
We could not find any results for:
Make sure your spelling is correct or try broadening your search.
| Name | Symbol | Market | Type |
|---|---|---|---|
| ASLAN Pharmaceuticals Ltd | NASDAQ:ASLN | NASDAQ | Depository Receipt |
| Price Change | % Change | Price | Bid Price | Offer Price | High Price | Low Price | Open Price | Traded | Last Trade | |
|---|---|---|---|---|---|---|---|---|---|---|
| 0.00 | 0.00% | 0.60 | 0.589 | 0.5891 | 0 | 00:00:00 |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 6-K/A
REPORT OF FOREIGN ISSUER
PURSUANT TO RULE 13a-16 OR 15d-16
OF THE SECURITIES EXCHANGE ACT OF 1934
July 6, 2023 (as amended on August 18, 2023)
(Commission File No. 001-38475)
ASLAN PHARMACEUTICALS LIMITED
(REG. NO. 289175)
(Translation of registrant’s name into English)
CAYMAN ISLANDS
(Jurisdiction of incorporation or organization)
3 Temasek Avenue
Level 18 Centennial Tower
Singapore 039190
(Address of registrant’s principal executive office)
Indicate by check mark whether the registrant files or will file annual reports under cover Form 20-F or Form 40-F.
Form 20-F ☒ Form 40-F ☐
Indicate by check mark if the registrant is submitting the Form 6-K in paper as permitted by Regulation S-T Rule 101 (b) (1):
Yes ☐ No ☒
Indicate by check mark if the registrant is submitting the Form 6-K in paper as permitted by Regulation S-T Rule 101 (b) (7):
Yes ☐ No ☒
Explanatory Note:
As previously reported in a Current Report on Form 6-K (the “Original 6-K”) filed on July 6, 2023 (the “Disclosure Date”), ASLAN Pharmaceuticals Limited (the “Company”) reported positive topline data from its Phase 2b dose-ranging study of eblasakimab in adult patients with moderate-to-severe atopic dermatitis, the TREK-AD study.
After reporting the topline data, the Company recently discovered a statistical programming error which resulted in two of its secondary endpoints being incorrectly calculated. Specifically, the corrections relate only to the scores previously disclosed for EASI-75 and EASI-90 across the four treatment arms and the placebo arm. The incorrect scores were reported in (i) the Original 6-K, (ii) the Company’s related press release announcing the data (the “Press Release”) and (iii) the investor presentation used in the Company’s webcast on the Disclosure Date and posted on the Company’s website (the “Investor Presentation”). The corrected results are set forth in Table 1 below.
Following this verification, the Company affirms that all other information disclosed in the Original 6-K, Press Release and Investor Presentation remain unchanged, including the percentage change from baseline in EASI score (primary endpoint), vIGA-AD scores and safety data.
The Company is filing this Amendment to Form 6-K to update the Original 6-K and to furnish the corrected Press Release, included as Exhibit 99.1 to this report and incorporated herein by reference, and the corrected Investor Presentation, included as Exhibit 99.2 to this report and incorporated herein by reference.
Table 1.
| Eblasakimab 600mg Q4W (n=59) |
Eblasakimab 400mg Q2W (n=56) |
Eblasakimab 300mg Q2W (n=58) |
Eblasakimab 400mg Q4W (n=59) |
Placebo (n=57) |
||||||||||||||||
| % of patients who achieved at least 75% improvement in EASI score from baseline (EASI-75) after 16 weeks of treatment |
|
52.0 (p=0.0040 |
) |
|
43.6 (p=0.0360 |
) |
|
51.2 (p=0.0050 |
) |
|
37.2 (p=0.1583 |
) |
24.4 | |||||||
| % of patients who achieved at least 90% improvement in EASI score from baseline (EASI-90) after 16 weeks of treatment |
|
27.6 (p=0.0080 |
) |
|
25.3 (p=0.0177 |
) |
|
30.8 (p=0.0028 |
) |
|
17.2 (p=0.1513 |
) |
7.9 | |||||||
The corrections referenced above do not change the key conclusions from the TREK-AD study. The positive data from the TREK-AD study (inclusive of the corrections referenced above) continue to establish eblasakimab, a potential first-in-class antibody, as the first biologic in moderate-to-severe atopic dermatitis to demonstrate a competitive efficacy profile with once-monthly dosing from initiation and support its advancement to Phase 3 development.
Restated 6-K:
Announcement of Positive Topline Data from Phase 2b Study of eblasakimab in Moderate-to-Severe Atopic Dermatitis
On July 6, 2023, ASLAN Pharmaceuticals Limited (the “Company”) announced positive topline data from its Phase 2b dose-ranging study of eblasakimab in adult patients with moderate-to-severe atopic dermatitis (AD), the TREK-AD (TRials with EblasaKimab in Atopic Dermatitis) study.
Eblasakimab met the primary endpoint of percent change from baseline in the Eczema Area and Severity Index (EASI) score at week 16 versus placebo, with statistical significance in three of the four dosing arms: 600mg dosed once every 4 weeks (600mg Q4W), which was numerically the best performing arm, 400mg dosed once every 2 weeks (400mg Q2W) and 300mg dosed once every 2 weeks (300mg Q2W).
Eblasakimab is the first biologic in moderate-to-severe AD to demonstrate competitive efficacy data with once-monthly dosing from initiation comparable to regimens dosing once every two weeks, supporting advancement to Phase 3.
600mg eblasakimab dosed once every four weeks in the TREK-AD study met the primary endpoint with statistical significance, with 52.0% of patients achieving EASI-75, 27.6% reaching EASI-90 and 31.2% achieving validated Investigator Global Assessment of Atopic Dermatitis (vIGA-AD) of 0 or 1.
Regimens dosing once every two weeks also met the primary endpoint with statistical significance, as well as meeting key secondary endpoints.
Eblasakimab’s unique loading dose regimen was observed to deliver rapid onset of action with statistically significant improvement in EASI score by week 4.
Eblasakimab was generally well-tolerated at all dose levels, with low rates of conjunctivitis and injection site reactions supporting the potential for a differentiated safety profile.
Key study results are set out below:
| • | Patients treated with eblasakimab 600mg Q4W, 400mg Q2W and 300mg Q2W saw a rapid onset of action in the first few weeks of treatment, with a statistically significant improvement in EASI score by week 4. Clinically meaningful improvements were achieved in other key efficacy measures compared to placebo (n=57) after 16 weeks of treatment, including: |
| • | Eblasakimab 600mg Q4W (n=59) |
| • | 52.0% of eblasakimab treated patients achieved a reduction of at least 75% from baseline (EASI-75), compared to 24.4% on placebo (p=0.0040). |
| • | 27.6% of eblasakimab treated patients achieved a reduction of at least 90% from baseline (EASI-90), compared to 7.9% on placebo (p=0.0080). |
| • | 31.2% of eblasakimab-treated patients achieved vIGA-AD score of 0 or 1 (clear or nearly clear skin), compared to 15.1% with placebo (p=0.0502). |
| • | 73.0% least squares (LS) mean reduction in EASI score from baseline, compared to 51.1% on placebo (p=0.0010). |
| • | Eblasakimab 400mg Q2W (n=56) |
| • | 43.6% of eblasakimab treated patients achieved EASI-75, compared to 24.4% on placebo (p=0.0360). |
| • | 25.3% of eblasakimab treated patients achieved EASI-90, compared to 7.9% on placebo (p=0.0177). |
| • | 32.6% of eblasakimab-treated patients achieved vIGA-AD score of 0 or 1, compared to 15.1% with placebo (p=0.0380). |
| • | 65.8% LS mean reduction in EASI score from baseline, compared to 51.1% on placebo (p=0.0294). |
| • | Eblasakimab 300mg Q2W (n=58) |
| • | 51.2% of eblasakimab treated patients achieved EASI-75, compared to 24.4% on placebo (p=0.0050). |
| • | 30.8% of eblasakimab treated patients achieved EASI-90, compared to 7.9% on placebo (p=0.0028). |
| • | 33.1% of eblasakimab-treated patients achieved vIGA-AD score of 0 or 1, compared to 15.1% with placebo (p=0.0327). |
| • | 69.8% LS mean reduction in EASI score from baseline, compared to 51.1% on placebo (p=0.0050). |
| • | Eblasakimab 400mg Q4W (n=59) |
The eblasakimab 400mg Q4W dosing arm (n=59) did not meet the primary or secondary endpoints with statistical significance.
| • | 37.2% of eblasakimab treated patients achieved EASI-75, compared to 24.4% on placebo (p=0.1583). |
| • | 17.2% of eblasakimab treated patients achieved EASI-90, compared to 7.9% on placebo (p=0.1513). |
| • | 15.0% of eblasakimab-treated patients achieved vIGA-AD score of 0 or 1, compared to 15.1% with placebo (p=0.7457). |
| • | 61.9% LS mean reduction in EASI score from baseline, compared to 51.1% on placebo (p=0.1053). |
| • | Overall, discontinuation rates were comparable between the treatment arms and higher in the placebo arm. No new safety signals were seen, and the frequency of adverse events (AEs) was comparable between treatment and placebo arms. The most frequently observed AEs across all treatment arms were nasopharyngitis (13.4% across all treatment arms compared to 8.8% for placebo), atopic dermatitis (8.6% compared to 7.0% for placebo), headache (6.9% compared to 7.0% for placebo) and upper respiratory tract infection (6.5% compared to 5.3% for placebo). Rates of conjunctivitis (5.6% compared to 1.8% for placebo), injection site reactions (4.7% compared to 1.8% for placebo) and herpes infections (3.4% compared to 5.3% for placebo) were low. |
Further information is set out in the press release (as corrected) attached hereto as Exhibit 99.1 and which is incorporated by reference herein.
A copy of the investor presentation (as corrected) used in the Company’s webcast on July 6, 2023 and posted on the Company’s website is attached hereto as Exhibit 99.2 and is incorporated by reference herein. Exhibit 99.2 is being furnished and shall not be deemed “filed” for the purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference into any filing made by the Company under the Securities Act of 1933, as amended, or the Exchange Act.
The information contained in this Form 6-K (except Exhibit 99.2) is hereby incorporated by reference into the Company’s Registration Statement on Form F-3 (File No. 333-252575), Registration Statement on Form F-3 (File No. 333-254768), Registration Statement on Form F-3 (File No. 333-270835), Registration Statement on Form F-3 (File No. 333-270837), Registration Statement on Form S-8 (File No. 333-252118), Registration Statement on Form S-8 (File No. 333-263843) and Registration Statement on Form S-8 (File No 333-270832).
Forward Looking Statements
This release contains forward-looking statements. These statements are based on the current beliefs and expectations of the management of the Company. These forward-looking statements may include, but are not limited to, statements regarding the Company’s business strategy and clinical development plans; the Company’s plans to develop and commercialize eblasakimab; the safety and efficacy of eblasakimab; the Company’s plans and expected timing with respect to clinical trials, clinical trial enrolment and clinical trial results for eblasakimab; and the potential for eblasakimab as a first-in-class treatment for AD. The Company’s estimates, projections and other forward-looking statements are based on management’s current assumptions and expectations of future events and trends, which affect or may affect the Company’s business, strategy, operations or financial performance, and inherently involve significant known and unknown risks and uncertainties. Actual results and the timing of events could differ materially from those anticipated in such forward-looking statements as a result of these risks and uncertainties, which include, without limitation, unexpected safety or efficacy data observed during preclinical or clinical studies; the fact that results of earlier studies and trials may not be predictive of future trial results; clinical site activation rates or clinical trial enrolment rates that are lower than expected; the impact of the COVID-19 pandemic or the ongoing conflict between Ukraine and Russia and bank failures on the Company’s business and the global economy; general market conditions; changes in the competitive landscape; and the Company’s ability to obtain sufficient financing to fund its strategic and clinical development plans. Other factors that may cause actual results to differ from those expressed or implied in such forward-looking statements are described in the Company’s Securities and Exchange Commission (“SEC”) filings and reports (Commission File No. 001-38475), including the Company’s Annual Report on Form 20-F filed with the SEC on March 24, 2023. All statements other than statements of historical fact are forward-looking statements. The words “believe,” “may,” “might,” “could,” “will,” “aim,” “estimate,” “continue,” “anticipate,” “intend,” “expect,” “plan,” or the negative of those terms, and similar expressions that convey uncertainty of future events or outcomes are intended to identify estimates, projections and other forward-looking statements. Estimates, projections and other forward-looking statements speak only as of the date they were made, and, except to the extent required by law, the Company undertakes no obligation to update or review any estimate, projection or forward-looking statement.
Exhibits
| Exhibit Number |
Exhibit Description | |
| 99.1 | Press release dated July 6, 2023 (Corrected) | |
| 99.2 | Investor Presentation dated July 6, 2023 (Corrected) | |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereto duly authorized.
| ASLAN PHARMACEUTICALS LIMITED (Registrant) | ||
| By: | /s/ Kiran Kumar Asarpota | |
| Name: | Kiran Kumar Asarpota | |
| Title: | Chief Operating Officer | |
Date: August 18, 2023
Exhibit 99.1

PRESS RELEASE
(restated August 18, 2023)
EBLASAKIMAB MONTHLY DOSING SHOWS POTENTIAL FOR BEST-IN-CLASS THERAPY IN POSITIVE PHASE 2B STUDY IN ATOPIC DERMATITIS
| • | Eblasakimab is the first biologic in moderate-to-severe atopic dermatitis to demonstrate a competitive efficacy profile with once-monthly dosing from initiation comparable to once every two weeks, supporting advancement to Phase 3. |
| • | 600mg eblasakimab dosed once every four weeks in the TREK-AD study met the primary endpoint with statistical significance, with 52.0% of patients achieving EASI-75, 27.6% reaching EASI-90 and 31.2% achieving vIGA-AD of 0 or 1. |
| • | Regimens dosing once every two weeks also met the primary endpoint with statistical significance, as well as meeting key secondary endpoints. |
| • | Unique loading dose regimen observed to deliver rapid onset of action with statistically significant improvement in EASI score by week 4. |
| • | Eblasakimab was generally well-tolerated at all dose levels, with low rates of conjunctivitis and injection site reactions supporting the potential for a differentiated safety profile. |
| • | Management to host webcast today, July 6, at 8:30 a.m. ET / 8:30 p.m. SGT. |
San Mateo, California, and Singapore, July 6, 2023 – ASLAN Pharmaceuticals (NASDAQ: ASLN), a clinical-stage, immunology-focused biopharmaceutical company developing innovative treatments to transform the lives of patients, today announced positive topline data from its Phase 2b dose-ranging study of eblasakimab in adult patients with moderate-to-severe atopic dermatitis (AD), the TREK-AD (TRials with EblasaKimab in Atopic Dermatitis) study. Eblasakimab met the primary endpoint of percent change from baseline in the Eczema Area and Severity Index (EASI) score at week 16 versus placebo with statistical significance in three dosing arms: 600mg dosed once every 4 weeks (600mg Q4W), which was numerically the best performing arm, 400mg dosed once every 2 weeks (400mg Q2W) and 300mg dosed once every 2 weeks (300mg Q2W).
Eblasakimab is a novel monoclonal antibody targeting the IL-13 receptor subunit of the Type 2 receptor, a key pathway driving several allergic inflammatory diseases. Eblasakimab’s unique mechanism of action has been observed to enable specific blockade of the Type 2 receptor, preventing signaling through both interleukin 4 (IL-4) and interleukin 13 (IL-13) – the key drivers of inflammation in AD, while sparing the Type 1 receptor.
“This is the first time we’ve seen a once-a-month treatment option deliver competitive efficacy data, which would be a game-changer for patients with AD,” said Eric L. Simpson, M.D., Frances J. Storrs Professor of Medical Dermatology at the Oregon Health and Science University and Lead Investigator in the TREK-AD study. “We haven’t seen much in the way of advancement since the launch of dupilumab, and there remains a huge unmet burden of disease experienced by patients. These results support eblasakimab’s potential to be a leading therapy for the treatment of AD, if approved.”
“We are delighted to announce these positive topline data from the TREK-AD study, which support recent translational studies we have published showing eblasakimab’s unique mechanism of action may provide a more efficient and targeted approach to blocking Type 2 signaling, which is responsible for allergic inflammation. Based on these results, we believe that eblasakimab could be a leading treatment in AD, if approved, getting more patients to EASI-75 or EASI-90, with fewer unwanted side effects, a rapid onset of action, and – uniquely – once-monthly dosing convenience,” said Dr Carl Firth, Chief Executive Officer of ASLAN Pharmaceuticals. “We look forward to advancing quickly into a Phase 3 program in AD, and exploring the broad range of indications where we would expect this drug candidate to be successful. We are grateful to the patients, investigators, and our team who have made such an important contribution to the development of eblasakimab.”

Key study results
In the TREK-AD Phase 2b study, 289 patients were randomized and treated in the intent-to-treat (ITT) population across five dosing arms in a 1:1:1:1:1 ratio to receive one of four doses of eblasakimab (300mg Q2W, 400mg Q2W, 400mg Q4W and 600mg Q4W) or placebo for 16 weeks. Key efficacy endpoints, including EASI-75 and a validated Investigator Global Assessment of Atopic Dermatitis (vIGA-AD) score of 0/1, the relevant endpoints for regulatory approval in Europe and the US respectively, are shown below:
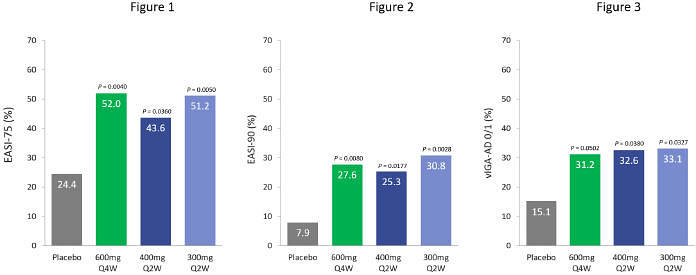
Patients treated with eblasakimab 600mg Q4W, 400mg Q2W and 300mg Q2W saw a rapid onset of action in the first few weeks of treatment, with a statistically significant improvement in EASI score by week 4. Clinically meaningful improvements were achieved in other key efficacy measures compared to placebo (n=57) after 16 weeks of treatment, including:
Eblasakimab 600mg Q4W (n=59)
| • | 52.0% of eblasakimab treated patients achieved a reduction of at least 75% from baseline (EASI-75), compared to 24.4% on placebo (p=0.0040). |
| • | 27.6% of eblasakimab treated patients achieved a reduction of at least 90% from baseline (EASI-90), compared to 7.9% on placebo (p=0.0080). |
| • | 31.2% of eblasakimab treated patients achieved vIGA-AD score of 0 or 1 (clear or nearly clear skin), compared to 15.1% with placebo (p=0.0502). |
| • | 73.0% least squares (LS) mean reduction in EASI score from baseline, compared to 51.1% on placebo (p=0.0010). |
Eblasakimab 400mg Q2W (n=56)
| • | 43.6% of eblasakimab treated patients achieved EASI-75, compared to 24.4% on placebo (p=0.0360). |
| • | 25.3% of eblasakimab treated patients achieved EASI-90, compared to 7.9% on placebo (p=0.0177). |

| • | 32.6% of eblasakimab treated patients achieved vIGA-AD score of 0 or 1, compared to 15.1% with placebo (p=0.0380). |
| • | 65.8% LS mean reduction in EASI score from baseline, compared to 51.1% on placebo (p=0.0294). |
Eblasakimab 300mg Q2W (n=58)
| • | 51.2% of eblasakimab treated patients achieved EASI-75, compared to 24.4% on placebo (p=0.0050). |
| • | 30.8% of eblasakimab treated patients achieved EASI-90, compared to 7.9% on placebo (p=0.0028). |
| • | 33.1% of eblasakimab treated patients achieved vIGA-AD score of 0 or 1, compared to 15.1% with placebo (p=0.0327). |
| • | 69.8% LS mean reduction in EASI score from baseline, compared to 51.1% on placebo (p=0.0050). |
Eblasakimab 400mg Q4W (n=59)
The eblasakimab 400mg Q4W dosing arm did not meet the primary or secondary endpoints with statistical significance.
| • | 37.2% of eblasakimab treated patients achieved EASI-75, compared to 24.4% on placebo (p=0.1583). |
| • | 17.2% of eblasakimab treated patients achieved EASI-90, compared to 7.9% on placebo (p=0.1513). |
| • | 15.0% of eblasakimab treated patients achieved vIGA-AD score of 0 or 1, compared to 15.1% with placebo (p=0.7457). |
| • | 61.9% LS mean reduction in EASI score from baseline, compared to 51.1% on placebo (p=0.1054). |
Overall, discontinuation rates were comparable between the active treatment arms and higher in the placebo arm. No new safety signals were seen and the frequency of adverse events (AEs) was comparable between active treatment and placebo arms. The most frequently observed AEs across all active treatment arms were nasopharyngitis (13.4% compared to 8.8% for placebo), atopic dermatitis (8.6% compared to 7.0% for placebo), headache (6.9% compared to 7.0% for placebo) and upper respiratory tract infection (6.5% compared to 5.3% for placebo). Rates of conjunctivitis (5.2% compared to 1.8% for placebo), injection site reactions (4.7% compared to 1.8% for placebo) and herpes infections (3.0% compared to 3.5% for placebo) were low.
Further data from the Phase 2b study, including patient reported outcomes and biomarker data, is expected to be available in Q4 2023 and submitted for presentation at a future scientific congress. ASLAN plans to meet with the US Food and Drug Administration for an end-of-Phase 2 meeting and expects to initiate a Phase 3 clinical development program for eblasakimab in 2024. The Company is also conducting the TREK-DX (TRials in EblasaKimab in Dupilumab eXperienced AD patients) study of eblasakimab in dupilumab-experienced patients and expects to announce topline data from that study in 1Q 2024.
About the Phase 2b TREK-AD study
The TREK-AD (TRials with EblasaKimab in Atopic Dermatitis) study is a randomized, double-blind, placebo-controlled, dose-ranging clinical trial, designed to evaluate the efficacy and safety of eblasakimab as monotherapy in biologic-naïve adult patients with moderate-to-severe AD who are candidates for systemic therapy. Patients from the US, Europe and Asia were randomized in equal ratios to four active treatment arms and one placebo arm:
| • | Arm 1: Received a loading dose of 600mg of eblasakimab at weeks 0 and 1, followed by 300mg eblasakimab dosed every two weeks. |
| • | Arm 2: Received a loading dose of 600mg of eblasakimab at weeks 0 and 1, followed by 400mg eblasakimab dosed every two weeks. |
| • | Arm 3: Received a loading dose of 600mg of eblasakimab at weeks 0, 1 and 2, followed by 400mg eblasakimab dosed every four weeks. |

| • | Arm 4: Received a loading dose of 600mg of eblasakimab at weeks 0, 1 and 2, followed by 600mg eblasakimab dosed every four weeks. |
| • | Arm 5: Placebo dosed at weeks 0 and 1 and every two weeks thereafter. |
The inclusion criteria for patients enrolled in the study included a clinical diagnosis of AD for at least one year, an EASI score of greater than or equal to 16, a vIGA-AD score of greater than or equal to 3 and a body surface area of AD involving at least 10% at screening and baseline. Following the end of the 16-week assessment period, patients were followed for an additional 12 weeks.
About eblasakimab
Eblasakimab is a potential first-in-class monoclonal antibody targeting the IL-13 receptor subunit of the Type 2 receptor, a key pathway driving several allergic inflammatory diseases. Eblasakimab’s unique mechanism of action enables specific blockade of the Type 2 receptor and has the potential to improve upon current biologics used to treat allergic disease. By blocking the Type 2 receptor, eblasakimab prevents signaling through both interleukin 4 (IL-4) and interleukin 13 (IL-13) – the key drivers of inflammation in AD.
Webcast
ASLAN’s management will host a webcast at 8:30 a.m. ET today, July 6, 2023, to discuss these data. The live webcast may be accessed by registering using this link: https://lifescievents.com/event/aslan/.
About ASLAN Pharmaceuticals
ASLAN Pharmaceuticals (Nasdaq: ASLN) is a clinical-stage, immunology-focused biopharmaceutical company developing innovative treatments to transform the lives of patients. ASLAN is developing eblasakimab, a potential first-in-class antibody targeting the IL-13 receptor in moderate-to-severe atopic dermatitis (AD) with the potential to improve upon current biologics used to treat allergic disease, and has recently reported positive topline data from a Phase 2b dose ranging study in moderate-to-severe AD. ASLAN is also developing farudodstat, a potent oral inhibitor of the enzyme dihydroorotate dehydrogenase (DHODH) as a potential first-in-class treatment for alopecia areata (AA) in a Phase 2a proof-of-concept trial with an interim readout expected in 1Q 2024. ASLAN has teams in San Mateo, California, and in Singapore. For additional information please visit the website or follow ASLAN on LinkedIn.

Forward-looking statements
This release contains forward-looking statements. These statements are based on the current beliefs and expectations of the management of ASLAN Pharmaceuticals Limited and/or its affiliates (the “Company”). These forward-looking statements may include, but are not limited to statements regarding the Company’s business strategy and clinical development plans; the Company’s plans to develop and commercialize eblasakimab; the safety and efficacy of eblasakimab, including its potential to be best-in-class; the Company’s plans and expected timing with respect to clinical trials, clinical trial enrolment and clinical trial results for eblasakimab; the potential of eblasakimab as a treatment for atopic dermatitis. The Company’s estimates, projections and other forward-looking statements are based on management’s current assumptions and expectations of future events and trends, which affect or may affect the Company’s business, strategy, operations, or financial performance, and inherently involve significant known and unknown risks and uncertainties. Actual results and the timing of events could differ materially from those anticipated in such forward-looking statements as a result of many risks and uncertainties, which include, without limitation, unexpected safety or efficacy data observed during preclinical or clinical studies; the fact that results of earlier studies and trials may not be predictive of future trial results; clinical site activation rates or clinical trial enrolment rates that are lower than expected; the impact of the COVID-19 pandemic or the ongoing conflict between Ukraine and Russia and bank failures on the Company’s business and the global economy; general market conditions; changes in the competitive landscape; and the Company’s ability to obtain sufficient financing to fund its strategic and clinical development plans. Other factors that may cause actual results to differ from those expressed or implied in such forward-looking statements are described in the Company’s US Securities and Exchange Commission filings and reports (Commission File No. 001- 38475), including the Company’s Annual Report on Form 20-F filed with the US Securities and Exchange Commission on March 24, 2023. All statements other than statements of historical fact are forward-looking statements. The words “believe,” “may,” “might,” “could,” “will,” “aim,” “estimate,” “continue,” “anticipate,” “intend,” “expect,” “plan,” or the negative of those terms, and similar expressions that convey uncertainty of future events or outcomes are intended to identify estimates, projections, and other forward-looking statements. Estimates, projections, and other forward-looking statements speak only as of the date they were made, and, except to the extent required by law, the Company undertakes no obligation to update or review any estimate, projection, or forward-looking statement.
Ends
Media and IR contacts
| Emma Thompson Spurwing Communications Tel: +65 6206 7350 Email: ASLAN@spurwingcomms.com |
Ashley R. Robinson LifeSci Advisors, LLC Tel: +1 (617) 430-7577 Email: arr@lifesciadvisors.com |
Exhibit 99.2

Eblasakimab Phase 2b TREK-AD Topline readout
6 July 2023
Restated 18 August 2023
NASDAQ: ASLN
Eblasakimab Phase 2b TREK-AD Topline readout
6 July 2023
Restated 18 August 2023
NASDAQ: ASLN

Legal disclaimer
This
presentation contains forward-looking statements. These statements are based on the current beliefs and expectations of the management of ASLAN Pharmaceuticals Limited (the “Company”). These forward-looking statements may include, but are
not limited to, statements regarding the
Company’s business strategy, the Company’s plans to develop and commercialize its product candidates, the safety
and efficacy of the Company’s product candidates, including their potential to be best-in-class, the Company’s plans and expected timing with respect to clinical trials, clinical trial enrolment and clinical trial results for its product
candidates, the Company’s plans and expected timing with respect to regulatory filings and approvals, the size and growth potential of the markets for the Company’s product candidates, and the potential for eblasakimab as a treatment for
atopic dermatitis. The Company’s estimates, projections and other forward-looking statements are based on management’s current assumptions and expectations of future events and trends, which affect or may affect the Company’s
business, strategy, operations or financial performance, and inherently involve significant known and unknown risks and uncertainties, which include, unexpected safety or efficacy data observed during preclinical or clinical studies; the fact that
results of earlier studies and trials may not be predictive of future trial results; clinical site activation rates or clinical trial enrolment rates that are lower than expected; the impact of the COVID-19 pandemic on the Company’s business
and the global economy; general market conditions; changes in the competitive landscape; and the Company’s ability to obtain sufficient financing to fund its strategic and clinical development plans. Actual results and the timing of events
could differ materially from those anticipated in such forward-looking statements as a result of these risks and uncertainties, which include, without limitation the risk factors described in the Company’s US Securities and Exchange Commission
filings and reports (Commission File No. 001-38475), including the Company’s Form 20-F filed with the U.S. Securities and Exchange Commission (the “SEC”) on March 24, 2023. This presentation discusses product candidates that are under
clinical study, and which have not yet been approved for marketing by the US Food and Drug Administration. No representation is made as to the safety or effectiveness of these product candidates for the use for which such product candidates are
being studied. Caution should be exercised when comparing data across trials of different products and product candidates. Differences existing between trial designs and patient populations and characteristics. The results across such trials may not
have interpretative value on our existing or future results. All statements other than statements of historical fact are forward-looking statements. The words “believe,” “view,” “may,” “might,”
“could,” “will,” “aim,” “estimate,” “continue,” “anticipate,” “intend,” “expect,” “plan,” or the negative of those terms, and similar expressions that convey
uncertainty of future events or outcomes are intended to identify estimates, projections and other forward-looking statements. Estimates, projections and other forward-looking statements speak only as of the date they were made, and, except to the
extent required by law, the Company undertakes no obligation to update or review any estimate, projection or forward-looking statement. 2
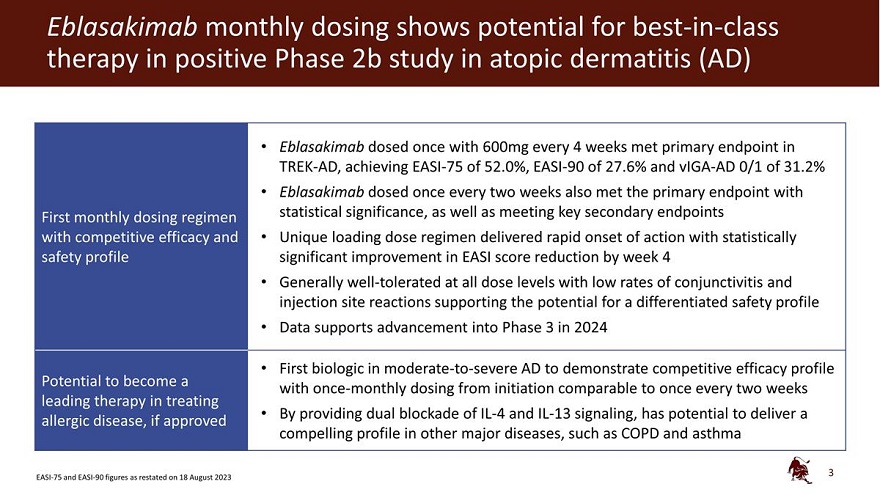
Eblasakimab monthly dosing shows potential for best-in-class therapy in positive Phase 2b study in atopic
dermatitis (AD)
First monthly dosing regimen with competitive efficacy and safety profile
Potential to become a leading therapy in treating allergic disease, if approved
Eblasakimab
dosed once with 600mg every 4 weeks met primary endpoint in TREK-AD, achieving EASI-75 of 52.0%, EASI-90 of 27.6% and vIGA-AD 0/1 of 31.2% Eblasakimab dosed once every two weeks also met the primary endpoint with statistical significance, as well as
meeting key secondary endpoints Unique loading dose regimen delivered rapid onset of action with statistically significant improvement in EASI score reduction by week 4 Generally well-tolerated at all dose levels with low rates of conjunctivitis and
injection site reactions supporting the potential for a differentiated safety profile Data supports advancement into Phase 3 in 2024
First biologic in
moderate-to-severe AD to demonstrate competitive efficacy profile with once-monthly dosing from initiation comparable to once every two weeks By providing dual blockade of IL-4 and IL-13 signaling, has potential to deliver a compelling profile in
other major diseases, such as COPD and asthma
3
EASI-75 and EASI-90 figures
as restated on 18 August 2023

Significant unmet needs exist despite existing and soon-to-be-approved therapies
Attributes related to
Efficacy
Safety
Dosing and Convenience
Treating comorbidities
Desirable
Dupilumab Tralokinumab Lebrikizumab1 Oral JAKi attributes
Efficacy profile comparable or
better than dupilumab2 Rapid onset of disease improvement3 ? Complete inhibition of Type 2 receptor without affecting Type 1 receptor4 Proven to block sensitization of human itch neurons to IL-13 and IL-45 No boxed warning or monitoring6 × Low
rates of conjunctivitis and Type 1 driven effects7 Convenient dosing: monthly injection from start of treatment or oral8 Potential for flexible dosing9 Stable at room temperature (no refrigeration required)10 ?
Effective in other atopic diseases11
1. Lebrikizumab is a candidate drug and not approved for
AD
2. Based on EASI-75 and IGA endpoints from monotherapy phase 3 studies
3.
For approved drugs, whether the label claims any form of fast or rapid effects on disease severity (EASI-75 or IGA). Approved label claim for candidate drugs not yet known.
4. Based on published preclinical and mechanistic data.
5. Based on published preclinical
data.
6. For approved drugs, based on label. For candidate drugs, based on monitoring requirements and reports of drug-related SAEs in phase 3
7. For approved drugs, based on label showing monotherapy conjunctivitis rate less than 5%. For candidate drugs, based on monotherapy phase 3 showing conjuncitivitis rates below 5%
8. For approved drugs, based on approved dosing regimens. For candidate drugs, based on regimens tested in phase 3 program at initiation of treatment (after
loading doses)
9. For approved drugs, based on adult dosing schedule that can be adjusted according to response, safety or other patient characteristics. For
candidate drugs, based on adjustments in dosing tested during monotherapy phase 3 10. For approved drugs, based on storage recommendations in label for periods of one month or longer. Storage requirements for candidate drugs not yet known.
11. Based on clinical trials that have delivered positive data.
For
illustrative purposes only. Not a head-to-head comparison. Caution should be exercised when comparing data across trials of different products and product candidates. Differences exist between trial designs and patient populations and
characteristics. The results across such trials may not have interpretative value on our existing or future results.
4
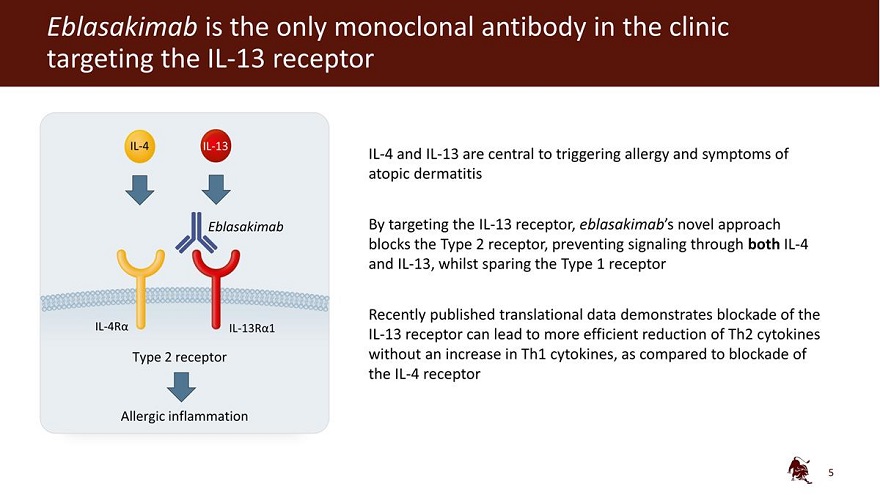
Eblasakimab is the only monoclonal antibody in the clinic targeting the IL-13 receptor IL-4 IL-13 Eblasakimab IL-4R 13R 1 Type 2 receptor Allergic inflammation IL-4 and IL-13 are central to triggering allergy and symptoms of atopic dermatitis By targeting the IL-13 receptor, eblasakimab’s novel approach blocks the Type 2 receptor, preventing signaling through both IL-4 and IL-13, whilst sparing the Type 1 receptor Recently published translational data demonstrates blockade of the IL-13 receptor can lead to more efficient reduction of Th2 cytokines without an increase in Th1 cytokines, as compared to blockade of the IL-4 receptor 5
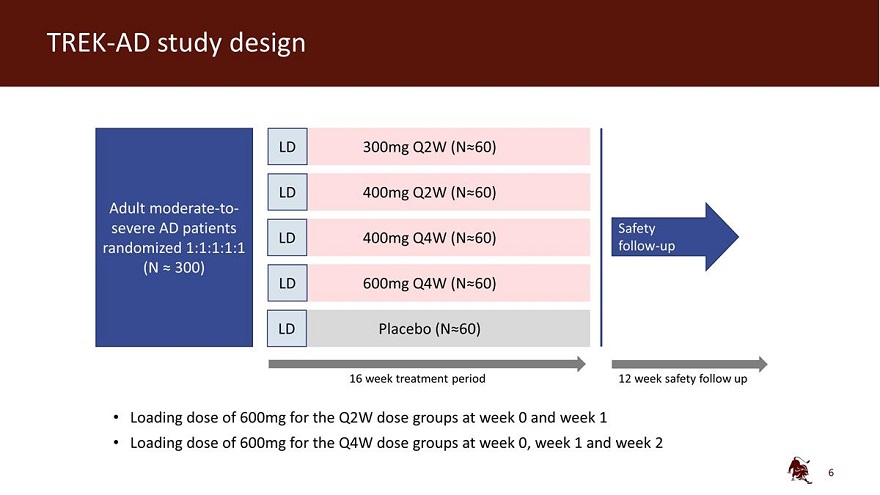
TREK-AD study design LD 300mg Q2W (N 60) LD 400mg Q2W (N 60) Adult moderate-to-severe AD patients Safety LD 400mg Q4W (N 60) randomized 1:1:1:1:1 follow-up (N 300) LD 600mg Q4W (N 60) LD Placebo (N 60) 16 week treatment period 12 week safety follow up Loading dose of 600mg for the Q2W dose groups at week 0 and week 1 Loading dose of 600mg for the Q4W dose groups at week 0, week 1 and week 2 6
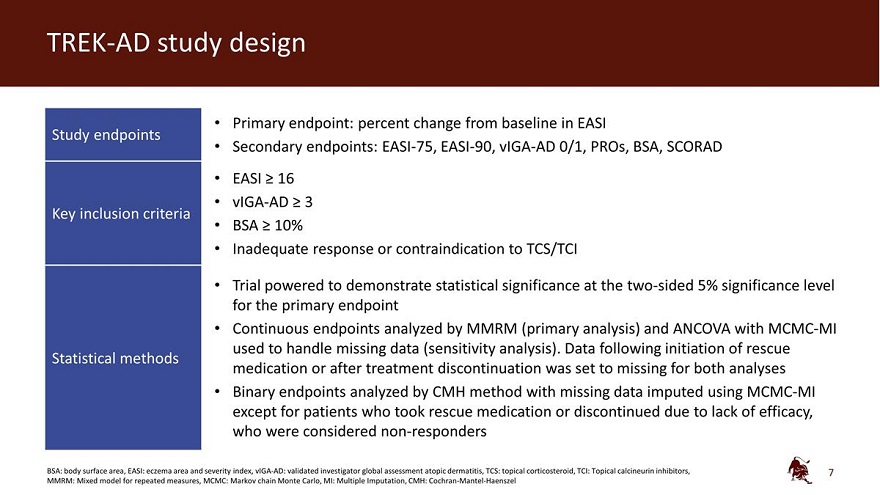
TREK-AD study design • Primary endpoint: percent change from baseline in EASI Study endpoints • Secondary endpoints: EASI-75, EASI-90, vIGA-AD 0/1, PROs, BSA, SCORAD EASI 16 vIGA-AD 3 Key inclusion criteria BSA 10% Inadequate response or contraindication to TCS/TCI Trial powered to demonstrate statistical significance at the two-sided 5% significance level for the primary endpoint Continuous endpoints analyzed by MMRM (primary analysis) and ANCOVA with MCMC-MI used to handle missing data (sensitivity analysis). Data following initiation of rescue Statistical methods medication or after treatment discontinuation was set to missing for both analyses Binary endpoints analyzed by CMH method with missing data imputed using MCMC-MI except for patients who took rescue medication or discontinued due to lack of efficacy, who were considered non-responders BSA: body surface area, EASI: eczema area and severity index, vIGA-AD: validated investigator global assessment atopic dermatitis, TCS: topical corticosteroid, TCI: Topical calcineurin inhibitors, MMRM: Mixed model for repeated measures, MCMC: Markov chain Monte Carlo, MI: Multiple Imputation, CMH: Cochran-Mantel-Haenszel 7
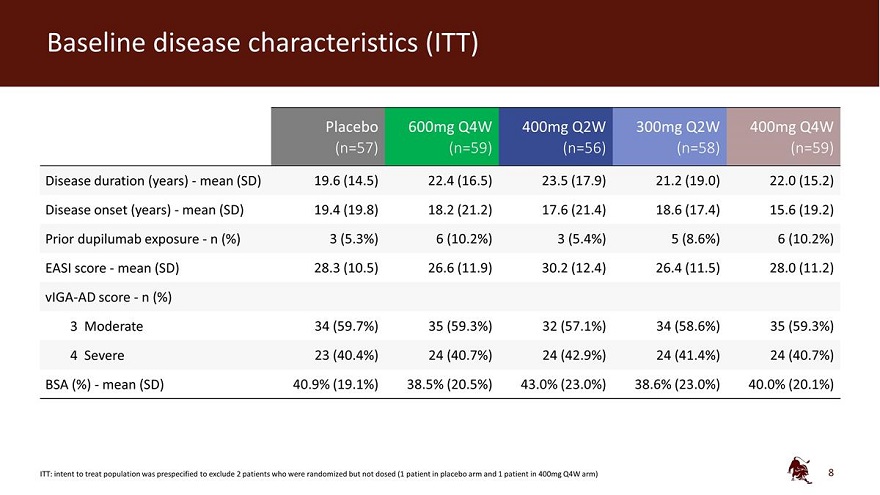
Baseline disease characteristics (ITT) Placebo 600mg Q4W 400mg Q2W 300mg Q2W 400mg Q4W (n=57) (n=59) (n=56) (n=58) (n=59) Disease duration (years) - mean (SD) 19.6 (14.5) 22.4 (16.5) 23.5 (17.9) 21.2 (19.0) 22.0 (15.2) Disease onset (years) - mean (SD) 19.4 (19.8) 18.2 (21.2) 17.6 (21.4) 18.6 (17.4) 15.6 (19.2) Prior dupilumab exposure - n (%) 3 (5.3%) 6 (10.2%) 3 (5.4%) 5 (8.6%) 6 (10.2%) EASI score - mean (SD) 28.3 (10.5) 26.6 (11.9) 30.2 (12.4) 26.4 (11.5) 28.0 (11.2) vIGA-AD score - n (%) 3 Moderate 34 (59.7%) 35 (59.3%) 32 (57.1%) 34 (58.6%) 35 (59.3%) 4 Severe 23 (40.4%) 24 (40.7%) 24 (42.9%) 24 (41.4%) 24 (40.7%) BSA (%) - mean (SD) 40.9% (19.1%) 38.5% (20.5%) 43.0% (23.0%) 38.6% (23.0%) 40.0% (20.1%) ITT: intent to treat population was prespecified to exclude 2 patients who were randomized but not dosed (1 patient in placebo arm and 1 patient in 400mg Q4W arm) 8
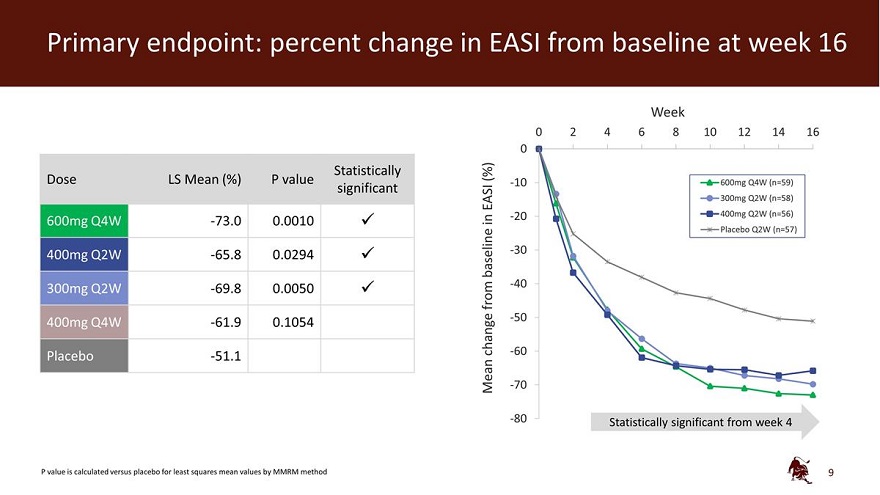
Primary endpoint: percent change in EASI from baseline at week 16 Week 0 2 4 6 8 10 12 14 16 0 Statistically (%) Dose LS Mean (%) P value -10 600mg Q4W (n=59) significant EASI 300mg Q2W (n=58) in -20 400mg Q2W (n=56) 600mg Q4W -73.0 0.0010 Placebo Q2W (n=57) 400mg Q2W -65.8 0.0294 baseline -30 -40 300mg Q2W -69.8 0.0050 from -50 400mg Q4W -61.9 0.1054 -change -60 Placebo -51.1 - Mean -70 -80 Statistically significant from week 4 P value is calculated versus placebo for least squares mean values by MMRM method 9
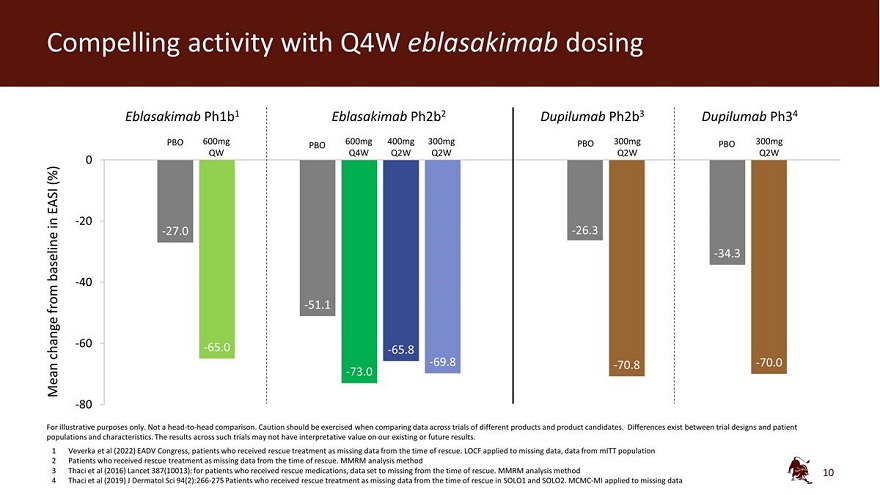
Compelling activity with Q4W eblasakimab dosing Eblasakimab Ph1b1 Eblasakimab Ph2b2 Dupilumab Ph2b3 Dupilumab Ph34 PBO 600mg 600mg 400mg 300mg PBO 300mg PBO 300mg QW PBO Q4W Q2W Q2W Q2W Q2W 0 (%) EASI in -20 -27.0 -26.3 baseline -34.3 -40 from -51.1 -60 -65.0 change -65.8 -69.8 -70.8 -70.0 Mean -73.0 -80 For illustrative purposes only. Not a head-to-head comparison. Caution should be exercised when comparing data across trials of different products and product candidates. Differences exist between trial designs and patient populations and characteristics. The results across such trials may not have interpretative value on our existing or future results. 1 Veverka et al (2022) EADV Congress, patients who received rescue treatment as missing data from the time of rescue. LOCF applied to missing data, data from mITT population 2 Patients who received rescue treatment as missing data from the time of rescue. MMRM analysis method 3 Thaci et al (2016) Lancet 387(10013): for patients who received rescue medications, data set to missing from the time of rescue. MMRM analysis method 4 Thaci et al (2019) J Dermatol Sci 94(2):266-275 Patients who received rescue treatment as missing data from the time of rescue in SOLO1 and SOLO2. MCMC-MI applied to missing data 10
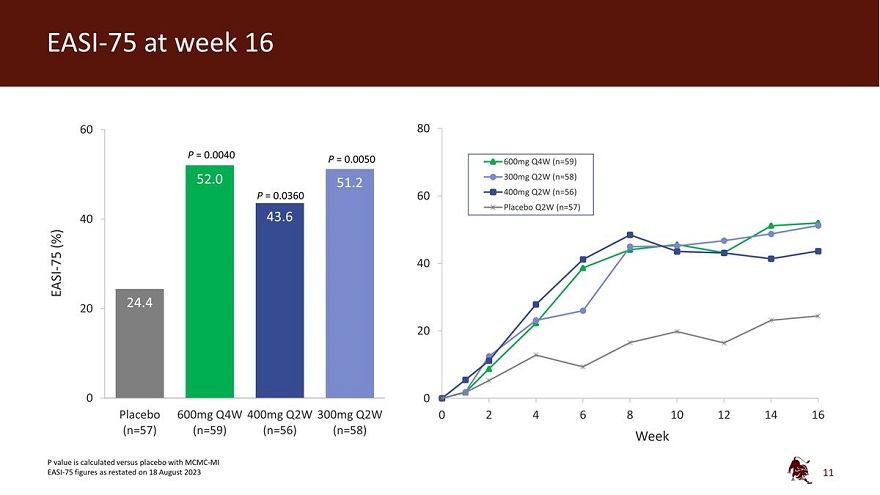
60 80 P = 0.0040 P = 0.0050 600mg Q4W (n=59) 300mg Q2W (n=58) P = 0.0360 60 400mg Q2W (n=56) Placebo Q2W (n=57) 40 (%) - 75 40 EASI 20 20 0 0 Placebo 600mg Q4W 400mg Q2W 300mg Q2W 0 2 4 6 8 10 12 14 16 (n=57) (n=59) (n=56) (n=58) Week P value is calculated versus placebo with MCMC-MI EASI-75 figures as restated on 18 August 2023 11
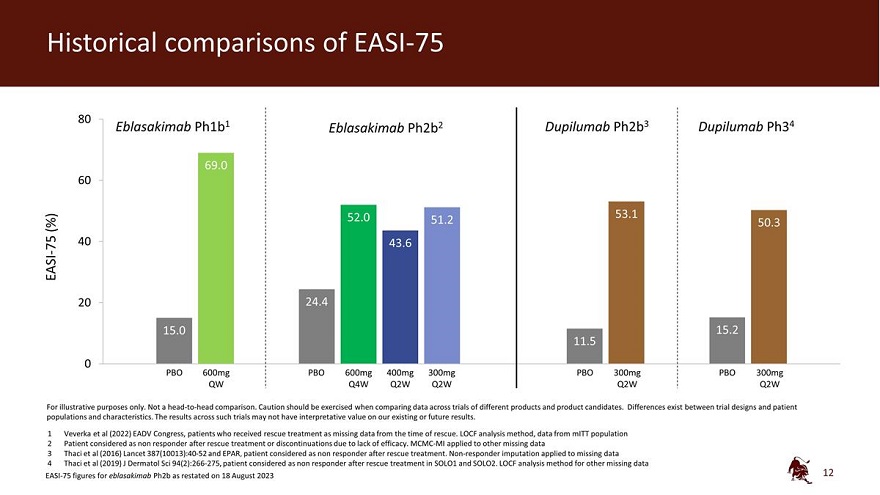
Historical comparisons of EASI-75 80 Eblasakimab Ph1b1 Eblasakimab Ph2b2 Dupilumab Ph2b3 Dupilumab Ph34 69.0 60 52.0 53.1 (%) 51.2 50.3 40 75 43.6 EASI - 20 24.4 15.0 15.2 11.5 0 PBO 600mg PBO 600mg 400mg 300mg PBO 300mg PBO 300mg QW Q4W Q2W Q2W Q2W Q2W For illustrative purposes only. Not a head-to-head comparison. Caution should be exercised when comparing data across trials of different products and product candidates. Differences exist between trial designs and patient populations and characteristics. The results across such trials may not have interpretative value on our existing or future results. 1 Veverka et al (2022) EADV Congress, patients who received rescue treatment as missing data from the time of rescue. LOCF analysis method, data from mITT population 2 Patient considered as non responder after rescue treatment or discontinuations due to lack of efficacy. MCMC-MI applied to other missing data 3 Thaci et al (2016) Lancet 387(10013):40-52 and EPAR, patient considered as non responder after rescue treatment. Non-responder imputation applied to missing data 4 Thaci et al (2019) J Dermatol Sci 94(2):266-275, patient considered as non responder after rescue treatment in SOLO1 and SOLO2. LOCF analysis method for other missing data EASI-75 figures for eblasakimab Ph2b as restated on 18 August 2023 12
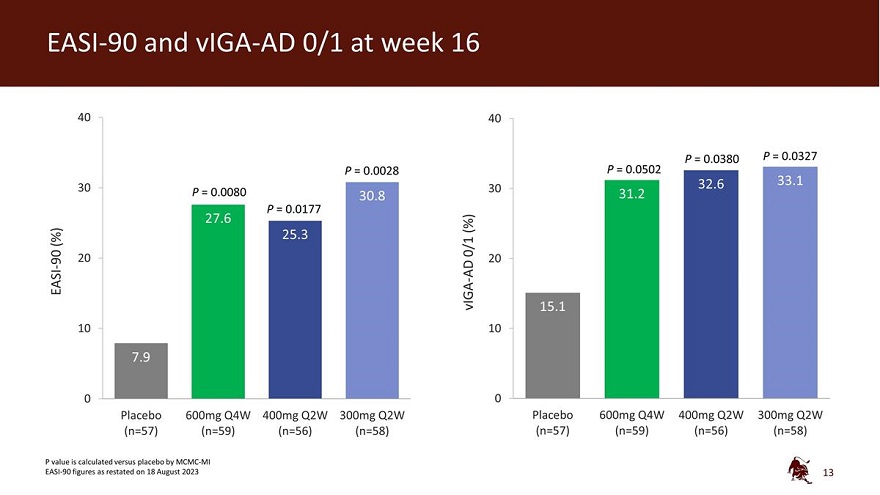
EASI-90 and vIGA-AD 0/1 at week 16 40 40 P = 0.0380 P = 0.0327 P = 0.0028 P = 0.0502 32.6 33.1 30 P = 0.0080 30 30.8 31.2 P = 0.0177 27.6 (%) (%) 25.3 0/1 90 20 20 - AD EASI vIGA - 15.1 10 10 7.9 0 0 Placebo 600mg Q4W 400mg Q2W 300mg Q2W Placebo 600mg Q4W 400mg Q2W 300mg Q2W (n=57) (n=59) (n=56) (n=58) (n=57) (n=59) (n=56) (n=58) P value is calculated versus placebo by MCMC-MI EASI-90 figures as restated on 18 August 202313

Historical comparisons of EASI-90 Eblasakimab Ph1b1 Eblasakimab Ph2b2 Dupilumab Ph2b3 Dupilumab Ph34 40 38.0 30 32.8 30.8 29.7 (%) 27.6 90 25.3 EASI - 20 15.0 10 7.9 7.4 3.3 0 PBO 600mg PBO 600mg 400mg 300mg PBO 300mg PBO 300mg QW Q4W Q2W Q2W Q2W Q2W For illustrative purposes only. Not a head-to-head comparison. Caution should be exercised when comparing data across trials of different products and product candidates. Differences exist between trial designs and patient populations and characteristics. The results across such trials may not have interpretative value on our existing or future results. 1 Veverka et al (2022) EADV Congress, patients who received rescue treatment as missing data from the time of rescue. LOCF analysis method, data from mITT population 2 Patient considered as non responder after rescue treatment or discontinuations due to lack of efficacy. MCMC-MI applied to other missing data 3 Thaci et al (2016) Lancet 387(10013):40-52 and EPAR, patient considered as non responder after rescue treatment. Non-responder imputation applied to missing data. 4 Thaci et al (2019) J Dermatol Sci 94(2):266-275, non-responder imputation: patients after rescue treatment or withdrawal from the study were considered as non responders in SOLO1 and SOLO2 EASI-90 figures for eblasakimab Ph2b as restated on 18 August 2023 14
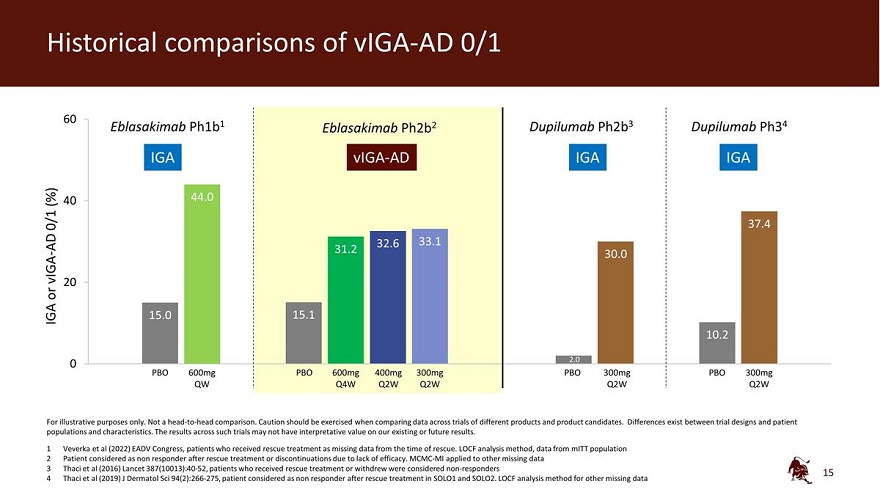
Historical comparisons of vIGA-AD 0/1 60 Eblasakimab Ph1b1 Eblasakimab Ph2b2 Dupilumab Ph2b3 Dupilumab Ph34 IGA vIGA-AD IGA IGA (%) 40 44.0 0/1 37.4 AD 32.6 33.1 31.2 vIGA - 30.0 20 or IGA 15.0 15.1 10.2 0 2.0 PBO 600mg PBO 600mg 400mg 300mg PBO 300mg PBO 300mg QW Q4W Q2W Q2W Q2W Q2W For illustrative purposes only. Not a head-to-head comparison. Caution should be exercised when comparing data across trials of different products and product candidates. Differences exist between trial designs and patient populations and characteristics. The results across such trials may not have interpretative value on our existing or future results. 1 Veverka et al (2022) EADV Congress, patients who received rescue treatment as missing data from the time of rescue. LOCF analysis method, data from mITT population 2 Patient considered as non responder after rescue treatment or discontinuations due to lack of efficacy. MCMC-MI applied to other missing data 3 Thaci et al (2016) Lancet 387(10013):40-52, patients who received rescue treatment or withdrew were considered non-responders 4 Thaci et al (2019) J Dermatol Sci 94(2):266-275, patient considered as non responder after rescue treatment in SOLO1 and SOLO2. LOCF analysis method for other missing data 15
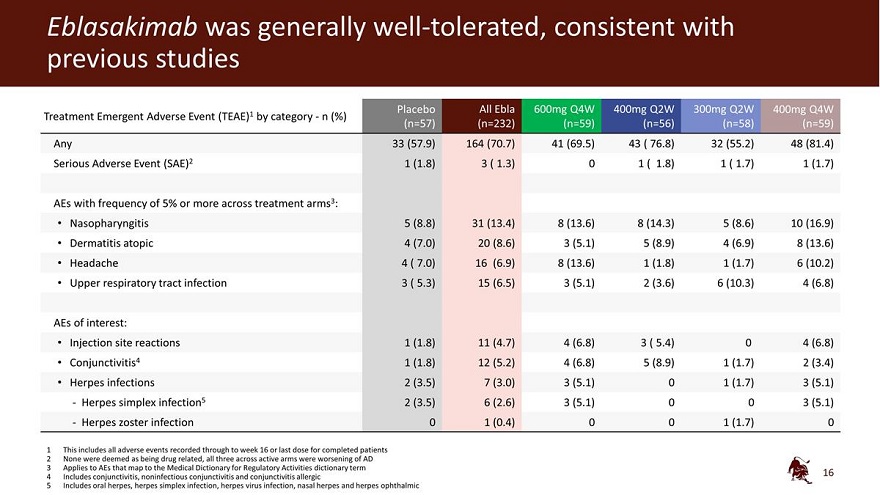
Eblasakimab was generally well-tolerated, consistent with previous studies Placebo All Ebla 600mg Q4W 400mg Q2W 300mg Q2W 400mg Q4W Treatment Emergent Adverse Event (TEAE)1 by category - n (%) (n=57) (n=232) (n=59) (n=56) (n=58) (n=59) Any 33 (57.9) 164 (70.7) 41 (69.5) 43 ( 76.8) 32 (55.2) 48 (81.4) Serious Adverse Event (SAE)2 1 (1.8) 3 ( 1.3) 0 1 ( 1.8) 1 ( 1.7) 1 (1.7) AEs with frequency of 5% or more across treatment arms3: Nasopharyngitis 5 (8.8) 31 (13.4) 8 (13.6) 8 (14.3) 5 (8.6) 10 (16.9) Dermatitis atopic 4 (7.0) 20 (8.6) 3 (5.1) 5 (8.9) 4 (6.9) 8 (13.6) Headache 4 ( 7.0) 16 (6.9) 8 (13.6) 1 (1.8) 1 (1.7) 6 (10.2) Upper respiratory tract infection 3 ( 5.3) 15 (6.5) 3 (5.1) 2 (3.6) 6 (10.3) 4 (6.8) AEs of interest: Injection site reactions 1 (1.8) 11 (4.7) 4 (6.8) 3 ( 5.4) 0 4 (6.8) Conjunctivitis4 1 (1.8) 12 (5.2) 4 (6.8) 5 (8.9) 1 (1.7) 2 (3.4) Herpes infections 2 (3.5) 7 (3.0) 3 (5.1) 0 1 (1.7) 3 (5.1) - Herpes simplex infection5 2 (3.5) 6 (2.6) 3 (5.1) 0 0 3 (5.1) - Herpes zoster infection 0 1 (0.4) 0 0 1 (1.7) 0 1 This includes all adverse events recorded through to week 16 or last dose for completed patients 2 None were deemed as being drug related, all three across active arms were worsening of AD 3 Applies to AEs that map to the Medical Dictionary for Regulatory Activities dictionary term 4 Includes conjunctivitis, noninfectious conjunctivitis and conjunctivitis allergic 5 Includes oral herpes, herpes simplex infection, herpes virus infection, nasal herpes and herpes ophthalmic 16
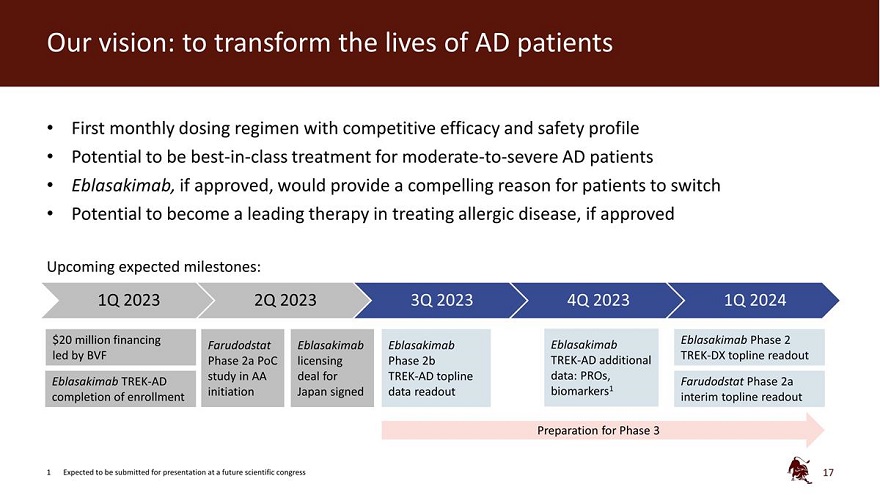
Our vision: to transform the lives of AD patients First monthly dosing regimen with competitive efficacy and safety profile Potential to be best-in-class treatment for moderate-to-severe AD patients Eblasakimab, if approved, would provide a compelling reason for patients to switch Potential to become a leading therapy in treating allergic disease, if approved Upcoming expected milestones: 1Q 2023 2Q 2023 3Q 2023 4Q 2023 1Q 2024 $20 million financing Eblasakimab Phase 2 Farudodstat Eblasakimab Eblasakimab Eblasakimab led by BVF TREK-DX topline readout Phase 2a PoC licensing Phase 2b TREK-AD additional study in AA deal for TREK-AD topline data: PROs, Eblasakimab TREK-AD Farudodstat Phase 2a completion of enrollment initiation Japan signed data readout biomarkers1 interim topline readout Preparation for Phase 3 1 Expected to be submitted for presentation at a future scientific congress 17

Q&A 18
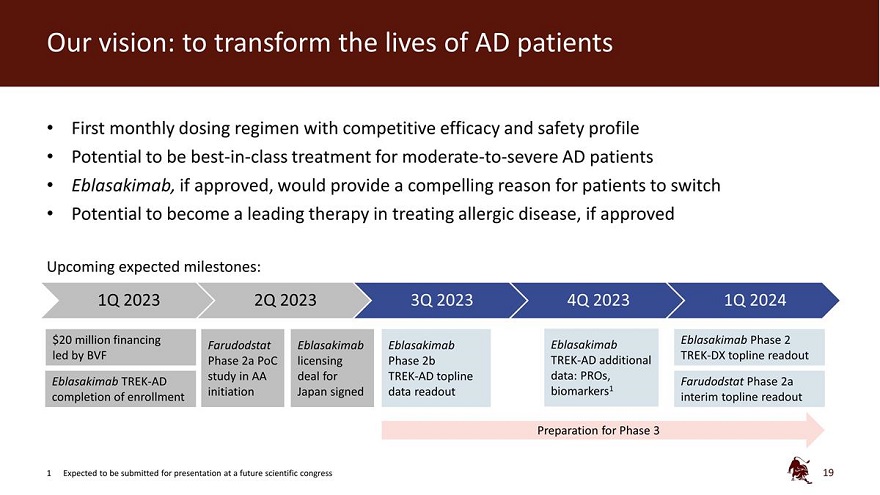
Our vision: to transform the lives of AD patients First monthly dosing regimen with competitive efficacy and safety profile Potential to be best-in-class treatment for moderate-to-severe AD patients Eblasakimab, if approved, would provide a compelling reason for patients to switch Potential to become a leading therapy in treating allergic disease, if approved Upcoming expected milestones: 1Q 2023 2Q 2023 3Q 2023 4Q 2023 1Q 2024 $20 million financing Eblasakimab Phase 2 Farudodstat Eblasakimab Eblasakimab Eblasakimab led by BVF TREK-DX topline readout Phase 2a PoC licensing Phase 2b TREK-AD additional study in AA deal for TREK-AD topline data: PROs, Eblasakimab TREK-AD Farudodstat Phase 2a completion of enrollment initiation Japan signed data readout biomarkers1 interim topline readout Preparation for Phase 3 1 Expected to be submitted for presentation at a future scientific congress 19

Eblasakimab Phase 2b TREK-AD Topline readout 6 July 2023 Restated 18 August 2023 NASDAQ: ASLN ASLAN PHARMACEUTICALS
1 Year ASLAN Pharmaceuticals Chart |
1 Month ASLAN Pharmaceuticals Chart |

It looks like you are not logged in. Click the button below to log in and keep track of your recent history.
Support: +44 (0) 203 8794 460 | support@advfn.com
By accessing the services available at ADVFN you are agreeing to be bound by ADVFN's Terms & Conditions