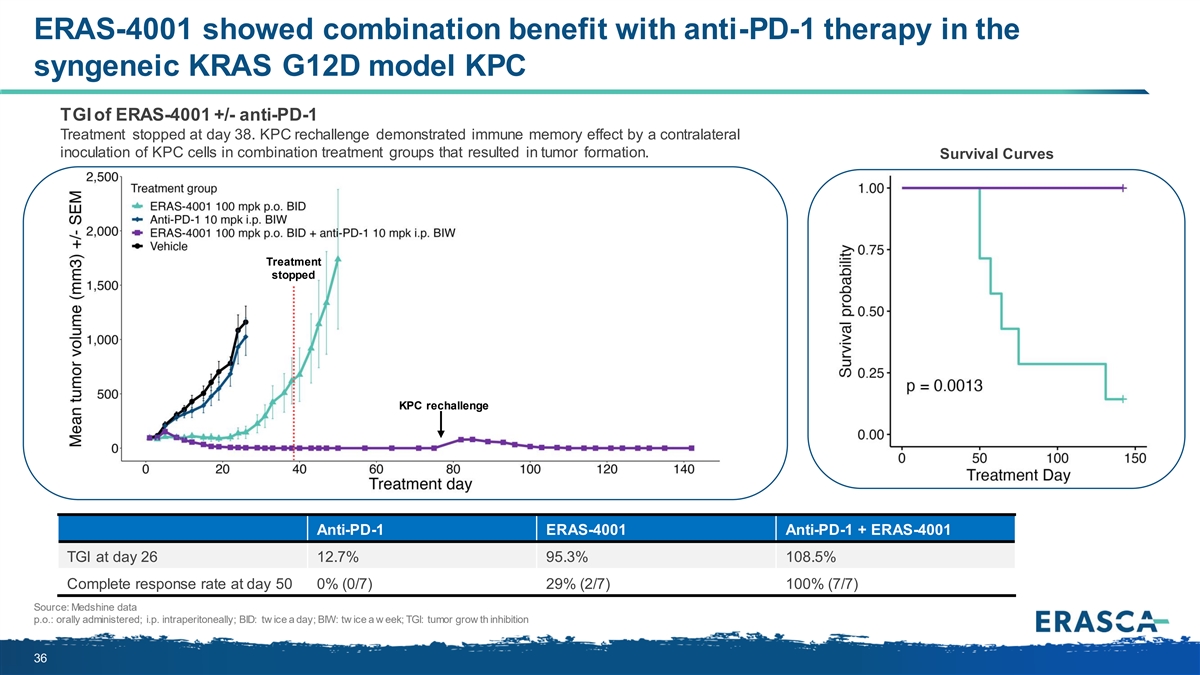
ERAS-4001 showed combination benefit with anti-PD-1 therapy in the syngeneic KRAS G12D model KPC TGI of ERAS-4001 +/- anti-PD-1 Treatment stopped at day 38. KPC rechallenge demonstrated immune memory effect by a contralateral inoculation of KPC cells in combination treatment groups that resulted in tumor formation. Survival Curves Treatment stopped KPC rechallenge Anti-PD-1 ERAS-4001 Anti-PD-1 + ERAS-4001 TGI at day 26 12.7% 95.3% 108.5% Complete response rate at day 50 0% (0/7) 29% (2/7) 100% (7/7) Source: Medshine data p.o.: orally administered; i.p. intraperitoneally; BID: tw ice a day; BIW: tw ice a w eek; TGI: tumor grow th inhibition 36
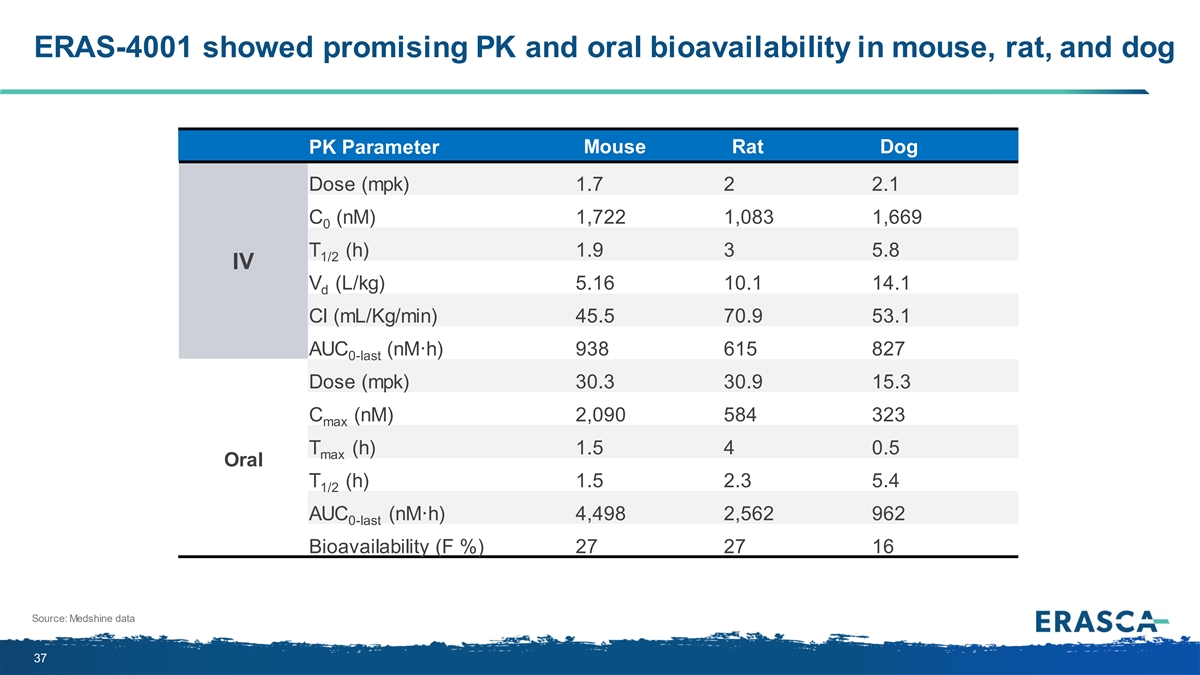
ERAS-4001 showed promising PK and oral bioavailability in mouse, rat, and dog PK Parameter Mouse Rat Dog Dose (mpk) 1.7 2 2.1 C (nM) 1,722 1,083 1,669 0 T (h) 1.9 3 5.8 1/2 IV V (L/kg) 5.16 10.1 14.1 d Cl (mL/Kg/min) 45.5 70.9 53.1 AUC (nM·h) 938 615 827 0-last Dose (mpk) 30.3 30.9 15.3 C (nM) 2,090 584 323 max T (h) 1.5 4 0.5 max Oral T (h) 1.5 2.3 5.4 1/2 AUC (nM·h) 4,498 2,562 962 0-last Bioavailability (F %) 27 27 16 Source: Medshine data 37
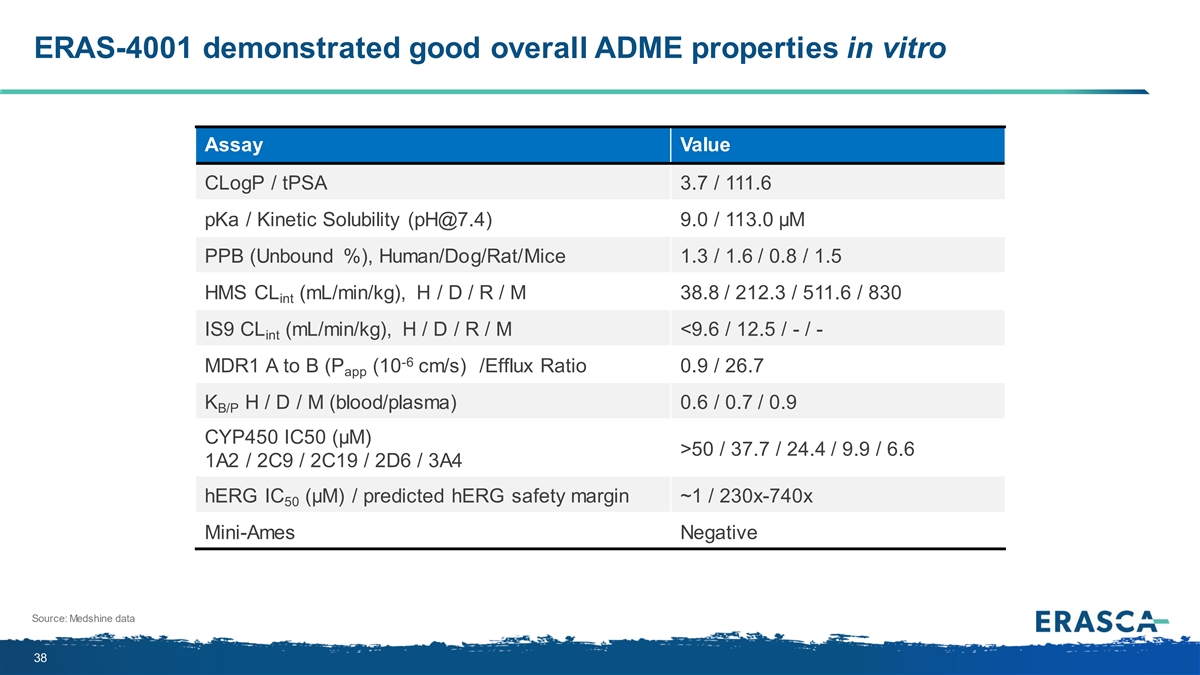
ERAS-4001 demonstrated good overall ADME properties in vitro Assay Value CLogP / tPSA 3.7 / 111.6 pKa / Kinetic Solubility (pH@7.4) 9.0 / 113.0 µM PPB (Unbound %), Human/Dog/Rat/Mice 1.3 / 1.6 / 0.8 / 1.5 HMS CL (mL/min/kg), H / D / R / M 38.8 / 212.3 / 511.6 / 830 int IS9 CL (mL/min/kg), H / D / R / M <9.6 / 12.5 / - / - int -6 MDR1 A to B (P (10 cm/s) /Efflux Ratio 0.9 / 26.7 app K H / D / M (blood/plasma) 0.6 / 0.7 / 0.9 B/P CYP450 IC50 (µM) >50 / 37.7 / 24.4 / 9.9 / 6.6 1A2 / 2C9 / 2C19 / 2D6 / 3A4 hERG IC (µM) / predicted hERG safety margin ~1 / 230x-740x 50 Mini-Ames Negative Source: Medshine data 38
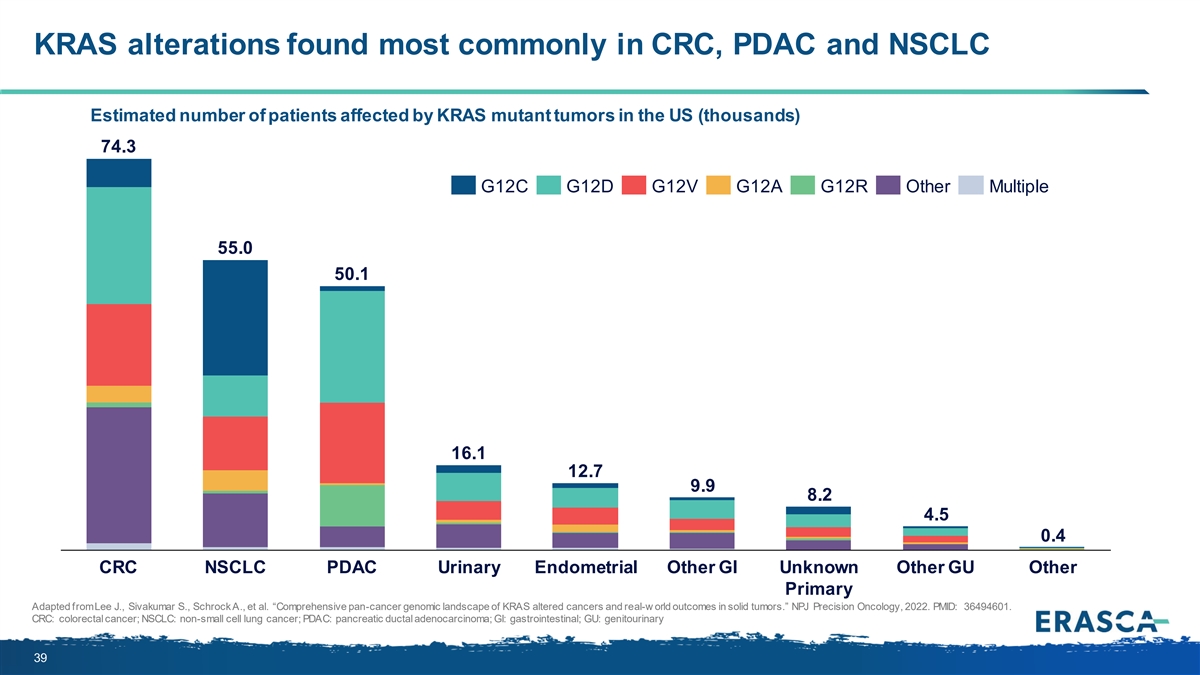
KRAS alterations found most commonly in CRC, PDAC and NSCLC Estimated number of patients affected by KRAS mutant tumors in the US (thousands) 74.3 G12C G12D G12V G12A G12R Other Multiple 55.0 50.1 16.1 12.7 9.9 8.2 4.5 0.4 CRC NSCLC PDAC Urinary Endometrial Other GI Unknown Other GU Other Primary Adapted from Lee J., Sivakumar S., Schrock A., et al. “Comprehensive pan-cancer genomic landscape of KRAS altered cancers and real-w orld outcomes in solid tumors.” NPJ Precision Oncology, 2022. PMID: 36494601. CRC: colorectal cancer; NSCLC: non-small cell lung cancer; PDAC: pancreatic ductal adenocarcinoma; GI: gastrointestinal; GU: genitourinary 39
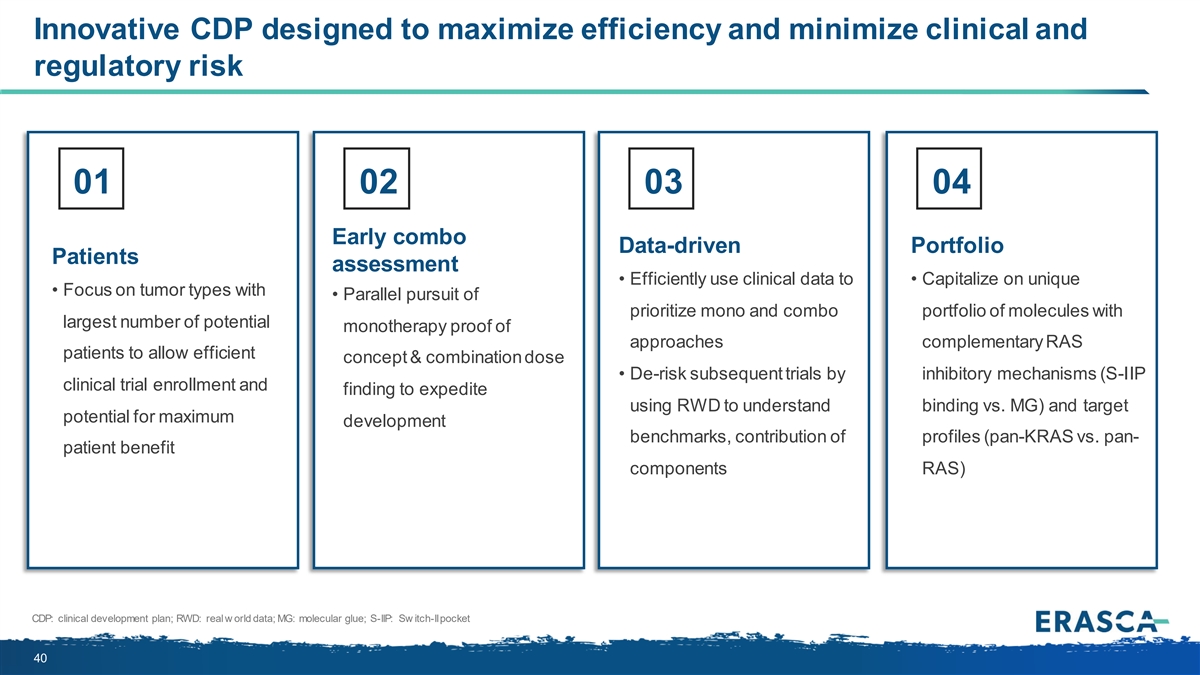
Innovative CDP designed to maximize efficiency and minimize clinical and regulatory risk 01 02 03 04 Early combo Data-driven Portfolio Patients assessment • Efficiently use clinical data to • Capitalize on unique • Focus on tumor types with • Parallel pursuit of prioritize mono and combo portfolio of molecules with largest number of potential monotherapy proof of approaches complementary RAS patients to allow efficient concept & combination dose • De-risk subsequent trials by inhibitory mechanisms (S-IIP clinical trial enrollment and finding to expedite using RWD to understand binding vs. MG) and target potential for maximum development benchmarks, contribution of profiles (pan-KRAS vs. pan- patient benefit components RAS) CDP: clinical development plan; RWD: real w orld data; MG: molecular glue; S-IIP: Sw itch-II pocket 40
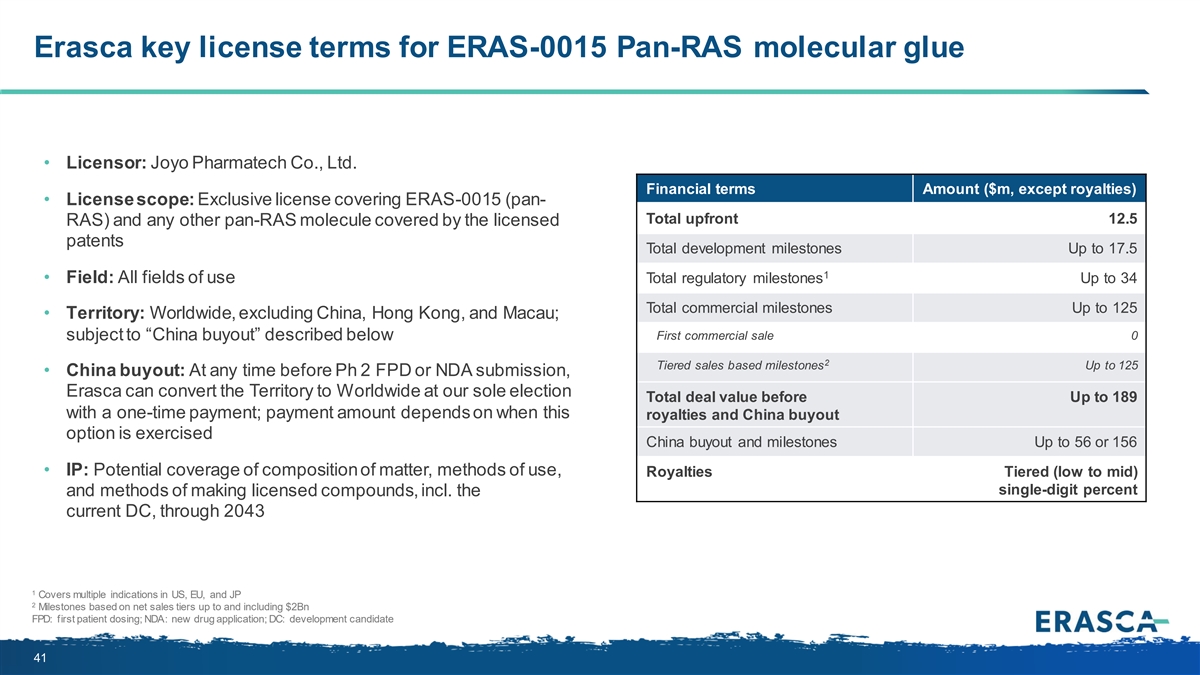
Erasca key license terms for ERAS-0015 Pan-RAS molecular glue • Licensor: Joyo Pharmatech Co., Ltd. Financial terms Amount ($m, except royalties) • License scope: Exclusive license covering ERAS-0015 (pan- Total upfront 12.5 RAS) and any other pan-RAS molecule covered by the licensed patents Total development milestones Up to 17.5 1 • Field: All fields of use Total regulatory milestones Up to 34 Total commercial milestones Up to 125 • Territory: Worldwide, excluding China, Hong Kong, and Macau; subject to “China buyout” described below First commercial sale 0 2 Tiered sales based milestones Up to 125 • China buyout: At any time before Ph 2 FPD or NDA submission, Erasca can convert the Territory to Worldwide at our sole election Total deal value before Up to 189 with a one-time payment; payment amount depends on when this royalties and China buyout option is exercised China buyout and milestones Up to 56 or 156 • IP: Potential coverage of composition of matter, methods of use, Royalties Tiered (low to mid) single-digit percent and methods of making licensed compounds, incl. the current DC, through 2043 1 Covers multiple indications in US, EU, and JP 2 Milestones based on net sales tiers up to and including $2Bn FPD: first patient dosing; NDA: new drug application; DC: development candidate 41
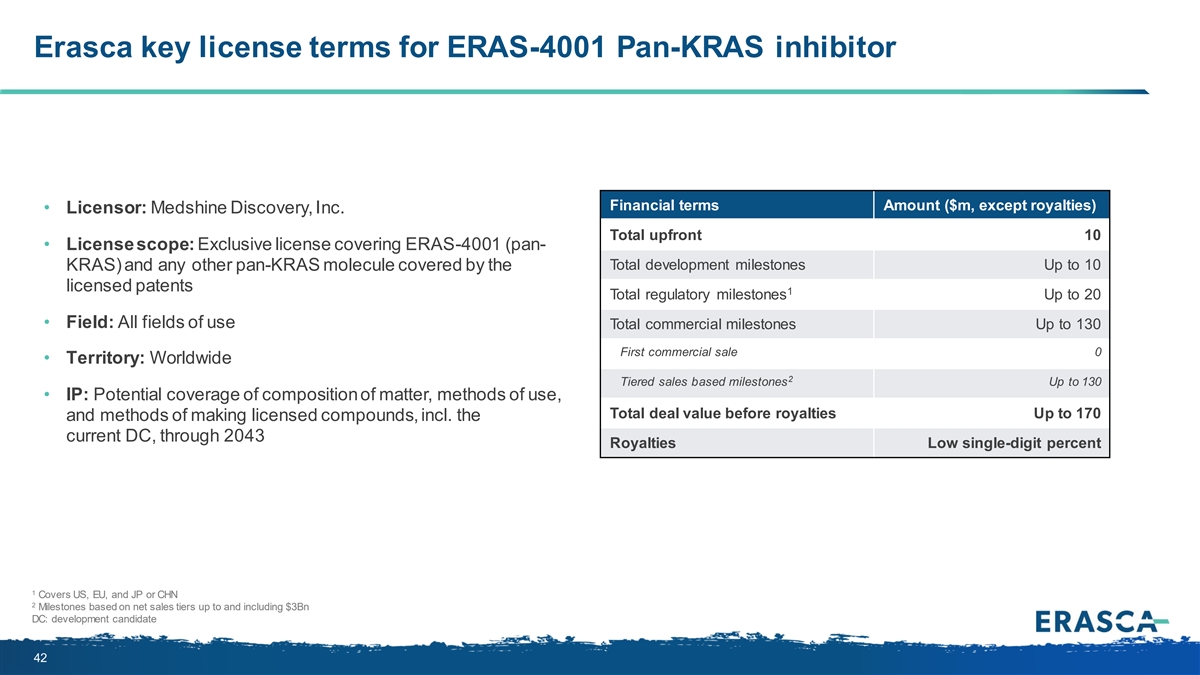
Erasca key license terms for ERAS-4001 Pan-KRAS inhibitor Financial terms Amount ($m, except royalties) • Licensor: Medshine Discovery, Inc. Total upfront 10 • License scope: Exclusive license covering ERAS-4001 (pan- Total development milestones Up to 10 KRAS) and any other pan-KRAS molecule covered by the licensed patents 1 Total regulatory milestones Up to 20 • Field: All fields of use Total commercial milestones Up to 130 First commercial sale 0 • Territory: Worldwide 2 Tiered sales based milestones Up to 130 • IP: Potential coverage of composition of matter, methods of use, Total deal value before royalties Up to 170 and methods of making licensed compounds, incl. the current DC, through 2043 Royalties Low single-digit percent 1 Covers US, EU, and JP or CHN 2 Milestones based on net sales tiers up to and including $3Bn DC: development candidate 42

Corporate Update
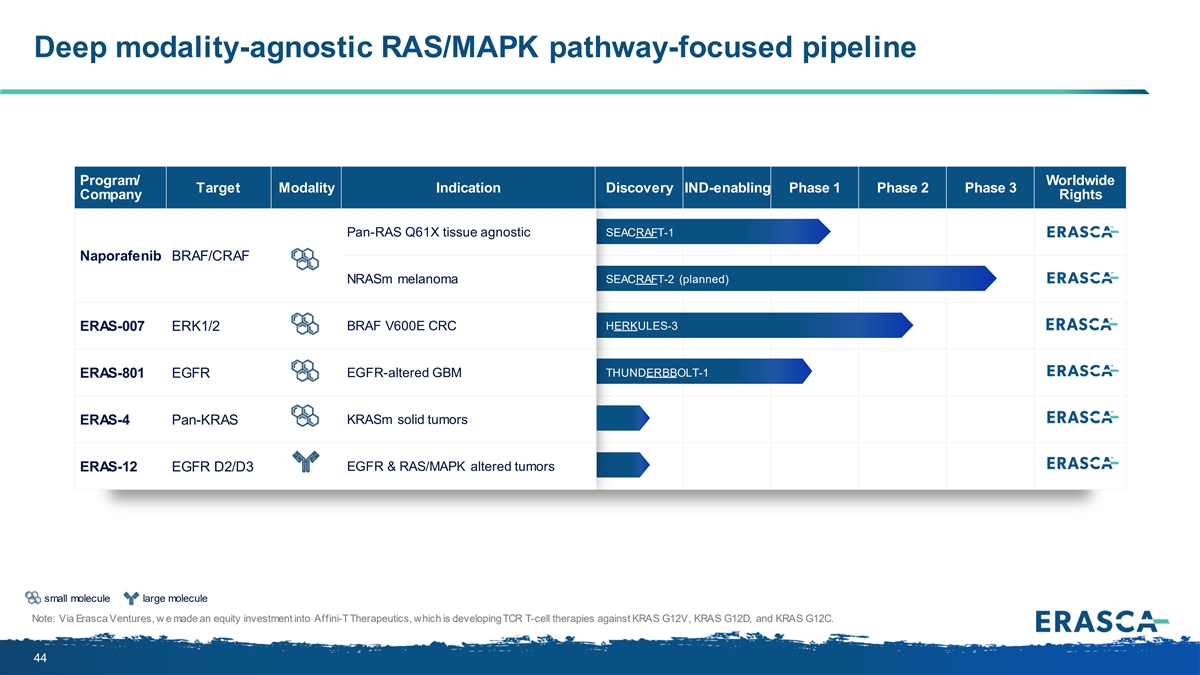
Deep modality-agnostic RAS/MAPK pathway-focused pipeline Program/ Worldwide Target Modality Indication Discovery IND-enabling Phase 1 Phase 2 Phase 3 Company Rights Pan-RAS Q61X tissue agnostic SEACRAFT-1 Naporafenib BRAF/CRAF NRASm melanoma SEACRAFT-2 (planned) BRAF V600E CRC HERKULES-3 ERAS-007 ERK1/2 THUNDERBBOLT-1 ERAS-801 EGFR EGFR-altered GBM KRASm solid tumors ERAS-4 Pan-KRAS ERAS-12 EGFR D2/D3 EGFR & RAS/MAPK altered tumors small molecule large molecule Note: Via Erasca Ventures, w e made an equity investment into Affini-T Therapeutics, which is developing TCR T-cell therapies against KRAS G12V, KRAS G12D, and KRAS G12C. 44
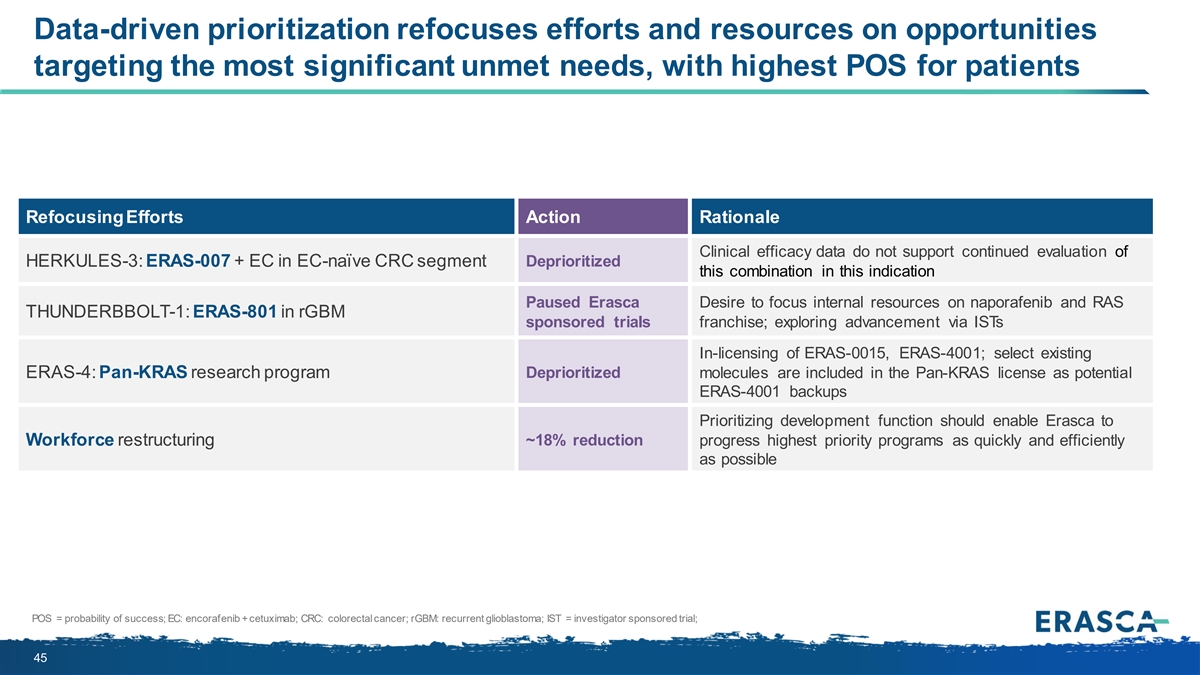
Data-driven prioritization refocuses efforts and resources on opportunities targeting the most significant unmet needs, with highest POS for patients Refocusing Efforts Action Rationale Clinical efficacy data do not support continued evaluation of Deprioritized HERKULES-3: ERAS-007 + EC in EC-naïve CRC segment this combination in this indication Paused Erasca Desire to focus internal resources on naporafenib and RAS THUNDERBBOLT-1: ERAS-801 in rGBM sponsored trials franchise; exploring advancement via ISTs In-licensing of ERAS-0015, ERAS-4001; select existing Deprioritized ERAS-4: Pan-KRAS research program molecules are included in the Pan-KRAS license as potential ERAS-4001 backups Prioritizing development function should enable Erasca to Workforce restructuring ~18% reduction progress highest priority programs as quickly and efficiently as possible POS = probability of success; EC: encorafenib + cetuximab; CRC: colorectal cancer; rGBM: recurrent glioblastoma; IST = investigator sponsored trial; 45

Erasca’s AURORAS and BOREALIS trials to address RAS opportunities During any long seafaring journey, the endless sea can often disorient a person’s sense of time and place. The appearance of the dawn, or aurora, provides a definitive sense of direction and a welcome signal to start the new day. At Erasca, helping patients is our guiding light. With the AURORAS and BOREALIS studies, we hope that our potent, selective, orally bioavailable Pan-RAS molecular glue ERAS-0015 and Pan-KRAS inhibitor ERAS-4001 can provide benefit to patients with (K)RASm solid tumors. 1. AURORAS-1 is expected to be the initial phase 1 trial that will assess the ERAS-0015 Pan-RAS molecular glue alone and 1 in combination for the treatment of patients with RASm solid tumors, for which an IND is targeted for H1 2025 2. BOREALIS-1 is expected to be the initial phase 1 trial that will assess the ERAS-4001 Pan-KRASi alone and in combination for the treatment of patients with KRASm solid tumors, for which an IND is targeted for Q1 2025 NOTE: Combination of the molecules with each other could be explored in future AURORA BOREALIS trial(s) 1 Timing of IND is subject to adjustment (could potentially move to Q3 2025) pending detailed program planning, driven predominantly by CMC timelines 46
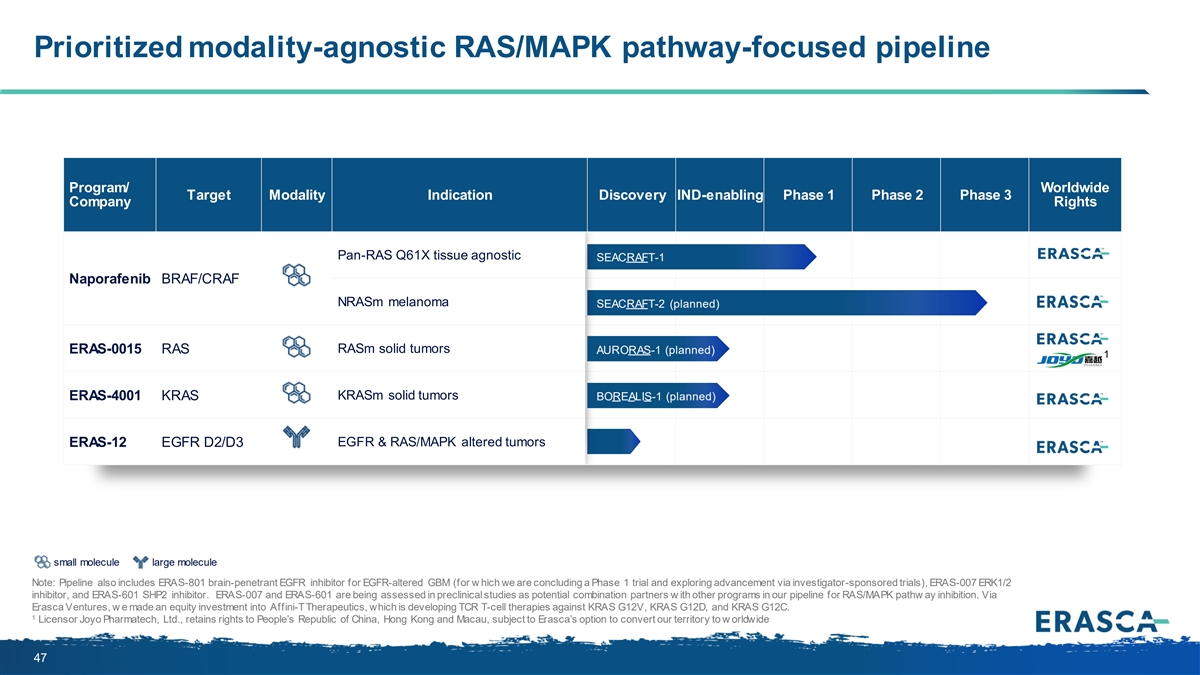
Prioritized modality-agnostic RAS/MAPK pathway-focused pipeline Program/ Worldwide Target Modality Indication Discovery IND-enabling Phase 1 Phase 2 Phase 3 Company Rights Pan-RAS Q61X tissue agnostic SEACRAFT-1 Naporafenib BRAF/CRAF NRASm melanoma SEACRAFT-2 (planned) ERAS-0015 RAS RASm solid tumors AURORAS-1 (planned) 1 ERAS-4001 KRAS KRASm solid tumors BOREALIS-1 (planned) EGFR & RAS/MAPK altered tumors ERAS-12 EGFR D2/D3 small molecule large molecule Note: Pipeline also includes ERAS-801 brain-penetrant EGFR inhibitor for EGFR-altered GBM (for w hich we are concluding a Phase 1 trial and exploring advancement via investigator-sponsored trials), ERAS-007 ERK1/2 inhibitor, and ERAS-601 SHP2 inhibitor. ERAS-007 and ERAS-601 are being assessed in preclinical studies as potential combination partners w ith other programs in our pipeline for RAS/MAPK pathw ay inhibition. Via Erasca Ventures, w e made an equity investment into Affini-T Therapeutics, which is developing TCR T-cell therapies against KRAS G12V, KRAS G12D, and KRAS G12C. 1 Licensor Joyo Pharmatech, Ltd., retains rights to People’s Republic of China, Hong Kong and Macau, subject to Erasca’s option to convert our territory to w orldwide 47
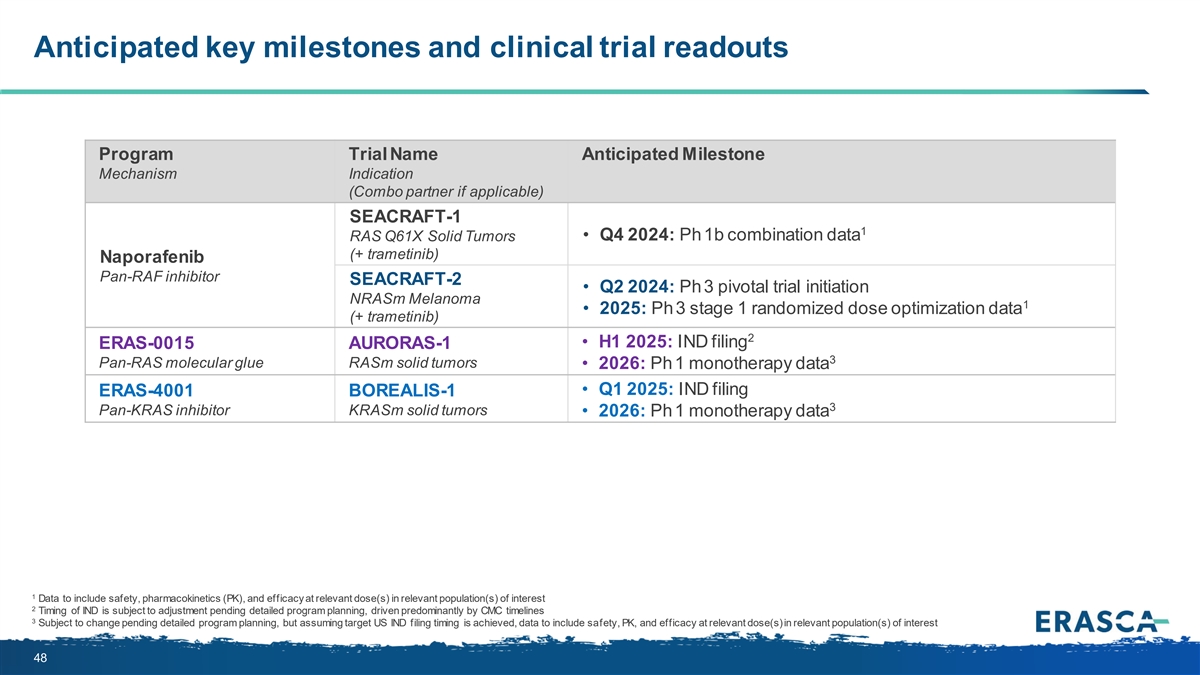
Anticipated key milestones and clinical trial readouts Program Trial Name Anticipated Milestone Mechanism Indication (Combo partner if applicable) SEACRAFT-1 1 RAS Q61X Solid Tumors • Q4 2024: Ph 1b combination data (+ trametinib) Naporafenib Pan-RAF inhibitor SEACRAFT-2 • Q2 2024: Ph 3 pivotal trial initiation NRASm Melanoma 1 • 2025: Ph 3 stage 1 randomized dose optimization data (+ trametinib) 2 • H1 2025: IND filing ERAS-0015 AURORAS-1 3 Pan-RAS molecular glue RASm solid tumors • 2026: Ph 1 monotherapy data • Q1 2025: IND filing ERAS-4001 BOREALIS-1 3 Pan-KRAS inhibitor KRASm solid tumors • 2026: Ph 1 monotherapy data 1 Data to include safety, pharmacokinetics (PK), and efficacy at relevant dose(s) in relevant population(s) of interest 2 Timing of IND is subject to adjustment pending detailed program planning, driven predominantly by CMC timelines 3 Subject to change pending detailed program planning, but assuming target US IND filing timing is achieved, data to include safety, PK, and efficacy at relevant dose(s) in relevant population(s) of interest 48

Thank You!
Document and Entity Information |
May 14, 2024 |
|---|---|
| Cover [Abstract] | |
| Amendment Flag | false |
| Entity Central Index Key | 0001761918 |
| Document Type | 8-K |
| Document Period End Date | May 14, 2024 |
| Entity Registrant Name | Erasca, Inc. |
| Entity Incorporation State Country Code | DE |
| Entity File Number | 001-40602 |
| Entity Tax Identification Number | 83-1217027 |
| Entity Address, Address Line One | 3115 Merryfield Row |
| Entity Address, Address Line Two | Suite 300 |
| Entity Address, City or Town | San Diego |
| Entity Address, State or Province | CA |
| Entity Address, Postal Zip Code | 92121 |
| City Area Code | (858) |
| Local Phone Number | 465-6511 |
| Written Communications | false |
| Soliciting Material | false |
| Pre Commencement Tender Offer | false |
| Pre Commencement Issuer Tender Offer | false |
| Security 12b Title | Common Stock, $0.0001 par value per share |
| Trading Symbol | ERAS |
| Security Exchange Name | NASDAQ |
| Entity Emerging Growth Company | true |
| Entity Ex Transition Period | false |
1 Year Erasca Chart |
1 Month Erasca Chart |

It looks like you are not logged in. Click the button below to log in and keep track of your recent history.
Support: +44 (0) 203 8794 460 | support@advfn.com
By accessing the services available at ADVFN you are agreeing to be bound by ADVFN's Terms & Conditions