
We could not find any results for:
Make sure your spelling is correct or try broadening your search.
| Share Name | Share Symbol | Market | Type |
|---|---|---|---|
| Autonomix Medical Inc | NASDAQ:AMIX | NASDAQ | Common Stock |
| Price Change | % Change | Share Price | Bid Price | Offer Price | High Price | Low Price | Open Price | Shares Traded | Last Trade | |
|---|---|---|---|---|---|---|---|---|---|---|
| 0.07 | 2.06% | 3.46 | 3.41 | 3.51 | 3.6144 | 3.30 | 3.32 | 75,083 | 00:59:27 |
As filed with the Securities and Exchange Commission on November 1, 2024.
Registration No. 333-___
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM S-1
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
AUTONOMIX MEDICAL, INC.
(Exact Name of Registrant as Specified in Its Charter)
|
Delaware |
3841 |
47-1607810 |
|
(State or Other Jurisdiction of Incorporation or Organization) |
(Primary Standard Industrial Classification Code Number) |
(I.R.S. Employer Identification Number) |
21 Waterway Avenue, Suite 300
The Woodlands, Texas 77380
(713) 588-6150
(Address, Including Zip Code, and Telephone Number, Including Area Code, of Registrant’s Principal Executive Offices)
Brad Hauser
Chief Executive Officer
21 Waterway Avenue, Suite 300
The Woodlands, Texas 77380
(713) 588-6150
(Name, Address, Including Zip Code, and Telephone Number, Including Area Code, of Agent For Service)
Copies to:
|
Cavas S. Pavri Johnathan Duncan ArentFox Schiff LLP 1717 K Street NW Washington, DC 20006 Telephone: (202) 724-6847 Fax: (202) 778-6460 |
Leslie Marlow, Esq. Patrick Egan, Esq. Blank Rome LLP 1271 Avenue of the Americas New York, NY 10020 Phone: (212) 885-5000 Fax: (212) 885-5001 |
Approximate date of commencement of proposed sale to the public: As soon as practicable after the effective date of this Registration Statement.
If any of the securities being registered on this form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, check the following box. ☒
If this form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company” and “emerging growth company” in Rule 12b-2 of the Exchange Act:
|
Large accelerated filer |
☐ |
Accelerated filer |
☐ |
|
Non-accelerated filer |
☒ |
Smaller reporting company |
☒ |
|
Emerging growth company |
☒ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 7(a)(2)(B) of the Securities Act. ☐
The Registrant hereby amends this Registration Statement on such date or dates as may be necessary to delay its effective date until the Registrant shall file a further amendment which specifically states that this Registration Statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933 or until the Registration Statement shall become effective on such date as the Commission, acting pursuant to said Section 8(a), may determine.
The information in this preliminary prospectus is not complete and may be changed. These securities may not be sold until the registration statement filed with the Securities and Exchange Commission is effective. This preliminary prospectus is not an offer to sell these securities and we are not soliciting offers to buy these securities in any jurisdiction where the offer or sale is not permitted.
|
Preliminary Prospectus |
Subject to Completion |
Dated November 1, 2024 |

Up to 698,812 Common Stock Units
Each Common Stock Unit Consisting of One Share of Common Stock and One Series A Warrant to Purchase One Share of Common Stock
Up to 698,812 Shares of Common Stock Issuable Upon Exercise of Series A Warrants
Up to 698,812 PFW Units
Each PFW Unit Consisting of One Pre-Funded Warrants to Purchase One Share of Common Stock and One Series A Warrant to Purchase One Share of Common Stock
Up to 698,812 Shares of Common Stock Issuable Upon Exercise of Pre-Funded Warrants
Up to 698,812 Shares of Common Stock Underlying the Series A Warrants
Representative Warrants to Purchase up to 41,928 Shares of Common Stock
Up to 41,928 Shares of Common Stock Issuable Upon Exercise of Representative Warrants
This is a firm commitment public offering of 698,812 of our common stock units (the “Common Stock Units”) of Autonomix Medical, Inc., a Delaware corporation (the "Company"), each Common Stock Unit consisting of: (i) one share of our common stock, par value $0.001 per share (the “Shares,” “common stock” or “Common Stock”), and (ii) a Series A warrant to purchase one share of our common stock (the “Series A Warrant” or the “Common Warrants”). The assumed public offering price for each Common Stock Unit is $14.31, based on the last reported sale price of our common stock as reported on the Nasdaq Capital Market (“Nasdaq”) on October 31, 2024. The Common Warrants will each have an assumed initial exercise price of $______ per share. The Common Warrants are exercisable immediately, subject to certain limitations described herein, and shall expire five years from the closing date of this offering. We are also offering the shares of our common stock that are issuable from time to time upon exercise of the Common Warrants.
We are also offering to each purchaser whose purchase of Common Stock Units in this offering would otherwise result in the purchaser, together with its affiliates and certain related parties, beneficially owning more than 4.99% (or, at the election of the purchaser, 9.99%) of our outstanding common stock immediately following the consummation of this offering, the opportunity to purchase, if the purchaser so chooses, up to 698,812 pre-funded warrant units (the “PFW Units” and together with the Common Stock Units, the “Units”), in lieu of Common Stock Units that would otherwise result in the purchaser’s beneficial ownership exceeding 4.99% (or, at the election of the purchaser, 9.99%) of our outstanding common stock. Each PFW Unit consists of: (i) one pre-funded warrant to purchase one share of our common stock (the “Pre-Funded Warrants”), and (ii) a Series A Warrant to purchase one share of our common stock. The Series A Warrants included in the PFW Units are identical to the Series A Warrants included in the Common Stock Units. Subject to limited exceptions, a holder of Pre-Funded Warrants will not have the right to exercise any portion of its Pre-Funded Warrants if the holder, together with its affiliates, would beneficially own in excess of 4.99% (or, at the election of the holder, 9.99%) of the number of shares of common stock outstanding immediately after giving effect to such exercise. Each Pre-Funded Warrant will be exercisable for one share of common stock at an exercise price of $0.001 per share of common stock. The public offering price per PFW Unit is equal to the public offering price per Common Stock Unit less $0.001. Each Pre-Funded Warrant will be exercisable upon issuance and will expire when exercised in full. We are also offering the shares of our common stock that are issuable from time to time upon exercise of the Pre-Funded Warrants and the Common Warrants issued with respect to them. For each PFW Unit we sell, the number of Common Stock Units we are offering will be decreased on a one-for-one basis.
Because one Series A Warrant is being sold together in this offering with each share of Common Stock included in the Common Stock Unit and, in the alternative, each PFW Unit, the number of Series A Warrants sold in this offering will not change as a result of a change in the mix of the Common Stock Units and PFW Units sold. The Shares of common stock in the Common Stock Units or the Pre-Funded Warrants in the PFW Units, as applicable, and the accompanying Series A Warrants, can only be purchased together in this offering but will be issued separately and will be immediately separable upon issuance.
Neither the Common Stock Units nor the PFW Units will be issued or certificated. The Common Stock Units, the PFW Units, the Shares of common stock, the Series A Warrants, the Pre-Funded Warrants and shares of common stock underlying the Series A Warrants and Pre-Funded Warrants offered hereby are sometimes collectively referred to herein as the “securities.”
Our common stock is listed on Nasdaq under the symbol “AMIX.” On October 31, 2024, the last reported sale price of our common stock on Nasdaq was $14.31 per share. There is no established public trading market for the Units, the Series A Warrants or the Pre-Funded Warrants, and we do not expect a market to develop. We do not intend to list the Units, the Series A Warrants or the Pre-Funded Warrants on Nasdaq or any other national securities exchange or automated quotation system. Without an active trading market, the liquidity of the Units, Common Warrants and Pre-Funded Warrants may be limited. The assumed public offering price used throughout this prospectus has been included for illustration purposes only. The actual offering price may differ materially from the assumed price used in the prospectus and will be determined by negotiations between us and the underwriters and may not be indicative of prices of the actual offering price.
We are an “emerging growth company” as defined in Section 2(a) of the Securities Act of 1933, as amended (the “Securities Act”) and we have elected to comply with certain reduced public company reporting requirements.
Investing in our securities involves a high degree of risk. See the section entitled “Risk Factors” beginning on page 7 of this prospectus for a discussion of risks that should be considered in connection with an investment in our securities.
Neither the Securities and Exchange Commission nor any other regulatory body has approved or disapproved of these securities or passed upon the accuracy or adequacy of this prospectus. Any representation to the contrary is a criminal offense.
|
Per Common Stock Unit |
Per PFW Unit |
Total |
||||||||||
|
Public offering price |
$ | $ | $ | |||||||||
|
Underwriting discounts and commissions(1) |
$ | $ | $ | |||||||||
|
Proceeds, before expenses, to us (2) |
$ | $ | $ | |||||||||
|
(1) |
Represents underwriting discounts equal to 8% per Common Stock Unit or PFW Unit, as applicable. |
|
(2) |
We have agreed to reimburse the underwriters for certain expenses and issue the representative of the underwriters warrants the (the "Representative Warrants”) to purchase up to 6% of the aggregate number of the Shares and the shares of common stock underlying the Pre-Funded Warrants sold in this offering inclusive of the over-allotment option, but excluding shares of Common Stock underlying the Series A Warrants, with an exercise price equal to 155% of the public offering price of the Common Stock Units sold in this offering. See “Underwriting” on page 80 for additional information regarding underwriting compensation. |
We have granted the underwriters an option for a period of up to 45 days from the date of this prospectus to purchase up to 104,821 additional shares of our common stock and/or Series A warrants to purchase up to an additional 104,822 shares of our common stock, or any combination thereof, as determined by the underwriters, at the assumed public offering price, less underwriting discounts and commissions, in each case solely to cover over-allotments, if any.
The underwriters expect to deliver the securities offered by this prospectus against payment on or about _________, 2024.
Ladenburg Thalmann
The date of this prospectus is _____________________, 2024
TABLE OF CONTENTS
|
Page |
|
|
ABOUT THIS PROSPECTUS |
ii |
|
PROSPECTUS SUMMARY |
1 |
|
RISK FACTORS |
8 |
|
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS |
29 |
|
USE OF PROCEEDS |
30 |
|
DIVIDEND POLICY |
30 |
|
DILUTION |
31 |
|
CAPITALIZATION |
32 |
|
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS |
33 |
|
BUSINESS |
41 |
|
MANAGEMENT |
63 |
|
EXECUTIVE AND DIRECTOR COMPENSATION |
66 |
|
CERTAIN RELATIONSHIPS AND RELATED PERSON TRANSACTIONS AND DIRECTOR INDEPENDENCE |
73 |
|
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS |
74 |
|
DESCRIPTION OF CAPITAL STOCK |
75 |
|
DESCRIPTION OF SECURITIES WE ARE OFFERING |
79 |
|
MATERIAL UNITED STATES FEDERAL INCOME TAX CONSIDERATIONS |
82 |
|
UNDERWRITING |
88 |
|
LEGAL MATTERS |
93 |
|
EXPERTS |
93 |
|
WHERE YOU CAN FIND MORE INFORMATION |
94 |
|
INDEX TO FINANCIAL STATEMENTS |
96 |
ABOUT THIS PROSPECTUS
This prospectus is part of a registration statement on Form S-1 that we filed with the United States Securities and Exchange Commission (“SEC”) to register the securities offered hereby under the Securities Act. We may also file a prospectus supplement or post-effective amendment to the registration statement of which this prospectus forms a part that may contain material information relating to these offerings. You should carefully read this prospectus before deciding to invest in our securities.
We have not, and the underwriters have not, authorized anyone to provide any information or to make any representations other than those contained in this prospectus or in any free writing prospectuses prepared by or on behalf of us or to which we have referred you. We take no responsibility for, and can provide no assurance as to the reliability of, any other information that others may give you. This prospectus is an offer to sell only the securities offered hereby, and only under circumstances and in jurisdictions where it is lawful to do so. The information contained in this prospectus or in any applicable free writing prospectus is current only as of its date, regardless of its time of delivery or any sale of our securities. Our business, financial condition, results of operations and prospects may have changed since that date.
For investors outside the United States: We have not, and the underwriters have not, done anything that would permit this offering or possession or distribution of this prospectus in any jurisdiction where action for that purpose is required, other than in the United States. Persons outside the United States who come into possession of this prospectus must inform themselves about, and observe any restrictions relating to, the offering of the securities and the distribution of this prospectus outside the United States.
This prospectus may contain references to trademarks belonging to other entities. Solely for convenience, trademarks and trade names referred to in this prospectus, including logos, artwork, and other visual displays, may appear without the ® or TM symbols. We do not intend our use or display of other companies’ trade names or trademarks to imply a relationship with, or endorsement or sponsorship of us by, any other company.
No dealer, salesperson or other person is authorized to give any information or to represent anything not contained in this prospectus. You must not rely on any unauthorized information or representations. This prospectus is an offer to sell only the securities offered hereby, but only under circumstances and in jurisdictions where it is lawful to do so. The information contained in this prospectus is current only as of its date.
This prospectus contains estimates and other statistical data made by independent parties and by us relating to market size and growth and other data about our industry. We obtained the industry and market data in this prospectus from our own research as well as from industry and general publications, surveys and studies conducted by third parties. This data involves a number of assumptions and limitations and contains projections and estimates of the future performance of the industries in which we operate that are subject to a high degree of uncertainty. We caution you not to give undue weight to such projections, assumptions and estimates.
PROSPECTUS SUMMARY
This summary highlights information contained elsewhere in this prospectus. This summary does not contain all of the information that you should consider before deciding to invest in our securities. You should read this entire prospectus carefully, including the “Risk Factors” section in this prospectus. References in this prospectus to “we”, “us”, “its”, “our” or the “Company” are to Autonomix Medical, Inc., as appropriate to the context.
Overview
We are a development stage medical device development company focused on advancing innovative technologies for sensing and treating disorders relating to the nervous system. Our first-in-class technology platform includes a catheter-based microchip-enabled sensing array that can detect and differentiate neural signals with a high degree of sensitivity as demonstrated in animal studies. We are initially developing our technology for patients with pancreatic cancer, a condition that can cause debilitating pain and needs a more effective solution. However, we believe our technology constitutes a platform with the potential to address dozens of indications in a range of areas including chronic pain management from all causes, hypertension, cardiovascular disease and a wide range of other nerve-related disorders.
We calculate sensitivity in units of minimum signal detection voltage in micro volts (uV) time area of the electrode (square millimeters). It is a combined measure that is related to the signal resolving power and spatial resolution of the system. For the BSC Orion, the nearest device on the market, the metrics are 10uV for signal detection levels, and roughly 0.4mm by 0.5mm for the electrode dimensions. For the Autonomix device, the metrics are <1uV for signal detection levels and roughly 0.02mm by 0.03mm for the electrode dimensions. The differences in these metrics result in a calculation of 3,000 times greater sensitivity for the Autonomix device. We believe, if we can recreate these results in clinical trials, this will enable a method of transvascular targeting, treating, and confirming treatment of diseases involving the nervous system throughout the body that is not currently available and may be capable of filling a wide range of unmet medical needs.
Our development efforts can be divided into to two sub parts: diagnostic and therapeutic, where diagnostic is focused on sensing and identifying neuronal activity that may be associated with a disorder with enough precision to enable targeted therapy with ablation. Our sensing catheter has already been developed sufficiently to demonstrate in animal models successful identification of a signal from a specific nerve bundle before ablation and confirmation of termination of that signal from the treated nerves after ablation. We are now in the process of improving the design of this catheter to meet the standards required for human use. In parallel with this effort, we are conducting a first-in-human demonstration of transvascular ablation to relieve pain associated with pancreatic cancer, with the intent to bring sensing and treatment together in a future pivotal clinical trial to enable the commercial launch of our technology. We are a development stage company, we have not completed any clinical trials and there is no guarantee that the results of any trials will produce positive results or that the results will support our claims.
We believe one of the most demanding aspects of our commercialization plan will be scaling up from our existing sensing prototype to a robust commercial version. Today, our sensing device is hand built and includes a combination of hand-crafted and 3D printed parts. We have not yet assembled or tested what will be the commercial version of our proposed device. Even if our proposed device is cleared for commercial use, there is no assurance that we will be able to successfully build such device on a commercial scale.
As of June 30, 2024, we had an accumulated deficit of $41.7 million, negative cash flows from operating activities of $1.9 million and working capital of $6.3 million, which raises substantial doubt about our ability to continue as a going concern. Further, we have incurred and expect to continue to incur significant costs in pursuit of our business plans. We cannot assure you that we will be successful in raising additional funds. These factors, among others, raise substantial doubt about our ability to continue as a going concern.
Recent Developments
Preliminary Second Quarter Results (Unaudited)
Based on information currently available, we estimate that as of September 30, 2024, cash and cash equivalents were approximately $5.2 million and cash used in operations for the second quarter ended September 30, 2024 was $1.6 million. Cash used in operations for the six months ended September 30, 2024 was $3.4 million.
Our estimate of our cash and cash equivalents as of September 30, 2024 and cash used in operations for the second quarter of 2024 are preliminary and actual results may differ from these estimates due to the completion of our closing procedures with respect to the three and six months ended September 30, 2024, final adjustments and other developments that may arise between now and the time the financial results for the three and six months ended September 30, 2024 are finalized. As such, these estimates should not be viewed as a substitute for our unaudited financial statements for the three and six months ended September 30, 2024, prepared in accordance with U.S. generally accepted accounting principles. Our expected results could change and are not necessarily indicative of the results to be achieved for three and six months ended September 30, 2024, or any future period. As a result of the foregoing considerations and the other limitations described herein, investors are cautioned not to place undue reliance on this preliminary financial information. We do not undertake any obligation to publicly update or revise these estimates, except as required by law.
Employment Agreements
On June 17, 2024, we entered into an employment agreement with Brad Hauser pursuant to which Mr. Hauser agreed to serve as our chief executive officer and president for an initial three-year period, which may be extended on a year-to-year basis. Mr. Hauser’s agreement provides for an initial annual base salary of $450,000 (subject to an annual review and increase at the discretion of our Compensation Committee) and a target annual bonus of 60% of his base salary. Pursuant to the agreement, Mr. Hauser was granted a ten-year option (the “Inducement Options”) to purchase 45,000 shares of common stock at an exercise price equal to the closing price of our common stock on the date of the employment agreement. The option vests in four equal annual installments (or 11,250 shares each installment) on each of the succeeding four anniversary dates of the execution of the employment agreement, provided Mr. Hauser is employed by us on each vesting date. In the event of a “change of control” or the termination of the agreement by us without “cause” or by Mr. Hauser for “good reason,” all of the unvested options shall immediately vest. The Inducement Options were granted outside of our 2023 Stock Plan as an inducement material to Mr. Hauser’s entering into employment with us in accordance with Nasdaq Stock Market Listing Rule 5635(c)(4). Commencing with the year ending March 31, 2025, Mr. Hauser will be eligible to receive annual option grants as determined by the Compensation Committee of the Board of Directors, based on criteria established by the Compensation Committee. The number of shares underlying the target annual option grant will be equal to $1,000,000 divided by the Black-Scholes value per share of our common stock on the date of grant.
On June 17, 2024, we entered into an employment agreement with Lori Bisson pursuant to which Ms. Bisson agreed to serve as our Executive Vice Chair and Strategic Adviser to the Chief Executive Officer (“Vice Chair”) for a two-year period. Ms. Bisson’s agreement provides for an initial annual base salary of $150,000 (subject to an annual review and increase at the discretion of our Compensation Committee) and a target annual bonus of 50% of her base salary. Pursuant to the agreement, Ms. Bisson continued to vest in the option grants issued to Ms. Bisson in her role as chief executive officer and president in accordance with the vesting schedule set out in her prior employment agreement. In the event of a “change of control” or the termination of the agreement by us without “cause” or by Ms. Bisson for “good reason,” all of the unvested options shall immediately vest. Ms. Bisson is entitled to any incentive compensation, including incentive compensation, for the fiscal year ended March 31, 2024 that has not been paid as of the date of the agreement. Commencing with the year ending March 31, 2025, Ms. Bisson will be eligible to receive annual option grants as determined by the Compensation Committee of the Board of Directors, based on criteria established by the Compensation Committee. Ms. Bisson agreed to waive any severance payments due to her in connection with the termination of the prior employment agreement that we entered into with her on June 30, 2023.
License Agreement
On July 10, 2024, we entered into a license agreement (the “Agreement”) with RF Innovations, Inc. (“RFI”), a privately held medical technology company, to license products utilizing RFI’s intellectual property related to its Apex 6 Radiofrequency Generator (the “Licensed Products”). The Apex 6 Generator is a United States Food and Drug Administration (“FDA”) cleared ablation technology designed to lesion neural tissue for pain management in the peripheral nervous system. Pursuant to the Agreement, RFI granted us a perpetual non-exclusive worldwide royalty free fully paid license related to the Licensed Products, provided that the license did not include the right to sell certain products to customers for the treatment of spine pain. In connection with the Agreement, we issued RFI 12,500 unregistered shares of our common stock as consideration for the license. The Agreement provides RFI the right to terminate the license if we breach any representation, warranty or covenant contained in the Agreement, subject to any relevant cure periods, or if we are subject to a bankruptcy or insolvency event.
Notice of failure to satisfy a continued listing rule or standard
On September 16, 2024, we a deficiency letter from the Listing Qualifications Department (the “Staff”) of the Nasdaq Stock Market (“Nasdaq”) notifying us that for the last 30 consecutive business days the closing bid price for our common stock had closed below the minimum $1.00 per share requirement for continued inclusion on the Nasdaq Capital Market pursuant to Nasdaq Listing Rule 5550(a)(2) (the “Bid Price Rule”). In accordance with Nasdaq Listing Rule 5810(c)(3)(A) (the “Compliance Period Rule”), we have been provided an initial period of 180 calendar days, or until March 17, 2025 (the “Compliance Date”), to regain compliance with the Bid Price Rule. If, at any time before the Compliance Date, the closing bid price for our common stock closes at $1.00 or more for a minimum of 10 consecutive business days as required under the Compliance Period Rule, the Staff will provide written notification to us that we comply with the Bid Price Rule, unless the Staff exercises its discretion to extend this 10 day period pursuant to Nasdaq Listing Rule 5810(c)(3)(H). If we are not in compliance with the Bid Price Rule by March 17, 2025, we may be afforded a second 180 calendar day period to regain compliance. To qualify, we would be required to meet the continued listing requirement for market value of publicly held shares and all other initial listing standards for The Nasdaq Capital Market, except for the minimum bid price requirement. In addition, we would be required to notify Nasdaq of our intent to cure the minimum bid price deficiency, which may include, if necessary, implementing a reverse stock split. If we do not regain compliance with the Bid Price Rule by the Compliance Date and are not eligible for an additional compliance period at that time, the Staff will provide written notification to us that our common stock may be delisted. We would then be entitled to appeal the Staff’s determination to a NASDAQ Listing Qualifications Panel and request a hearing. There can be no assurance that, if we do appeal the delisting determination by the Staff to the NASDAQ Listing Qualifications Panel, that such appeal would be successful.
Submission of matters to a vote of security holders
On October 17, 2024, we held our annual meeting, our stockholders approved an amendment to our certificate of incorporation (the “Amendment”) to effect a reverse stock split of the outstanding shares of our common stock, at a split ratio of between 1-for-2 and 1-for-50 as determined by our board of directors in their sole discretion, prior to the one-year anniversary of the annual meeting. Pursuant to such authority granted by our stockholders, our board of directors approved a 1-for-20 reverse stock split of our common stock and the filing of the Amendment to effectuate the reverse stock split. The Amendment was filed with the Secretary of State of the State of Delaware and the reverse stock split became effective in accordance with the terms of the Amendment at 11:59 p.m. Eastern Time on October 24, 2024 (the “Effective Time”). The Amendment provided that, at the Effective Time, every 20 shares of our issued and outstanding common stock was automatically combined into one issued and outstanding share of common stock, without any change in par value per share, which will remain $0.001. Unless the context expressly dictates otherwise, all reference to share and per share amounts referred to herein reflect the 1-for-20 reverse stock split.
Preliminary results from proof-of-concept human clinical trial
On October 28, 2024, we highlighted positive preliminary results from the first five “lead-in” patients in our ongoing proof-of-concept human clinical trial (the “Trial”) evaluating the safety and effectiveness of delivering transvascular energy to ablate relevant problematic nerves and mitigate pain in patients with pancreatic cancer pain. Three patients were treated with femoral access and two were treated with brachial access. All patients treated with femoral access positively responded to treatment while patients treated with brachial access showed no improvement in their pain scores (or worsened). The three patients in the responder group showed a reduction in pain assessed using the Visual Analog Scale (“VAS”) from a mean pre-procedure score of 8.0 to a mean score of 1.33 at 4-6 weeks post-procedure. Additionally, all responding patients were able to completely eliminate their opioid use at 4-6 weeks post-procedure.
On October 31, 2024, we highlighted positive preliminary results from the first 15 patients, including five (5) "lead-in" patients. 11 patients were treated with femoral access and three (3) were treated with brachial access. Patients treated with femoral access responded positively to treatment while patients treated with brachial access showed no improvement in their pain scores. One (1) patient could not receive treatment due to a more severe stenosis than what appeared on pre-screening scans and is not included in the modified intent to treat population. The result of the 11 patients in the responder group showed that 79% of patients responded with a mean 4.96 reduction of pain on the VAS pain scale (from baseline of 7.82 to 2.86) at 7 days post-procedure. Through 7 days post-procedure, the study showed a decrease in opioid demand and no responding patient needed dose increase; No responding patient needed opioids after their 24-hour post- procedure follow-up visit. Responding patients reported a mean 66% improvement in overall health status at 7 days post-procedure. When evaluating the total treated population, including non-responders, the mean reduction in the VAS pain score was a 3.64 point reduction, or 48%, in pain scores reported pre-procedure to Day 7 post-procedure.
Company Information
Our principal executive offices are located at 21 Waterway Avenue, Suite 300, The Woodlands, Texas 77380 and our telephone number is (713) 588-6150. Our website address is www.autonomix.com. The information on or accessible through our website is not part of this prospectus.
Risk Factors Summary
Our business is subject to a number of risks of which you should be aware before making an investment decision. You should carefully consider all of the information set forth in this prospectus and, in particular, should evaluate the specific factors set forth in the section titled “Risk Factors” before deciding whether to invest in our common stock. Among these important risks are the following:
Risks Related to Our Overall Business
|
● |
The report of our independent registered public accounting firm expresses substantial doubt about our ability to continue as a going concern. |
|
● |
We have no approved products, and we cannot assure you that we will generate revenue or become profitable in the future. |
|
● |
We intend to utilize a single manufacturer for the manufacture of our lead product candidate and expect to continue to do so for commercial products. Risks associated with the manufacturing of our products could reduce our gross margins and negatively affect our operating results. |
|
● |
We are a developmental stage company and have not yet had a history of generating revenue. |
|
● |
Our business may be adversely affected by the state of the global economy, uncertainties in global financial markets, and possible trade tariffs and trade restrictions. |
|
● |
We have no experience in assembling and testing our products and may encounter problems or delays in the assembly of our products or fail to meet certain regulatory requirements which could result in an adverse effect on our business and financial results. |
|
● |
Rapidly changing technology in life sciences could make the products we are developing obsolete. |
|
● |
Adverse developments affecting the financial services industry, such as actual events or concerns involving liquidity, defaults, or non-performance by financial institutions or transactional counterparties, could adversely affect our current and projected business operations and our financial condition and results of operations. |
|
● |
Catastrophic events and disaster recovery may disrupt business continuity. |
|
● |
We may fail to meet the Sarbanes-Oxley regulations and may lack the financial controls and safeguards required of public companies. |
|
● |
While our Company’s management is working to improve our internal controls and procedures, at present management has determined that our internal controls were deemed to be inadequate, which could cause our financial reporting to be unreliable and lead to misinformation being disseminated to the public. |
Risks Related to Government Regulation and Product Approvals
|
● |
There is no guarantee that the FDA will grant 510(k) or de novo clearance or a premarket approval application (“PMA”) of our future products and failure to obtain necessary clearances or approvals for our future products would adversely affect our ability to grow our business. |
|
● |
Modifications to our future products may require new regulatory clearances or approvals or may require us to recall or cease marketing our products until clearances or approvals are obtained. |
|
● |
The results of our future clinical trials may not support our product candidate claims or may result in the discovery of adverse side effects. |
|
● |
We plan to conduct our initial Proof of Concept trial outside the United States and to present the relevant data from this trial to the FDA in an effort to minimize the clinical requirements for clearance in the United States. There is no assurance that the FDA will accept this data. |
|
● |
Our clinical studies could be delayed or otherwise adversely affected by many factors. |
|
● |
Even if our products are cleared or approved by the FDA, if we or our suppliers fail to comply with ongoing FDA requirements, or if we experience unanticipated problems with our products, these products could be subject to restrictions or withdrawal from the market. |
|
● |
Our products may in the future be subject to product recalls that could harm our business and financial results. |
|
● |
If our products cause or contribute to a death or a serious injury, or malfunction in certain ways, we will be subject to medical device reporting regulations, which can result in voluntary corrective actions or enforcement actions. |
|
● |
Certain parts used in the manufacturing of our equipment may experience shortages in global supply which could impact our ability to manufacture our device for customers or maintain research and development timelines. |
|
● |
U.S. legislative or FDA regulatory reforms may make it more difficult and costly for us to obtain regulatory approval of our product candidates and to manufacture, market and distribute our products after approval. |
|
● |
Because of the specialized nature of our business, the termination of relationships with our key employees, consultants and advisors may prevent us from successfully operating our business, including developing our products, conducting clinical studies, commercializing our products and obtaining any necessary financing. |
|
● |
Failure to secure and maintain adequate coverage and reimbursement from third-party payers could adversely affect acceptance of our products, if approved, and reduce our revenues. |
|
● |
We may not be successful in securing and maintaining reimbursement codes necessary to facilitate accurate and timely billing for our products or physician services attendant to our products. |
|
● |
If we are unable to establish good relationships with physicians, our business could be negatively affected. |
|
● |
There is no assurance that Medicare or the Medicare Administrative Contractors will provide coverage or adequate payment rates for our products. |
Risks Related to Our Intellectual Property and to Our Information Technology
|
● |
Our business and operations would suffer in the event of third-party computer system failures, cyber-attacks on third-party systems or deficiency in our cyber security. |
|
● |
Artificial intelligence presents risks and challenges that can impact our business, including by posing security risks to our confidential information, proprietary information and personal data. |
|
● |
Cybersecurity risks and cyber incidents could adversely affect our business and disrupt operations. |
|
● |
We may incur substantial costs as a result of proceedings relating to patent and other intellectual property rights. |
|
● |
If third parties claim that our products infringe their intellectual property rights, we may be forced to expend significant financial resources and management time defending against such actions and our financial condition and our results of operations could suffer. |
|
● |
If we are unable to protect the intellectual property used in our products, others may be able to copy our innovations which may impair our ability to compete effectively in our markets. |
|
● |
We may be subject to claims that our employees have wrongfully used or disclosed alleged trade secrets of their former employers. |
|
● |
If we are unable to protect the confidentiality of our proprietary information and know-how, the value of our technology and products could be adversely affected. |
Risks Related to this Offering
| ● | There are material limitations with making preliminary estimates of our results for the period ended September 30, 2024. Our independent registered public accounting firm has not conducted an audit or review of, and does not express an opinion or any other form of assurance with respect to, the preliminary unaudited results. It is possible that we, or our independent registered public accounting firm, may identify items that require us to make adjustments to the preliminary estimates of operating loss and net loss before income taxes from continuing operations. |
|
● |
We have broad discretion in how we use the proceeds of this offering and may not use these proceeds effectively, which could affect our results of operations and cause our common stock to decline. |
|
● |
We will require substantial funding, which may not be available to us on acceptable terms, or at all, and, if not so available, may require us to delay, limit, reduce or cease our operations. |
|
● |
Purchasers in this offering will experience immediate and substantial dilution in net tangible book value. |
|
● |
Your ownership may be diluted if additional capital stock is issued to raise capital, to finance acquisitions or in connection with strategic transactions. |
|
● |
If our stock price fluctuates after the offering, you could lose a significant part of your investment. |
|
● |
This offering may cause the trading price of our common stock to decrease. |
|
● |
We have never paid dividends on our capital stock, and we do not anticipate paying dividends in the foreseeable future. |
Risks Related to our Common Stock
| ● | We completed a reverse stock split on October 24, 2024 in an effort to regain compliance with Nasdaq listing rules and we cannot predict the effect that such reverse stock split will have on the market price for shares of our common stock. |
|
● |
Concentration of ownership of our common stock among our existing executive officers and directors may prevent new investors from influencing significant corporate decisions. |
|
● |
Techniques employed by short sellers may in the future drive down the market price of our common stock. |
|
● |
If securities or industry analysts do not publish research or reports about us, or if they adversely change their recommendations regarding our common stock, then our stock price and trading volume could decline. |
|
● |
As an “emerging growth company” under the Jumpstart Our Business Startups Act, or JOBS Act, we are permitted to, and intend to, rely on exemptions from certain disclosure requirements. |
|
● |
Our failure to meet the continued listing requirements of the Nasdaq could result in de-listing of our common stock. |
|
● |
Our Certificate of Incorporation includes a forum selection provision, which could result in less favorable outcomes to the plaintiff(s) in any action against us. |
|
● |
The requirements of being a public company may strain our resources, divert management’s attention and affect our ability to attract and retain qualified board members. |
|
● |
We may be at an increased risk of securities class action litigation. |
The Offering
|
Common Units we are offering |
Up to 698,812 Common Stock Units, each Common Stock Unit consisting of one share of our common stock and one Common Warrant, at an assumed public offering price of $14.31 per Common Stock Unit, which is equal to the last sale price of our common stock as reported by Nasdaq on October 31, 2024.
The Common Stock Units will not initially be certificated or issued in stand-alone form. The shares of common stock and the Common Warrants comprising the Common Stock Units are immediately separable upon issuance and will be issued separately in this offering. |
|
Common stock outstanding immediately before this offering |
1,152,149 shares |
|
Common stock outstanding immediately after this offering |
1,850,961 shares of common stock (1,955,783 shares if the underwriter exercises its option to cover over-allotments, if any), excluding shares of common stock issuable upon exercise of the Common Warrants and assuming no Pre-Funded Warrants are sold. |
|
PFW Units offered |
We are also offering to those purchasers, if any, whose purchase of Common Stock Units in this offering would otherwise result in the purchaser, together with its affiliates and certain related parties, beneficially owning more than 4.99% (or, at the election of the purchaser, 9.99%) of our outstanding shares of common stock immediately following the consummation of this offering, the opportunity to purchase, if such purchasers so choose, up to _____ PFW Units, in lieu of Common Stock Units that would otherwise result in any such purchaser’s beneficial ownership exceeding 4.99% of our outstanding shares of common stock. Each PFW Unit consists of: (i) one Pre-Funded Warrant to purchase one share of our common stock and (ii) one Series A Warrant. The purchase price of each PFW Unit will equal the public offering price at which Common Stock Units are being sold to the public in this offering, minus $0.001 and the exercise price of each Pre-Funded Warrant will be $0.001 per share. See “Description of Securities Being Offered.” This prospectus also relates to the offering of the Shares issuable upon exercise of the Pre-Funded Warrants.
For each PFW Unit we sell, the number of Common Stock Units we are offering will be decreased on a one-for-one basis. Because one Series A Warrants is being sold together in this offering with each Common Stock Unit and, in the alternative, each PFW Unit, the number of Series A Warrants sold in this offering will not change as a result of a change in the mix of the Common Stock Units and PFW Units sold. This offering also relates to the offering of the shares of common stock issuable upon exercise of the Pre-Funded Warrants. |
| Series A Warrants |
Each Common Stock Unit or PFW Unit purchased in this offering, as the case may be, will include one Series A warrant to purchase one share of our common stock. The Series A Warrants have an exercise price of $______ per share of common stock (_____% of the public offering price per Unit), will be immediately exercisable and will expire five years from the date of issuance. The shares of common stock in the Common Stock Units or the Pre-Funded Warrants in the PFW Units, as applicable, and the accompanying Series A Warrants, can only be purchased together in this offering but will be issued separately and will be immediately separable upon issuance. This offering also relates to the offering of the shares of common stock issuable upon exercise of the Series A Warrants. Each Series A Warrant is exercisable for one share of common stock, subject to adjustment in the event of stock dividends, stock splits, stock combinations, reclassifications, reorganizations or similar events affecting our common stock.
A holder of Series A Warrants may not exercise any portion of a Series A Warrant to the extent that the holder, together with its affiliates and any other person or entity acting as a group, would own more than 4.99% (or, at the election of the holder, such limit may be increased to up to 9.99%) of our outstanding shares of common stock after exercise, as such ownership percentage is determined in accordance with the terms of the Series A Warrants, except that upon notice from the holder to us, the holder may waive such limitation up to a percentage, not in excess of 9.99%. This prospectus also relates to the offering of the common stock issuable upon exercise of the Series A Warrants.
To better understand the terms of the Common Warrants, you should carefully read the “Description of Securities We Are Offering” section of this prospectus. You should also read the form of Common Warrant, which is filed as an exhibit to the registration statement that includes this prospectus. |
|
Over-allotment option |
We have granted the underwriter an option for a period of 45 days from the date of this prospectus to purchase up to an additional 104,822 Shares of common stock and/or 104,822 Common Warrants, or any combination thereof, at a purchase price per additional Share equal to the public offering price per Share, less the underwriting discount.
Because the Common Warrants will not be listed on a national securities exchange or other nationally recognized trading market, the underwriters will be unable to satisfy any over-allotment of Shares and Common Warrants without exercising the underwriters’ over-allotment option with respect to the Common Warrants. As a result, the underwriters will exercise their over-allotment option for all of the Common Warrants which are over-allotted, if any, at the time of the initial offering of the Shares and the Common Warrants. However, because our common stock is publicly traded, the underwriters may satisfy some or all of the over-allotment of shares of our common stock, if any, by purchasing shares in the open market and will have no obligation to exercise the over-allotment option with respect to our common stock. |
|
Lock-up Agreements |
We have agreed with the underwriters not to sell additional equity securities for a period of 90 days after the effective date of this Offering. Our directors and officers have agreed with the underwriters not to offer for sale, sell, contract to sell, pledge or otherwise dispose of any of their Shares of our common stock or securities convertible into our common stock, subject to certain exceptions, for a period of 90 days after the date of this prospectus, which restriction may be waived in the discretion of the underwriter. |
|
Representative Warrants |
We have agreed to issue to the Representative of the underwriters warrants, or the Representative Warrants, to purchase up to 6% of the total number of Shares underlying units sold in this offering, including Shares underlying PFW Units, or 41,928 shares of common stock (or 48,218 shares of common stock, assuming the exercise of the over-allotment option in full), as a portion of the compensation payable to the underwriters in connection with this offering. The Representative Warrants will be immediately exercisable at any time, and from time to time, in whole or in part, commencing 180 days from the commencement of sales of the securities in this offering at an exercise price equal to 155% of the public offering price of the Shares sold in this offering, expire on the fifth anniversary of the commencement of sales in this offering, and are otherwise in substantially similar form to the Common Warrants issued in the offering. The Representative Warrants and the Shares of common stock underlying the Representative Warrants are being registered on the registration statement of which this prospectus is a part. See “Underwriting” on page 80 of this prospectus. |
|
Use of proceeds |
We estimate that the net proceeds from this offering will be approximately $9.0 million, based on an assumed public offering price of $14.31 per share, which is the closing price of our common stock as reported on Nasdaq on October 31, 2024, after deducting the underwriting discounts and estimated offering expenses payable by us. We intend to use the proceeds from this offering primarily to fund our clinical trial, for other research and development, for development of intellectual property, and for working capital. See “Use of Proceeds.” |
|
Risk Factors |
An investment in our securities involves a high degree of risk. See “Risk Factors” beginning on page 7 of this prospectus and the other information included and incorporated by reference in this prospectus for a discussion of the risk factors you should carefully consider before deciding to invest in our securities. |
|
Nasdaq listing symbol |
Our common stock is listed on The Nasdaq Capital Market under the symbol “AMIX.” |
The number of shares of common stock to be outstanding after this is based on 1,152,120 shares outstanding as of October 1, 2024 and excludes:
|
● |
112,534 shares of common stock underlying outstanding warrants at a weighted average exercise price of $8.26 per share; |
|
● |
216,483 shares of common stock underlying outstanding options with a weighted average exercise price of $36.43 per share; |
|
● |
33,250 shares of common stock underlying outstanding convertible notes at a weighted average conversion price of $40.00 per share; |
|
● |
75,633 shares available for future issuance under the Autonomix Medical, Inc. 2023 Stock Plan; |
|
● |
698,812 shares of common stock underlying the Common Warrants issuable in this offering; and |
|
● |
41,928 shares of common stock underlying the Representative Warrants issuable to the underwriters in connection with this offering. |
Except as otherwise indicated, the information in this prospectus assumes no exercise of options or warrants, or conversion of convertible notes.
Unless otherwise indicated, this prospectus reflects and assumes no exercise by the underwriters of their over-allotment option.
The information discussed above is illustrative only and will adjust based on the actual public offering price and other terms of this offering determined at pricing.
RISK FACTORS
Investing in our securities involves a high degree of risk. Before investing in our securities, you should consider carefully the risks and uncertainties discussed hereunder “Risk Factors”. You should carefully consider each of the following risks, together with all other information set forth in this prospectus, including the financial statements and the related notes, before making a decision to buy our securities. If any of the following risks actually occurs, our business could be harmed. In that case, the trading price of our common stock could decline, and you may lose all or part of your investment.
Risks Related to Our Overall Business
The report of our independent registered public accounting firm dated May 31, 2024 expresses substantial doubt about our ability to continue as a going concern.
As of June 30, 2024, we had an accumulated deficit of $41.7 million, negative cash flows from operating activities of $1.9 million and working capital of $6.3 million. The report of our independent registered public accounting firm dated May 31, 2024, expresses substantial doubt about our ability to continue as a going concern. Further, we have incurred, and expect to continue to incur, significant costs in pursuit of our business plans. We cannot assure you that our plans to raise sufficient capital to fund our business will be successful. These factors, among others, raise substantial doubt about our ability to continue as a going concern. The financial statements contained elsewhere in this prospectus do not include any adjustments that might result from our inability to raise additional capital or our inability to continue as a going concern.
We have no approved products, and we cannot assure you that we will generate revenue or become profitable in the future.
Our products may never be cleared by the United States Food & Drug Administration (“FDA”) or any other foreign regulator or become commercially viable or accepted for use. We have incurred significant losses since our inception and expect to continue to experience operating losses and negative cash flow for the foreseeable future. We expect to expend significant resources on hiring of personnel, continued scientific and product research and development, product testing and preclinical and clinical investigation, intellectual property development and prosecution, marketing and promotion, capital expenditures, working capital, general and administrative expenses, and fees and expenses associated with our capital raising efforts. We expect to incur costs and expenses related to consulting costs, hiring of scientists, engineers, science and other operational personnel, and the continued development of relationships with strategic partners.
We intend to utilize a single manufacturer for the manufacture of our lead product candidate and expect to continue to do so for commercial products. Risks associated with the manufacturing of our products could reduce our gross margins and negatively affect our operating results.
We do not have any manufacturing facilities or direct manufacturing personnel. We currently rely, and expect to continue to rely, on a single manufacturer for the manufacture of our lead product candidate for commercial manufacture. As such, we are subject to numerous risks relating to our reliance on a single manufacturer. If they encounter problems in manufacturing our product candidate, then our business could be significantly impacted. These problems include:
|
● |
inability to secure product components in a timely manner, insufficient quantities or on commercially reasonable terms; |
|
● |
failure to increase production to meet demand; |
|
● |
inability to modify production lines to enable us to efficiently produce future products or implement changes in current products in response to regulatory requirements; |
|
● |
difficulty identifying and qualifying alternative manufacturers in a timely manner; |
|
● |
inability to establish agreements with future third-party manufacturers or to do so on acceptable terms; or |
|
● |
potential damage to or destruction of our manufacturers' equipment or facilities. |
If demand for our future products increases, our manufacturer will need to invest additional resources to purchase components, hire and train employees, and enhance their manufacturing processes. If they fail to increase production capacity efficiently, our sales may not increase in line with our expectations and our operating margins could fluctuate or decline. We do not have a long-term agreement with our manufacturer and there is no assurance that they will continue to provide us with manufacturing services in the future.
We are a developmental stage company and have not yet had a history of generating revenue.
As a development-stage entity, we have not generated any revenues. Investors are subject to all the risks incident to the creation and development of a new business and each investor should be prepared to withstand a complete loss of investment. We have not emerged from the development stage and may be unable to raise further equity. These factors raise substantial doubt about our ability to continue as a going concern.
Our business may be adversely affected by the state of the global economy, uncertainties in global financial markets, and possible trade tariffs and trade restrictions.
Our operations and performance will depend significantly on worldwide economic and geopolitical conditions. Uncertainty about global economic conditions could result in potential customers postponing purchases of our future products in response to tighter credit, unemployment, negative financial news and/or declines in income or asset values and other macroeconomic factors, which could have a material negative effect on demand for our future products and, accordingly, on our business, results of operations or financial condition. For example, current global financial markets continue to reflect uncertainty, which has been heightened by the COVID-19 pandemic and the ongoing military conflict between Russia and Ukraine and the ongoing conflict in Israel. Given these uncertainties, there could be further disruptions to the global economy, financial markets and consumer confidence. If economic conditions deteriorate unexpectedly, our business and results of operations could be materially and adversely affected. For example, our future customers, including our distributors and their customers, may have trouble obtaining the working capital and other financing necessary to support historical or projected purchasing patterns, which could negatively affect our results of operations.
Recent global economic slowdowns could continue and potentially result in certain economies dipping into economic recessions, including in the United States. Additionally, increased inflation around the world, including in the United States, applies pressure to our costs. Continued economic slowdowns or recessions and inflationary pressures could have a negative impact on our business, including decreased demand, increased costs, and other challenges. Government actions to address economic slowdowns and increased inflation, including increased interest rates, also could result in negative impacts to our growth.
General trade tensions between the United States and China have been escalating, and any economic and political uncertainty caused by the United States tariffs imposed on goods from China, among other potential countries, and any corresponding tariffs or currency devaluations from China or such other countries in response, may negatively impact, demand and/or increase the cost for our future products. Additionally, Russia’s invasion of Ukraine in early 2022 triggered significant sanctions from U.S. and European countries. Resulting changes in U.S. trade policy could trigger retaliatory actions by Russia, its allies and other affected countries, including China, resulting in a potential trade war. Furthermore, if the conflict between Russia and Ukraine continues for a prolonged period of time, or if other countries, including the U.S., become involved in the conflict, we could face significant adverse effects to our business and financial condition. For example, if our supply or customer arrangements are disrupted due to expanded sanctions or involvement of countries where we have operations or relationships in the future, our business could be materially disrupted. Further, the use of cyberattacks could expand as part of the conflict, which could adversely affect our ability to maintain or enhance our cyber-security and data protection measures.
The inability to obtain adequate financing from debt or capital sources in the future could force us to self-fund strategic initiatives or even forego certain opportunities, which in turn could potentially harm our performance.
We have no experience in assembling and testing our products and may encounter problems or delays in the assembly of our products or fail to meet certain regulatory requirements which could result in an adverse effect on our business and financial results.
We have no experience in assembling and testing our planned device and no experience in doing so on a commercial scale. To become profitable, we must assemble and test our planned device in commercial quantities in compliance with regulatory requirements and at an acceptable cost. Increasing our capacity to assemble and test our products on a commercial scale will require us to improve internal efficiencies. We may encounter a number of difficulties in increasing our assembly and testing capacity, including:
|
● |
managing production yields; |
|
● |
maintaining quality control and assurance; |
|
● |
providing component and service availability; |
|
● |
maintaining adequate control policies and procedures; |
|
● |
hiring and retaining qualified personnel; and |
|
● |
complying with state, federal and foreign regulations. |
If we are unable to satisfy commercial demand for our planned device due to our inability to assemble and test our planned device, our ability to generate revenue would be impaired, market acceptance of our products could be adversely affected and customers may instead purchase or use, our competitors’ products.
Rapidly changing technology in life sciences could make the products we are developing obsolete.
The medical device and life-science industry in general is characterized by rapid and significant technological changes, frequent new product introductions and enhancements and evolving industry standards. Our future success will depend on our ability to continually develop and then improve the products that we design and to develop and introduce new products that address the evolving needs of our customers on a timely and cost-effective basis.
Adverse developments affecting the financial services industry, such as actual events or concerns involving liquidity, defaults, or non-performance by financial institutions or transactional counterparties, could adversely affect our current and projected business operations and our financial condition and results of operations.
Actual events involving limited liquidity, defaults, non-performance, or other adverse developments that affect financial institutions, transactional counterparties or other companies in the financial services industry or the financial services industry generally or concerns or rumors about any events of these kinds or other similar risks, have in the past and may in the future lead to market-wide liquidity problems. For example, on March 10, 2023, Silicon Valley Bank, or SVB, was closed by the California Department of Financial Protection and Innovation, which appointed the Federal Deposit Insurance Corporation, or the FDIC, as receiver. Similarly, on March 12, 2023, Signature Bank Corp., or Signature, and Silvergate Capital Corp. were each swept into receivership. Although the Department of the Treasury, the Federal Reserve and the FDIC ensured that all depositors of SVB would have access to all of their money after only one business day of closure, including funds held in uninsured deposit accounts, borrowers under credit agreements, letters of credit and certain other financial instruments with SVB, Signature or any other financial institution that is placed into receivership by the FDIC may be unable to access undrawn amounts thereunder. Although we were not a borrower under or party to any material letter of credit or any other such instruments with SVB, Signature or any other financial institution currently in receivership, if we enter into any such instruments and any of our lenders or counterparties to such instruments were to be placed into receivership, we may be unable to access such funds. In addition, if any of our partners, suppliers or other parties with whom we conduct business are unable to access funds pursuant to such instruments or lending arrangements with such a financial institution, such parties’ ability to pay their obligations to us or to enter into new commercial arrangements requiring additional payments to us could be adversely affected. In this regard, counterparties to credit agreements and arrangements with these financial institutions, and third parties such as beneficiaries of letters of credit (among others), may experience direct impacts from the closure of these financial institutions and uncertainty remains over liquidity concerns in the broader financial services industry. Similar impacts have occurred in the past, such as during the 2008-2010 financial crisis. Inflation and rapid increases in interest rates have led to a decline in the trading value of previously issued government securities with interest rates below current market interest rates. Although the U.S. Department of Treasury, FDIC and Federal Reserve Board have announced a program to provide up to $25 billion of loans to financial institutions secured by certain of such government securities held by financial institutions to mitigate the risk of potential losses on the sale of such instruments, widespread demands for customer withdrawals or other liquidity needs of financial institutions for immediately liquidity may exceed the capacity of such program.
Our access to funding sources and other credit arrangements in amounts adequate to finance or capitalize our current and projected future business operations could be significantly impaired by factors that affect us, any financial institutions with which we enter into credit agreements or arrangements directly, or the financial services industry or economy in general. These factors could include, among others, events such as liquidity constraints or failures, the ability to perform obligations under various types of financial, credit or liquidity agreements or arrangements, disruptions or instability in the financial services industry or financial markets or concerns or negative expectations about the prospects for companies in the financial services industry. These factors could involve financial institutions or financial services industry companies with which we have financial or business relationships but could also include factors involving financial markets or the financial services industry generally.
The results of events or concerns that involve one or more of these factors could include a variety of material and adverse impacts on our current and projected business operations and our financial condition and results of operations. These risks include, but may not be limited to, the following:
|
● |
delayed access to deposits or other financial assets or the uninsured loss of deposits or other financial assets; |
|
● |
inability to enter into credit facilities or other working capital resources; |
|
● |
potential or actual breach of contractual obligations that require us to maintain letters of credit or other credit support arrangements; or |
|
● |
termination of cash management arrangements and/or delays in accessing or actual loss of funds subject to cash management arrangements. |
In addition, investor concerns regarding the U.S. or international financial systems could result in less favorable commercial financing terms, including higher interest rates or costs and tighter financial and operating covenants, or systemic limitations on access to credit and liquidity sources, thereby making it more difficult for us to acquire financing on acceptable terms or at all. Any decline in available funding or access to our cash and liquidity resources could, among other risks, adversely impact our ability to meet our operating expenses or other obligations, financial or otherwise, result in breaches of our financial and/or contractual obligations or result in violations of federal or state wage and hour laws. Any of these impacts, or any other impacts resulting from the factors described above or other related or similar factors, could have material adverse impacts on our liquidity and our current and/or projected business operations and financial condition and results of operations.
In addition, any further deterioration in the macroeconomic economy or financial services industry could lead to losses or defaults by our partners, vendors, or suppliers, which in turn, could have a material adverse effect on our current and/or projected business operations and results of operations and financial condition. For example, a partner may fail to make payments when due, default under their agreements with us, become insolvent or declare bankruptcy, or a supplier may determine that it will no longer deal with us as a customer. In addition, a vendor or supplier could be adversely affected by any of the liquidity or other risks that are described above as factors that could result in material adverse impacts on us, including but not limited to delayed access or loss of access to uninsured deposits or loss of the ability to draw on existing credit facilities involving a troubled or failed financial institution. The bankruptcy or insolvency of any partner, vendor or supplier, or the failure of any partner to make payments when due, or any breach or default by a partner, vendor or supplier, or the loss of any significant supplier relationships, could cause us to suffer material losses and may have a material adverse impact on our business.
Catastrophic events and disaster recovery may disrupt business continuity.
A disruption or failure of our systems or operations in the event of a natural disaster or severe weather event, including, but not limited to, earthquakes, wildfires, droughts, flooding, tornadoes, hurricanes or tsunamis, health pandemic, such as an influenza outbreak within our workforce, or man-made catastrophic event could cause delays in completing sales, continuing production or performing other critical functions of our business, particularly if a catastrophic event were to occur at our premises. Global climate change could result in certain natural disasters occurring more frequently or with greater intensity. Any of these events could severely affect our ability to conduct normal business operations and, as a result, our operating results could be adversely affected. There may also be secondary impacts that are unforeseeable as well, such as impacts on our customers, which could cause delays in new orders, delays in completing sales or even order cancellations.
We may fail to meet the Sarbanes-Oxley regulations and may lack the financial controls and safeguards required of public companies.
Ensuring that we have adequate internal financial and accounting controls and procedures in place to produce accurate financial statements on a timely basis is a costly and time-consuming effort that needs to be re-evaluated frequently. Our management has concluded that our internal controls over financial reporting are ineffective and has identified material weaknesses in our internal controls in areas such as the lack of segregation of duties; general technology controls; and financial statement reporting. While management is working to remediate the material weaknesses, there is no assurance that such changes, when economically feasible and sustainable, will remediate the identified material weaknesses or that the controls will prevent or detect future material weaknesses. If we are not able to maintain effective internal control over financial reporting, our financial statements, including related disclosures, may be inaccurate, which could have a material adverse effect on our business. We may discover additional material weaknesses in our internal financial and accounting controls and procedures that need improvement from time to time.
Management is responsible for establishing and maintaining adequate internal control over financial reporting to provide reasonable assurance regarding the reliability of our financial reporting and the preparation of financial statements for external purposes in accordance with United States generally accepted accounting principles. Management does not expect that our internal control over financial reporting will prevent or detect all errors and all fraud. A control system, no matter how well designed and operated, can provide only reasonable, not absolute, assurance that the control system’s objectives will be met. Because of the inherent limitations in all control systems, no evaluation of controls can provide absolute assurance that misstatements due to error or fraud will not occur or that all control issues and instances of fraud, if any, within our company will have been detected.
We are required to comply with Section 404 of the Sarbanes-Oxley Act in connection with our future annual and quarterly reports on Form 10-K and Form 10-Q, commencing with the Form 10-K for the year ended March 31, 2025. We expect to expend significant resources in developing the necessary documentation and testing procedures required by Section 404. We cannot be certain that the actions we will be taking to improve our internal controls over financial reporting will be sufficient, or that we will be able to implement our planned processes and procedures in a timely manner. In addition, if we are unable to produce accurate financial statements on a timely basis, investors could lose confidence in the reliability of our financial statements, which could cause the market price of our common stock to decline and make it more difficult for us to finance our operations and growth.
While our Company’s management is working to improve our internal controls and procedures, at present management has determined that our internal controls were deemed to be inadequate, which could cause our financial reporting to be unreliable and lead to misinformation being disseminated to the public.
Our management is responsible for establishing and maintaining adequate internal control over financial reporting. As defined in Rule 13a-15(f) under the Securities Exchange Act of 1934, as amended (the “Exchange Act”), internal control over financial reporting is a process designed by, or under the supervision of, the principal executive and principal financial officer and effected by the board of directors, management and other personnel, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles and includes those policies and procedures that:
|
● |
pertain to the maintenance of records that in reasonable detail accurately and fairly reflect the transactions and dispositions of our assets; |
|
● |
provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the Company are being made only in accordance with authorizations of management and/or directors of the Company; and |
|
● |
provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of the Company’s assets that could have a material effect on the financial statements. |
We are required to include a report of management on the effectiveness of our internal control over financial reporting. We expect to incur additional expenses and diversion of management’s time as a result of performing the system and process evaluation, testing and remediation required in order to comply with the management certification requirements.
Presently, we do not have a sufficient number of employees to segregate responsibilities and may be unable to afford increasing our staff or engaging outside consultants or professionals to overcome our lack of employees. During the course of our testing, we may identify other deficiencies that we may not be able to timely remediate. If we cannot provide reliable financial reports or prevent fraud, our business and operating results could be harmed, investors could lose confidence in our reported financial information, and the trading price of our common stock, if a market ever develops, could drop significantly.
Our Certificate of Incorporation and Bylaws, each as amended to date, provide for indemnification of officers and directors at the expense of the Company and limit their liability that may result in a major cost to us and hurt the interests of our stockholders because corporate resources may be expended for the benefit of officers and/or directors.
Our Certificate of Incorporation and Bylaws, each as amended to date, provide for the indemnification of our officers and directors. We have been advised that, in the opinion of the SEC, indemnification for liabilities arising under federal securities laws is against public policy as expressed in the Securities Act and is therefore, unenforceable.
Risks Related to Government Regulation and Product Approvals
There is no guarantee that the FDA will grant 510(k) or de novo clearance or a premarket approval application (“PMA”) of our future products and failure to obtain necessary clearances or approvals for our future products would adversely affect our ability to grow our business.
Our lead product candidate will require FDA clearance of a 510(k) or de novo application or may require FDA approval of a PMA. The FDA may not approve or clear these products for the indications that are necessary or desirable for successful commercialization. Indeed, the FDA may refuse our requests for premarket clearance or premarket approval of new products, new intended uses or modifications to existing products. Failure to receive clearance or approval for our products would have an adverse effect on our ability to continue or expand our business.
If we fail to obtain and maintain regulatory approvals and clearances, or are unable to obtain, or experience significant delays in obtaining, FDA clearances or approvals for our device, our future products or product enhancements, our ability to commercially distribute and market these products could suffer.
Our products will be subject to rigorous regulation by the FDA and numerous other federal, state and foreign governmental authorities. The process of obtaining regulatory clearances or approvals to market a medical device can be costly and time consuming, and we may not be able to obtain these clearances or approvals on a timely basis, if at all. In particular, the FDA permits commercial distribution of a new medical device only after the device has received clearance under Section 510(k) of the Federal Food, Drug and Cosmetic Act, or is the subject of an approved premarket approval application, or PMA, unless the device is specifically exempt from those requirements. The FDA will clear marketing of a lower risk medical device through the 510(k) process if the manufacturer demonstrates that the new product is substantially equivalent to other 510(k)-cleared products or through a de novo process if substantial equivalence is not available. High risk devices deemed to pose the greatest risk, such as life-sustaining, life-supporting, or implantable devices, or devices not deemed substantially equivalent to a previously cleared device, require the approval of a PMA. The PMA process is more costly, lengthy and uncertain than the 510(k) or de novo clearance processes. A PMA application must be supported by extensive data, including, but not limited to, technical, preclinical, clinical trial, manufacturing and labeling data, to demonstrate to the FDA’s satisfaction the safety and efficacy of the device for its intended use. We believe our current product candidate will require clearance through the 510(k) or de novo process.
Modifications to our future products may require new regulatory clearances or approvals or may require us to recall or cease marketing our products until clearances or approvals are obtained.
Modifications to our future products may require new regulatory approvals or clearances, including 510(k) clearances or premarket approvals, or require us to recall or cease marketing the modified devices until these clearances or approvals are obtained. The FDA requires device manufacturers to initially make and document a determination of whether or not a modification requires a new approval, supplement or clearance. A manufacturer may determine that a modification could not significantly affect safety or efficacy and does not represent a major change in its intended use, so that no new 510(k) clearance is necessary. However, the FDA can review a manufacturer's decision and may disagree. The FDA may also on its own initiative determine that a new clearance or approval is required. Once we have a commercialized product, we may make modifications in the future that we believe do not or will not require additional clearances or approvals. If the FDA disagrees and requires new clearances or approvals for these modifications, we may be required to recall and to stop marketing our products as modified, which could require us to redesign our products and harm our operating results. In these circumstances, we may be subject to significant enforcement actions.
Where we determine that modifications to our products require a new 510(k) clearance or premarket approval application, we may not be able to obtain those additional clearances or approvals for the modifications or additional indications in a timely manner, or at all. Obtaining clearances and approvals can be a time-consuming process, and delays in obtaining required future clearances or approvals would adversely affect our ability to introduce new or enhanced products in a timely manner, which in turn would harm our future growth.
The results of our future clinical trials may not support our product candidate claims or may result in the discovery of adverse side effects.
We have not completed any clinical trials and we cannot be certain that their results will support our product candidate claims or that the FDA will agree with our conclusions regarding them. Success in pre-clinical studies and early clinical trials does not ensure that later clinical trials will be successful, and we cannot be sure that the later trials will replicate the results of prior trials and pre-clinical studies. The clinical trial process may fail to demonstrate that our product candidates are safe and effective for the proposed indicated uses, which could cause us to abandon a product candidate and may delay development of others. Any delay or termination of our clinical trials will delay the filing of our product submissions and, ultimately, our ability to commercialize our product candidates and generate revenues. It is also possible that patients enrolled in clinical trials will experience adverse side effects that are not currently part of the product candidate’s profile.
We plan to conduct our initial Proof of Concept trial outside the United States and to present the relevant data from this trial to the FDA in an effort to minimize the clinical requirements for clearance in the United States. There is no assurance that the FDA will accept this data.
Human trials are often designed to begin with a Proof of Concept trial and then progress to a “Pivotal” or approval, trial. We intend our Proof of Concept trial outside the United States. Upon completion, we plan to present the relevant data from this study to the FDA in a pre-submission meeting to request “Breakthrough Status” in an effort to minimize the clinical requirements for clearance in the United States. The first trial is not designed to replace the trial that will be required by the FDA to support our submissions for clearance in the United States, but rather to potentially impact the size of that trial. There is no assurance that the FDA will accept the data from our international trial or that they will not require us to conduct additional studies to supplement this trial. Any additional trials that we are required to conduct will be costly and time-consuming, and may require us to raise additional financing, for which we have no commitments.
Our clinical studies could be delayed or otherwise adversely affected by many factors, including difficulties in enrolling patients.
Clinical testing can be costly and take many years and the outcome is uncertain and susceptible to varying interpretations. Moreover, success in pre-clinical and early clinical studies does not ensure that large-scale studies will be successful or predict final results. Acceptable results in early studies may not be replicable in later studies. A number of companies have suffered significant setbacks in advanced clinical studies, even after promising results in earlier studies. Negative or inconclusive results or adverse events or incidents during a clinical study could cause the clinical study to be redone or terminated. In addition, failure to appropriately construct clinical studies could result in high rates of adverse events or incidents, which could cause a clinical study to be suspended, redone or terminated. Our failure or the failure of third-party participants in our studies to comply with their obligations to follow protocols and/or legal requirements may also result in our inability to use the affected data in our submissions to regulatory authorities.
The timely completion of clinical studies depends, among other things, on our ability to enroll a sufficient number of patients who remain in the study until its conclusion. We may experience difficulties in patient enrollment in our clinical studies for a variety of reasons, including:
|
● |
the severity of the disease under investigation; |
|
● |
the limited size and nature of the patient population; |
|
● |
the patient eligibility criteria defined in our protocol and other clinical study protocols; |
|
● |
the nature of the study protocol, including the attractiveness of, or the discomforts and risks associated with, the treatments received by enrolled subjects; |
|
● |
difficulties and delays in clinical studies that may occur as a result of future pandemics; |
|
● |
the ability to obtain institutional review board (“IRB”) approval at clinical study locations; |
|
● |
clinicians’ and patients’ perceptions as to the potential advantages, disadvantages and side effects of our products in relation to other available therapies, including any new drugs or treatments that may be approved for the indications we are pursuing; |
|
● |
the possibility or perception that enrolling in a product’s clinical study may limit the patient’s ability to enroll in future clinical studies for other therapies due to protocol restrictions; |
|
● |
the possibility or perception that our software is not secure enough to maintain patient privacy; |
|
● |
the ability to monitor patients adequately during and after treatment; |
|
● |
the availability of appropriate clinical study investigators, support staff, drugs and other therapeutic supplies and proximity of patients to clinical sites; |
|
● |
physicians’ or our ability to obtain and maintain patient consents; and |
|
● |
the risk that patients enrolled in clinical studies will choose to withdraw from or otherwise not be able to complete a clinical study. |
If we have difficulty enrolling and retaining a sufficient number or diversity of patients to conduct our clinical studies as planned, or encounter other difficulties, we may need to delay, terminate or modify ongoing or planned clinical studies, any of which would have an adverse effect on our business.
Even if our products are cleared or approved by the FDA, if we or our suppliers fail to comply with ongoing FDA requirements, or if we experience unanticipated problems with our products, these products could be subject to restrictions or withdrawal from the market.
Any product for which we obtain clearance or approval, and the manufacturing processes, reporting requirements, post-approval clinical data and promotional activities for such product, will be subject to continued regulatory review, oversight and periodic inspections by the FDA and other domestic and foreign regulatory bodies. In particular, we, and our suppliers, will be required to comply with FDA’s Quality System Regulations, or QSR, which covers the methods and documentation of the design, testing, production, control, quality assurance, labeling, packaging, storage and shipping of any product for which we obtain clearance or approval. FDA enforces the QSR and other regulations through periodic inspections. The failure by us or one of our suppliers to comply with applicable statutes and regulations administered by the FDA, or the failure to timely and adequately respond to any adverse inspectional observations or product safety issues, could result in, among other things, any of the following enforcement actions:
|
● |
untitled letters, warning letters, fines, injunctions, consent decrees and civil penalties; |
|
● |
unanticipated expenditures to address or defend such actions; |
|
● |
customer notifications for repair, replacement, refunds; |
|
● |
recall, detention or seizure of our products; |
|
● |
operating restrictions or partial suspension or total shutdown of production; |
|
● |
refusing or delaying our requests for premarket clearance or premarket approval of new products or modified products; |
|
● |
operating restrictions; |
|
● |
withdrawing premarket clearances on PMA approvals that have already been granted; |
|
● |
refusal to grant export approval for our products; or |
|
● |
criminal prosecution. |
If any of these actions were to occur, it would harm our reputation and cause our product sales and profitability to suffer and may prevent us from generating revenue. Furthermore, our key component suppliers may not be in compliance with all applicable regulatory requirements which could result in our failure to produce our products on a timely basis and in the required quantities, if at all.
Even if regulatory clearance or approval of a product is granted, such clearance or approval may be subject to limitations on the intended uses for which the product may be marketed and reduce our potential to successfully commercialize the product and generate revenue from the product. If the FDA determines that our promotional materials, labeling, training or other marketing or educational activities constitute promotion of an unapproved use, it could request that we cease or modify our training or promotional materials or subject us to regulatory enforcement actions. It is also possible that other federal, state or foreign enforcement authorities might take action if they consider our training or other promotional materials to constitute promotion of an unapproved use, which could result in significant fines or penalties under other statutory authorities, such as laws prohibiting false claims for reimbursement.
In addition, we may be required to conduct costly post-market testing and surveillance to monitor the safety or effectiveness of our products, and we must comply with medical device reporting requirements, including the reporting of adverse events and malfunctions related to our products. Later discovery of previously unknown problems with our products, including unanticipated adverse events or adverse events of unanticipated severity or frequency, manufacturing problems, or failure to comply with regulatory requirements such as QSR, may result in changes to labeling, restrictions on such products or manufacturing processes, withdrawal of the products from the market, voluntary or mandatory recalls, a requirement to repair, replace or refund the cost of any medical device we manufacture or distribute, fines, suspension of regulatory approvals, product seizures, injunctions or the imposition of civil or criminal penalties which would adversely affect our business, operating results and prospects.
Our products may in the future be subject to product recalls that could harm our reputation, business, and financial results.
The FDA and similar foreign governmental authorities have the authority to require the recall of commercialized products in the event of material deficiencies or defects in design or manufacture. In the case of the FDA, the authority to require a recall must be based on an FDA finding that there is a reasonable probability that the device would cause serious injury or death. Manufacturers may, under their own initiative, recall a product if any material deficiency in a device is found. A government-mandated or voluntary recall by us or one of our distributors could occur as a result of component failures, manufacturing errors, design or labeling defects or other deficiencies and issues. Recalls of any of our products would divert managerial and financial resources and have an adverse effect on our financial condition and results of operations. The FDA requires that certain classifications of recalls be reported to FDA within 10 working days after the recall is initiated. Companies are required to maintain certain records of recalls, even if they are not reportable to the FDA. We may initiate voluntary recalls involving our products in the future that we determine do not require notification of the FDA. If the FDA disagrees with our determinations, they could require us to report those actions as recalls. A future recall announcement could harm our reputation with customers and negatively affect our sales. In addition, the FDA could take enforcement action for failing to report the recalls when they were conducted.
If our products cause or contribute to a death or a serious injury, or malfunction in certain ways, we will be subject to medical device reporting regulations, which can result in voluntary corrective actions or agency enforcement actions.
Under the FDA medical device reporting regulations, medical device manufacturers are required to report to the FDA information that a device has or may have caused or contributed to a death or serious injury or has malfunctioned in a way that would likely cause or contribute to death or serious injury if the malfunction of the device or one of our similar devices were to recur. If we fail to report these events to the FDA within the required timeframes, or at all, FDA could take enforcement action against us. Any such adverse event involving our products also could result in future voluntary corrective actions, such as recalls or customer notifications, or agency action, such as inspection or enforcement action. Any corrective action, whether voluntary or involuntary, as well as defending ourselves in a lawsuit, will require the dedication of our time and capital, distract management from operating our business, and may harm our reputation and financial results.
Certain parts used in the manufacturing of our equipment may experience shortages in global supply which could impact our ability to manufacture our device for customers or maintain research and development timelines.
There are a number of component parts used in the manufacture of our device that are used by many manufacturers in a variety of products. We will compete with other manufacturers for the supply of these components. Additionally, certain parts that are currently in our design may be discontinued by our supplier requiring us to find alternative parts. This issue may require us to change the design of our device or purchase significant inventories of these parts in order to protect against manufacturing delays. Design changes may require FDA approval. We may not be able to procure alternative components or adequate raw material inventories which would result in an inability to produce our device.
U.S. legislative or FDA regulatory reforms may make it more difficult and costly for us to obtain regulatory approval of our product candidates and to manufacture, market and distribute our products after approval is obtained.
From time to time, legislation is drafted and introduced in Congress that could significantly change the statutory provisions governing the regulatory approval, manufacture and marketing of regulated products or the reimbursement thereof. In addition, FDA regulations and guidance are often revised or reinterpreted by the FDA in ways that may significantly affect our business and our products. Any new regulations or revisions or reinterpretations of existing regulations may impose additional costs or lengthen review times of future products. In addition, FDA regulations and guidance are often revised or reinterpreted by the agency in ways that may significantly affect our business and our products. It is impossible to predict whether legislative changes will be enacted, or FDA regulations, guidance or interpretations changed, and what the impact of such changes, if any, may be.
Because of the specialized nature of our business, the termination of relationships with our key employees, consultants and advisors may prevent us from successfully operating our business, including developing our products, conducting clinical studies, commercializing our products and obtaining any necessary financing.
We are highly dependent on the members of our executive team, the loss of whose services may adversely impact the achievement of our objectives. While we have entered into employment or consulting agreements with each of our key executives, any of them could leave our employment at any time. We do not have “key person” insurance on any of our employees. The loss of the services of one or more of our current employees might impede the achievement of our business objectives.
The competition for qualified personnel in the medical device fields is intense and we rely heavily on our ability to attract and retain qualified scientific, technical, and managerial personnel. Our future success depends upon our ability to attract, retain and motivate highly skilled employees. In order to commercialize our products successfully, we will be required to expand our workforce, particularly in the areas of research and development and clinical studies, finance, accounting and reporting, sales and marketing and supply chain management. These activities will require the addition of new personnel and the development of additional expertise by existing management personnel. We face intense competition for qualified individuals from numerous pharmaceutical, biopharmaceutical and biotechnology companies, as well as academic and other research institutions. We may not be able to attract and retain these individuals on acceptable terms or at all. Failure to do so could materially harm our business.
Failure to secure and maintain adequate coverage and reimbursement from third-party payers could adversely affect acceptance of our products, if approved, and reduce our revenues.
Assuming we receive approval of our products, we expect that the vast majority of our revenues will come from third-party payers, either directly to us in markets where we plan to provide our device candidates to patients, or indirectly via payments made to hospitals or other entities, which may in the future provide our device candidates to patients.
In the U.S., private payers cover the largest segment of the population, with the remainder either uninsured or covered by governmental payers. The majority of the third-party payers outside the U.S. are government agencies, government sponsored entities or other payers operating under significant regulatory requirements from national or regional governments.
Third-party payers may decline to cover and reimburse certain procedures, supplies or services. Additionally, some third-party payers may decline to cover and reimburse our products for a particular patient even if the payer has a favorable coverage policy addressing our products or previously approved reimbursement for our products. Additionally, private and government payers may consider the cost of a treatment in approving coverage or in setting reimbursement for the treatment.
Private and government payers around the world are increasingly challenging the prices charged for medical products and services. Additionally, the containment of healthcare costs has become a priority of governments around the world. Adoption of additional price controls and cost-containment measures, and adoption of more restrictive policies in jurisdictions with existing controls and measures, could further limit our revenues and operating results. If third-party payers do not consider our products or the combination of our products with additional treatments to be cost-justified under a required cost-testing model, they may not cover our products for their populations or, if they do, the level of reimbursement may not be sufficient to allow us to sell our products on a profitable basis.
Reimbursement for the treatment of patients with medical devices around the world is governed by complex mechanisms established on a national or sub-national level in each country. These mechanisms vary widely among countries, can be informal, somewhat unpredictable, and evolve constantly, reflecting the efforts of these countries to reduce public spending on healthcare. As a result, obtaining and maintaining reimbursement for the treatment of patients with medical devices has become more challenging globally. We cannot guarantee that the use of our products will receive reimbursement approvals and cannot guarantee that our existing reimbursement approvals will be maintained in any country.
Our failure to secure or maintain adequate coverage or reimbursement for our products by third-party payers in the U.S., or in the other jurisdictions in which we market our products, could have a material adverse effect on our business, revenues and results of operations.
We may not be successful in securing and maintaining reimbursement codes necessary to facilitate accurate and timely billing for our products or physician services attendant to our products.
Third-party payers, healthcare systems, government agencies or other groups often issue reimbursement codes to facilitate billing for products and physician services used in the delivery of healthcare. Within the U.S., the billing codes most directly related to our products are contained in the Healthcare Common Procedure Coding System (“HCPCS code set”). The HCPCS code set contains Level I codes that describe physician services, also known as Common Procedural Terminology codes (“CPT codes”) and Level II codes that primarily describe products. Centers for Medicare & Medicaid Services (“CMS”) is responsible for issuing the HCPCS Level II codes. The American Medical Association issues HCPCS Level I codes.
No HCPCS codes or CPT codes currently exist to describe physician services related to the delivery of therapy using our products. We may not be able to secure HCPCS codes and CPT codes for physician services related to our products. Our future revenues and results may be affected by the absence of CPT codes, as physicians may be less likely to prescribe the therapy when there is no certainty that adequate reimbursement will be available for the time, effort, skill, practice expense and malpractice costs required to provide the therapy to patients.
Outside the U.S., we have not secured codes to describe our products or to document physician services related to the delivery of therapy using our products. The failure to obtain and maintain these codes could affect the future growth of our business.
If we are unable to establish good relationships with physicians, our business could be negatively affected.
Our business model will require us to build and maintain good relationships with physicians who will have a significant source of patients that will generate treatment revenues for both the physician and the Company. If we are unable to establish good relationships with physicians and maintain them, it will jeopardize our ability to generate future revenues.
There is no assurance that Medicare or the Medicare Administrative Contractors will provide coverage or adequate payment rates for our products.
We anticipate that a significant portion of patients using our products will be beneficiaries under the Medicare fee-for-service program. Failure to secure or maintain coverage or maintain adequate reimbursement from Medicare would reduce our revenues and may also affect the coverage and reimbursement decisions of other third-party payers in the U.S. and elsewhere.
Medicare may classify our medical device as durable medical equipment (“DME”). Medicare has the authority to issue national coverage determinations or to defer coverage decisions to its regional Medicare Administrative Contractors (“MACs”). The fact that only two MACs administer the entire DME program may negatively affect our ability to petition individual medical policy decision-makers at the MACs for coverage. The absence of a positive coverage determination or a future restriction to existing coverage from Medicare or the DME MACs would materially affect our future revenues.
Additionally, Medicare has the authority to publish the reimbursement amounts for DME products. Medicare may in the future publish reimbursement amounts for our products that do not reflect then-current prices for our products. Medicare fee schedules are frequently referenced by private payers in the U.S. and around the world. Medicare’s publication of reimbursement amounts for our products that are below our products’ established prices could materially reduce our revenues and operating results with respect to non-Medicare payers in the U.S. and our other active markets.
Even if our products were authorized by Medicare, CMS requires prior authorization for certain DME items. Claims for such items that did not receive prior authorization before they were furnished to a beneficiary will be automatically denied. In the event Medicare adds one of our products to the list of items requiring prior authorization, our ability to bill and secure reimbursement for patients who would otherwise be covered to use our product under the Medicare fee-for-service program may be reduced.
We cannot provide any assurance that we can access transitional, expedited, or expanded Medicare coverage for our products. CMS is expected to issue rules regarding coverage of emerging technologies; however, no specific information is available about the content of the expected rules and we cannot provide any assurance that any new rules regarding emerging technologies would be applicable to our future products.
Risks Related to Our Intellectual Property and to Our Information Technology
Our business and operations would suffer in the event of third-party computer system failures, cyber-attacks on third-party systems or deficiency in our cyber security.
We rely on information technology (“IT”) systems, including third-party “cloud based” service providers, to keep financial records, maintain laboratory data, clinical data, and corporate records, to communicate with staff and external parties and to operate other critical functions. This includes critical systems such as email, other communication tools, electronic document repositories and archives. If any of these third-party information technology providers are compromised due to computer viruses, unauthorized access, malware, natural disasters, fire, terrorism, war and telecommunication failures, electrical failures, cyber-attacks or cyber-intrusions over the internet, then sensitive emails or documents could be exposed or deleted. Similarly, we could incur business disruption if our access to the internet is compromised, and we are unable to connect with third-party IT providers. The risk of a security breach or disruption, particularly through cyber-attacks or cyber intrusion, including by computer hackers, foreign governments and cyber terrorists, has generally increased as the number, intensity and sophistication of attempted attacks and intrusions from around the world have increased. In addition, we rely on those third parties to safeguard important confidential personal data regarding our employees and subjects enrolled in our clinical trials. If a disruption event were to occur and cause interruptions in a third-party IT provider’s operation, it could result in a disruption of our drug development programs. For example, the loss of clinical trial data from completed, ongoing or planned clinical trials could result in delays in our regulatory approval efforts and significantly increase our costs to recover or reproduce the data. To the extent that any disruption or security breach results in a loss of or damage to our data or applications, or inappropriate disclosure of confidential or proprietary information, we could incur liability and development of our product candidates could be delayed or could fail.
Artificial intelligence presents risks and challenges that can impact our business, including by posing security risks to our confidential information , proprietary information and personal data.
Issues in the development and use of artificial intelligence, combined with an uncertain regulatory environment, may result in reputational harm, liability, or other adverse consequences to our business operations. As with many technological innovations, artificial intelligence presents risks and challenges that could impact our business. We may adopt and integrate generative artificial intelligence tools into our systems for specific use cases reviewed by legal and information security. Our vendors may incorporate generative artificial intelligence tools into their offerings without disclosing this use to us, and the providers of these generative artificial intelligence tools may not meet existing or rapidly evolving regulatory or industry standards with respect to privacy and data protection and may inhibit our or our vendors’ ability to maintain an adequate level of service and experience. If we, our vendors, or our third-party partners experience an actual or perceived breach or privacy or security incident because of the use of generative artificial intelligence, we may lose valuable intellectual property and confidential information and our reputation and the public perception of the effectiveness of our security measures could be harmed. Further, bad actors around the world use increasingly sophisticated methods, including the use of artificial intelligence, to engage in illegal activities involving the theft and misuse of personal information, confidential information, and intellectual property. Any of these outcomes could damage our reputation, result in the loss of valuable property and information, and adversely impact our business.
Cybersecurity risks and cyber incidents could adversely affect our business and disrupt operations.
Cyber incidents can result from deliberate attacks or unintentional events. These incidents can include, but are not limited to, gaining unauthorized access to digital systems for purposes of misappropriating assets or sensitive information, corrupting data, or causing operational disruption. The result of these incidents could include, but are not limited to, disrupted operations, misstated financial data, liability for stolen assets or information, increased cybersecurity protection costs, litigation and reputational damage adversely affecting customer or investor confidence. We are in the process of implementing systems and processes to focus on identification, prevention, mitigation and resolution. However, these measures cannot provide absolute security, and our systems may be vulnerable to cybersecurity breaches such as viruses, hacking, and similar disruptions from unauthorized intrusions. In addition, we rely on third party service providers to perform certain services, such as payroll and tax services. Any failure of our systems or third-party systems may compromise our sensitive information and/or personally identifiable information of our employees or patient health information subject to HIPAA confidentiality requirements. While we are in the process of securing cyber insurance to potentially cover certain risks associated with cyber incidents, there can be no assurance the insurance will be sufficient to cover any such liability.
We may incur substantial costs as a result of litigation or other proceedings relating to patent and other intellectual property rights.
We may from time to time seek to enforce our intellectual property rights against infringers when we determine that a successful outcome is probable and may lead to an increase in the value of the intellectual property. If we choose to enforce our patent rights against a party, then that individual or company has the right to ask the court to rule that such patents are invalid or should not be enforced. Additionally, the validity of our patents and the patents we have licensed may be challenged if a petition for post grant proceedings such as inter-parties review and post grant review is filed within the statutorily applicable time with the U.S. Patent and Trademark Office (USPTO). These lawsuits and proceedings are expensive and would consume time and resources and divert the attention of managerial and scientific personnel even if we were successful in stopping the infringement of such patents. In addition, there is a risk that the court will decide that such patents are not valid and that we do not have the right to stop the other party from using the inventions. There is also the risk that, even if the validity of such patents is upheld, the court will refuse to stop the other party on the ground that such other party's activities do not infringe our intellectual property rights. In addition, in recent years the U.S. Supreme Court modified some tests used by the USPTO in granting patents over the past 20 years, which may decrease the likelihood that we will be able to obtain patents and increase the likelihood of a challenge of any patents we obtain or license.
If third parties claim that our products infringe their intellectual property rights, we may be forced to expend significant financial resources and management time defending against such actions and our financial condition and our results of operations could suffer.
Third parties may claim that our products infringe their patents and other intellectual property rights. Identifying third-party patent rights can be particularly difficult because, in general, patent applications can be maintained in secrecy for a prolonged period after their earliest priority date. Historically, there has been substantial litigation regarding patents and other intellectual property rights in the medical device and related industries. If a competitor were to challenge our patents or other intellectual property rights, or assert that our products infringe its patent or other intellectual property rights, we could incur substantial litigation costs, be forced to make expensive changes to our product design, pay royalties or other fees to license rights in order to continue manufacturing and selling our products, or pay substantial damages. Third-party infringement claims, regardless of their outcome, would not only consume our financial resources but also divert our management’s time and effort.
If we are unable to protect the intellectual property used in our products, others may be able to copy our innovations which may impair our ability to compete effectively in our markets.
The strength of our patents involves complex legal and scientific questions and can be uncertain. These patent applications may be challenged or fail to result in issued patents, or if issued, these patents and our existing patents may be too narrow to prevent third-parties from developing or designing around our intellectual property and in that event, we may lose competitive advantage, which could result in harm to our business.
We may be subject to claims that our employees have wrongfully used or disclosed alleged trade secrets of their former employers.
As is common in the medical device industry, we may employ individuals who were previously employed at other medical device companies, including our competitors or potential competitors. We may be subject to claims that these employees, or we, have used or disclosed trade secrets or other proprietary information of their former employers. Litigation may be necessary to defend against these claims. Even if we are successful in defending against these claims, litigation could result in substantial costs and be a distraction to management.
If we are unable to protect the confidentiality of our proprietary information and know-how, the value of our technology and products could be adversely affected.
In addition to patented technology, we rely upon, among other things, unpatented proprietary technology, processes, trade secrets and know-how. Any involuntary disclosure to or misappropriation by third-parties of our confidential or proprietary information could enable competitors to duplicate or surpass our technological achievements, potentially eroding our competitive position in our market. We seek to protect confidential or proprietary information in part by confidentiality agreements with our employees, consultants and third-parties. While we require all of our employees, consultants, advisors and any third-parties who have access to our proprietary know-how, information and technology to enter into confidentiality agreements, we cannot be certain that this know-how, information and technology will not be disclosed or that competitors will not otherwise gain access to our trade secrets or independently develop substantially equivalent information and techniques. These agreements may be terminated or breached, and we may not have adequate remedies for any such termination or breach. Furthermore, these agreements may not provide meaningful protection for our trade secrets and know-how in the event of unauthorized use or disclosure. To the extent that any of our staff were previously employed by other pharmaceutical, medical technology or biotechnology companies, those employers may allege violations of trade secrets and other similar claims in relation to their medical device development activities for us.
Risks Related to this Offering
There are material limitations with making preliminary estimates of our results for the period ended September 30, 2024, prior to the completion of our and our auditors’ financial review procedures for such period. Our independent registered public accounting firm has not conducted an audit or review of, and does not express an opinion or any other form of assurance with respect to, the preliminary unaudited results. It is possible that we, or our independent registered public accounting firm, may identify items that require us to make adjustments to the preliminary estimates of operating loss and net loss before income taxes from continuing operations.
The preliminary cash flow estimates contained in “Prospectus Summary - Preliminary Second Quarter Results (Unaudited)” are not a comprehensive statement of our cash flows for the period ended September 30, 2024. Our financial statements for the period ended September 30, 2024, will not be available until after this offering is completed and, consequently, will not be available to you prior to investing in this offering. Our actual results for the period ended September 30, 2024, may differ materially from the preliminary estimates we have provided as a result of the completion of our financial closing procedures, final adjustments, and other developments arising between now and the time that our financial results for such periods are finalized. You should not place undue reliance on the preliminary estimates, and the preliminary estimates are not necessarily indicative of the results to be expected in the future. The preliminary financial data included herein has been prepared by, and are the responsibility of, management. Forvis Mazars LLP, our independent registered public accounting firm, has not audited, reviewed, compiled, or performed any procedures with respect to such preliminary estimates. Accordingly, Forvis Mazars LLP does not express an opinion or any other form of assurance with respect thereto.
We have broad discretion in how we use the proceeds of this offering and may not use these proceeds effectively, which could affect our results of operations and cause our common stock to decline.
We will have considerable discretion in the application of the net proceeds of this offering. We intend to use the net proceeds from this offering primarily to fund our clinical trial, for other research and development, and for working capital. As a result, investors will be relying upon management’s judgment with only limited information about our specific intentions for the use of the net proceeds of this offering. We may use the net proceeds for purposes that do not yield a significant return or any return at all for our stockholders. In addition, pending their use, we may invest the net proceeds from this offering in a manner that does not produce income or that loses value.
We will require substantial funding, which may not be available to us on acceptable terms, or at all, and, if not so available, may require us to delay, limit, reduce or cease our operations.
We are using the proceeds from this offering to, among other uses, advance our lead product candidate toward commercial launch. Developing medical device products, including conducting clinical trials, is expensive. We will require substantial additional future capital in order to complete clinical development and commercialize our product candidate. If the FDA requires that we perform additional studies or clinical trials, our expenses would further increase beyond what we currently expect and the anticipated timing of any potential clearance of our product candidate would likely be delayed. Further, there can be no assurance that the costs we will need to incur to obtain regulatory approval will not increase.
We will continue to require substantial additional capital to continue our clinical development and commercialization activities. Because successful development of our product candidates is uncertain, we are unable to estimate the actual amount of funding we will require to complete research and development and commercialize our products under development.
We estimate that we will require additional financing of approximately $40 million to fund our operations through initial commercial launch. We believe that our existing cash and cash equivalents plus the proceeds from this offering will be sufficient to meet our projected operating requirements into but not beyond the second calendar quarter of 2025. Such projections are subject to changes in our internally funded preclinical and clinical activities, including unplanned preclinical and clinical activity. The timing and costs of clinical trials are difficult to predict and as such the foregoing estimates may prove to be inaccurate. We have no commitments for such additional needed financing and will likely be required to raise such financing through the sale of additional equity or debt securities.
The amount and timing of our future funding requirements will depend on many factors, including but not limited to:
|
● |
whether our plan for clinical trials will be completed on a timely basis; |
|
|
● |
the progress, costs, results of and timing of our clinical trials; |
|
|
● |
the outcome, costs and timing of seeking and obtaining FDA clearance and any other regulatory approvals; |
|
|
● |
the costs associated with securing and establishing commercialization and manufacturing capabilities; |
|
|
● |
market acceptance of our product candidates; |
|
|
● |
the costs of acquiring, licensing or investing in businesses, products, product candidates and technologies; |
|
|
● |
our ability to maintain, expand and enforce the scope of our intellectual property portfolio, including the amount and timing of any payments we may be required to make, or that we may receive, in connection with the licensing, filing, prosecution, defense and enforcement of any patents or other intellectual property rights; |
|
|
● |
our need and ability to hire additional management and scientific and medical personnel; |
|
|
● |
the effect of competing product candidates and new product approvals; and |
|
|
● |
our need to implement additional internal systems and infrastructure, including financial and reporting systems. |
Some of these factors are outside of our control. We may seek additional funding through a combination of equity offerings, debt financings, government or other third-party funding, commercialization, marketing and distribution arrangements and other collaborations, strategic alliances and licensing arrangements. Additional funding may not be available to us on acceptable terms or at all. In addition, the terms of any financing may adversely affect the holdings or the rights of our stockholders.
If we are unable to obtain funding on a timely basis, we may be required to significantly curtail one or more of our research or development programs. We also could be required to seek funds through arrangements with collaborative partners or otherwise that may require us to relinquish rights to some of our technologies or product candidates or otherwise agree to terms unfavorable to us.
Purchasers in this offering will experience immediate and substantial dilution in net tangible book value.
The public offering price per share will be substantially higher than the pro forma as adjusted net tangible book value per share of our common stock after giving effect to this offering. Assuming the sale of 698,812 shares of our common stock at an assumed public offering price of $14.31 per share, the closing sale price per share of our common stock on Nasdaq on October 31, 2024, after deducting the underwriting discounts and commissions and estimated offering expenses payable by us, you will incur immediate dilution in pro forma as adjusted net tangible book value of approximately $6.60 per share. As a result of the dilution to investors purchasing securities in this offering, investors may receive significantly less than the purchase price paid in this offering, if anything, in the event of the liquidation of our company. See the section entitled “Dilution” below for a more detailed discussion of the dilution you will incur if you participate in this offering. To the extent shares are issued under outstanding options, warrants or convertible notes at exercise or conversion prices lower than the public offering price of our common stock in this offering, you will incur further dilution.
Your ownership may be diluted if additional capital stock is issued to raise capital, to finance acquisitions or in connection with strategic transactions.
We will require additional, substantial financing in order to commercialize our product candidate. We intend to seek to raise additional funds for our operations, to finance acquisitions or to develop strategic relationships by issuing equity or convertible debt securities in addition to the securities issued in this offering, which would reduce the percentage ownership of our existing stockholders. Our board of directors has the authority, without action or vote of the stockholders, to issue all or any part of our authorized but unissued shares of common or preferred stock. Our certificate of incorporation authorizes us to issue up to 500,000,000 shares of common stock and 10,000,000 shares of preferred stock. Future issuances of common or preferred stock would reduce your influence over matters on which stockholders vote and would be dilutive to earnings per share. In addition, any newly issued preferred stock could have rights, preferences and privileges senior to those of the common stock. Those rights, preferences and privileges could include, among other things, the establishment of dividends that must be paid prior to declaring or paying dividends or other distributions to holders of our common stock or providing for preferential liquidation rights. These rights, preferences and privileges could negatively affect the rights of holders of our common stock, and the right to convert such preferred stock into shares of our common stock at a rate or price that would have a dilutive effect on the outstanding shares of our common stock.
If our stock price fluctuates after the offering, you could lose a significant part of your investment.
The market price of our common stock could be subject to wide fluctuations in response to, among other things, the risk factors described in this prospectus, and other factors beyond our control, such as fluctuations in the valuation of companies perceived by investors to be comparable to us. Furthermore, the stock markets have experienced price and volume fluctuations that have affected and continue to affect the market prices of equity securities of many companies. These fluctuations often have been unrelated or disproportionate to the operating performance of those companies. These broad market and industry fluctuations, as well as general economic, political, and market conditions, such as recessions, interest rate changes or international currency fluctuations, may negatively affect the market price of our common stock. In the past, many companies that have experienced volatility in the market price of their stock have been subject to securities class action litigation. We may be the target of this type of litigation in the future. Securities litigation against us could result in substantial costs and divert our management’s attention from other business concerns, which could seriously harm our business.
This offering may cause the trading price of our common stock to decrease.
The price per share, together with the number of shares of common stock we issue if this offering is completed, may result in an immediate decrease in the market price of our common stock. This decrease may continue after the completion of this offering.
We have never paid dividends on our capital stock, and we do not anticipate paying dividends in the foreseeable future.
We have never paid dividends on any of our capital stock and currently intend to retain any future earnings to fund the growth of our business. We may also enter into credit agreements or other borrowing arrangements in the future that will restrict our ability to declare or pay cash dividends on our common stock. Any determination to pay dividends in the future will be at the discretion of our board of directors and will depend on our financial condition, operating results, capital requirements, general business conditions and other factors that our board of directors may deem relevant. As a result, capital appreciation, if any, of the securities will be the sole source of gain, if any, for the foreseeable future.
Risks Related to our Common Stock
We completed a reverse stock split on October 24, 2024 in an effort to regain compliance with Nasdaq listing rules and we cannot predict the effect that such reverse stock split will have on the market price for shares of our common stock.
Our board of directors approved a 1-for-20 reverse stock split of our common stock, which became effective at 11:59 p.m. Eastern Time on October 24, 2024. We cannot predict the effect that the reverse stock split will have on the market price for shares of our common stock, and the history of similar reverse stock splits for companies in like circumstances has varied. Some investors may have a negative view of a reverse stock split. Even if the reverse stock split has a positive effect on the market price for shares of our common stock, performance of our business and financial results, general economic conditions and the market perception of our business, and other adverse factors which may not be in our control could lead to a decrease in the price of our common stock following the reverse stock split.
Furthermore, even if the reverse stock split does result in an increased market price per share of our common stock, the market price per share following the reverse stock split may not increase in proportion to the reduction of the number of shares of our common stock outstanding before the implementation of the reverse stock split. Accordingly, even with an increased market price per share, the total market capitalization of shares of our common stock after a reverse stock split could be lower than the total market capitalization before the reverse stock split. Also, even if there is an initial increase in the market price per share of our common stock after a reverse stock split, the market price many not remain at that level.
If the market price of shares of our common stock declines following the reverse stock split, the percentage decline as an absolute number and as a percentage of our overall market capitalization may be greater than would occur in the absence of the reverse stock split due to decreased liquidity in the market for our common stock. Accordingly, the total market capitalization of our common stock following the reverse stock split could be lower than the total market capitalization before the reverse stock split.
There is no public market for the Common Warrants or Pre-Funded Warrants being offered in this offering.
There is no public trading market for the Common Warrants or Pre-Funded Warrants being offered in this offering, and we do not expect a market to develop. In addition, we do not intend to apply to list the Common Warrants or Pre-Funded Warrants on any securities exchange or nationally recognized trading system, including Nasdaq. Without an active market, the liquidity of the Common Warrants and Pre-Funded Warrants will be limited.
Holders of Common Warrants and/or Pre-Funded Warrants purchased in this offering will have no rights as holders of our common stock with respect to the shares of common stock underlying such Common Warrants or Pre-Funded Warrants until such holders exercise their Common Warrants or Pre-Funded Warrants and acquire our common stock.
Except as set forth in the Common Warrants and the Pre-Funded Warrants, until holders of Common Warrants or Pre-Funded Warrants acquire shares of our common stock upon exercise of the Common Warrants or Pre-Funded Warrants, holders of Common Warrants or Pre-Funded Warrants will have no rights with respect to the shares of our common stock underlying such Common Warrants or Pre-Funded Warrants including with respect to voting rights, See “Description of Securities We Are Offering” for rights with respect to dividends. Upon exercise of the Common Warrants or Pre-Funded Warrants, the holders will be entitled to exercise the rights of a holder of our common stock only as to matters for which the record date occurs after the exercise date.
Provisions of the Series A Warrants could discourage an acquisition of us by a third party.
The Series A Warrants provide that in the event of a “Fundamental Transaction” (as defined in the related warrant agreement, which generally includes any merger with another entity, the sale, transfer or other disposition of all or substantially all of our assets to another entity, or the acquisition by a person of more than 50% of our common stock), each Series A Warrant holder will have the right at any time prior to the consummation of the Fundamental Transaction to require us to repurchase the common warrant for a purchase price in cash equal to the Black-Scholes value (as calculated under the warrant agreement) of the then remaining unexercised portion of such Series A Warrant on the date of such Fundamental Transaction, which may materially adversely affect our financial condition and/or results of operations and may prevent or deter a third party from acquiring us.
Concentration of ownership of our common stock among our existing executive officers and directors may prevent new investors from influencing significant corporate decisions.
Our executive officers and directors, and their affiliates, who are our principal stockholders, in the aggregate, beneficially own approximately 30.7% of our outstanding common stock prior to this offering. As a result, these persons, acting together, would be able to significantly influence all matters requiring stockholder approval, including the election and removal of directors, any merger, consolidation, sale of all or substantially all of our assets, or other significant corporate transactions. The minority stockholders have no way of overriding decisions made by our principal stockholders. This level of control may also have an adverse impact on the market value of our shares because our principal stockholders may institute or undertake transactions, policies or programs that result in losses and may not take any steps to increase our visibility in the financial community and/or may sell sufficient numbers of shares to significantly decrease our price per share.
Future issuances of our common stock underlying warrants and convertible notes could cause the market price of our common stock to decline and would result in the dilution of your holdings.
As of the date of this prospectus, we have outstanding warrants to purchase 112,534 shares of our common stock underlying at a weighted average exercise price of $8.26 per share; and outstanding convertible notes to purchase 33,250 shares of our common stock at an average conversion price of $40.00. Future issuances of our common stock underlying convertible securities could cause the market price of our common stock to decline. We cannot predict the effect, if any, of future issuances of our securities, on the price of our common stock. In all events, future issuances of our stock would result in the dilution of your holdings. In addition, the perception that new issuances of our securities could occur, could adversely affect the market price of our common stock.
If our stock price fluctuates, you could lose a significant part of your investment.
The market price of our common stock may be subject to wide fluctuations in response to, among other things, the risk factors described in this filing and other factors beyond our control, such as fluctuations in the valuation of companies perceived by investors to be comparable to us. Furthermore, the stock markets have experienced price and volume fluctuations that have affected and continue to affect the market prices of equity securities of many companies. These fluctuations often have been unrelated or disproportionate to the operating performance of those companies. These broad market and industry fluctuations, as well as general economic, political, and market conditions, such as recessions, interest rate changes or international currency fluctuations, may negatively affect the market price of our common stock. In the past, many companies that have experienced volatility in the market price of their stock have been subject to securities class action litigation. We may be the target of this type of litigation in the future. Securities litigation against us could result in substantial costs and divert our management’s attention from other business concerns, which could seriously harm our business.
Techniques employed by short sellers may in the future drive down the market price of our common stock.
Short selling is the practice of selling securities that the seller does not own but rather has borrowed from a third-party with the intention of buying identical securities back at a later date to return to the lender. The short seller hopes to profit from a decline in the value of the securities between the sale of the borrowed securities and the purchase of the replacement shares, as the short seller expects to pay less in that purchase than it received in the sale. As it is in the short seller’s best interests for the price of the stock to decline, many short sellers publish, or arrange for the publication of, negative opinions regarding the relevant issuer and its business prospects in order to create negative market momentum and generate profits for themselves after selling a stock short. These short attacks have led to selling of shares in the market. Issuers that have common stock with limited trading volumes and/or have been susceptible to relatively high volatility levels, can be particularly vulnerable to such short seller attacks. The publication of any such articles regarding us in the future may bring about a temporary, or possibly long-term, decline in the market price of our common stock. If we continue to be the subject of unfavorable allegations, we may have to expend a significant amount of resources to investigate such allegations and/or defend ourselves. While we would strongly defend against any such short seller attacks, we may be constrained in the manner in which we can proceed against the relevant short seller by applicable state law or issues of commercial confidentiality. Such a situation could be costly, and time-consuming, and could be distracting for our management team.
If securities or industry analysts do not publish research or reports about us, or if they adversely change their recommendations regarding our common stock, then our stock price and trading volume could decline.
The trading market for our common stock is influenced by the research and reports that industry or securities analysts publish about us, our industry and our market. If no analyst elects to cover us and publish research or reports about us, the market for our common stock could be severely limited and our stock price could be adversely affected. As a small-cap company who has recently completed its initial public offering (“IPO”) pursuant to Regulation A, we are more likely than our larger competitors to lack coverage from securities analysts. In addition, even if we receive analyst coverage, if one or more analysts ceases coverage of us or fails to regularly publish reports on us, we could lose visibility in the financial markets, which in turn could cause our stock price or trading volume to decline. If one or more analysts who elect to cover us issue negative reports or adversely change their recommendations regarding our common stock, our stock price could decline.
As an “emerging growth company” under the Jumpstart Our Business Startups Act, or JOBS Act, we are permitted to, and intend to, rely on exemptions from certain disclosure requirements.
As an “emerging growth company” under the JOBS Act, we are permitted to, and intend to, rely on exemptions from certain disclosure requirements. We are an emerging growth company until the earliest of:
|
● |
the last day of the fiscal year during which we have total annual gross revenues of $1.235 billion or more; |
|
● |
the last day of the fiscal year following the fifth anniversary of the date of the first sale of our common equity pursuant to an effective registration statement under the Securities Act; |
|
● |
the date on which we have, during the previous 3-year period, issued more than $1 billion in non-convertible debt; or |
|
● |
the date on which we are deemed a “large accelerated issuer” as defined under the federal securities laws. |
For so long as we remain an emerging growth company, we will not be required to:
|
● |
have an auditor report on our internal control over financial reporting pursuant to the Sarbanes-Oxley Act of 2002; |
|
● |
comply with any requirement that may be adopted by the Public Company Accounting Oversight Board regarding mandatory audit firm rotation or a supplement to the auditor’s report providing additional information about the audit and the financial statements (auditor discussion and analysis); |
|
● |
submit certain executive compensation matters to shareholders advisory votes pursuant to the “say on frequency” and “say on pay” provisions (requiring a non-binding shareholder vote to approve compensation of certain executive officers) and the “say on golden parachute” provisions (requiring a non-binding shareholder vote to approve golden parachute arrangements for certain executive officers in connection with mergers and certain other business combinations) of the Dodd-Frank Wall Street Reform and Consumer Protection Act of 2010; |
|
● |
include detailed compensation discussion and analysis in our filings under the Securities Exchange Act of 1934, as amended, and instead may provide a reduced level of disclosure concerning executive compensation; |
|
● |
may present only two years of audited financial statements and only two years of related Management’s Discussion and Analysis of Financial Condition and Results of Operations, or MD&A; and |
|
● |
are eligible to claim longer phase-in periods for the adoption of new or revised financial accounting standards under §107 of the JOBS Act. |
We intend to take advantage of all of these reduced reporting requirements and exemptions.
|
● |
Certain of these reduced reporting requirements and exemptions will already be available to us due to the fact that we also qualify as a “smaller reporting company” under Commission rules. For instance, smaller reporting companies are not required to obtain an auditor attestation and report regarding management’s assessment of internal control over financial reporting; are not required to provide a compensation discussion and analysis; are not required to provide a pay-for-performance graph or CEO pay ratio disclosure; and may present only two years of audited financial statements and related MD&A disclosure. |
We cannot predict if investors will find our securities less attractive due to our reliance on these exemptions. If investors were to find our common stock less attractive as a result of our election, we may have difficulty raising all of the proceeds in any future offering.
Our failure to meet the continued listing requirements of the Nasdaq could result in de-listing of our common stock.
If we fail to satisfy the continued listing requirements of the Nasdaq, such as the minimum closing bid price requirement, the Nasdaq may take steps to de-list our common stock. Such a de-listing would likely have a negative effect on the price of our common stock and would impair your ability to sell or purchase our common stock when you wish to do so. In the event of a de-listing, we would take actions to try to restore our compliance with the Nasdaq marketplace rules, but our common stock may not be listed again, and such actions may not stabilize the market price or improve the liquidity of our common stock, prevent our common stock from dropping below the Nasdaq minimum bid price requirement or prevent future non-compliance with the Nasdaq marketplace rules.
Our Certificate of Incorporation includes a forum selection provision, which could result in less favorable outcomes to the plaintiff(s) in any action against us.
Our Certificate of Incorporation includes a forum selection provision that requires any claims against us by stockholders not arising under the federal securities laws to be brought in the Court of Chancery State in the state of Delaware. This forum selection provision may limit investors’ ability to bring claims in judicial forums that they find favorable to such disputes and may discourage lawsuits with respect to such claims. In addition, this forum selection provision may impose additional litigation costs on stockholders in pursuing the claims identified above, particularly if the stockholders do not reside in or near the State of Delaware.
The requirements of being a public company may strain our resources, divert management’s attention and affect our ability to attract and retain qualified board members.
As a public company, we incur accounting, legal and other expenses that we did not incur as a private company. We incur costs associated with our public company reporting requirements. We also incur costs associated with corporate governance requirements, including requirements under the Sarbanes-Oxley Act of 2002, as well as rules and regulations implemented by the United States Securities and Exchange Commission (“SEC”) and Nasdaq. We expect these rules and regulations to increase our legal and financial compliance costs and to make some activities more time-consuming and costly. Furthermore, these rules and regulations could make it more difficult or costlier for us to obtain certain types of insurance, including director and officer liability insurance, and we may be forced to accept reduced policy limits and coverage or incur substantially higher costs to obtain the same or similar coverage. The impact of these requirements could also make it more difficult for us to attract and retain qualified persons to serve on our board of directors, our board committees or as executive officers. We are currently evaluating and monitoring developments with respect to these rules and regulations and we cannot predict or estimate the amount of additional costs we may incur or the timing of such costs.
We may be at an increased risk of securities class action litigation.
Historically, securities class action litigation has often been brought against a company following a decline in the market price of its securities. This risk is especially relevant for us because biotechnology companies have experienced significant stock price volatility in recent years. If we were to be sued, it could result in substantial costs and a diversion of management’s attention and resources, which could harm our business.
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus contains certain forward-looking statements that involve substantial risks and uncertainties. All statements contained in this prospectus, other than statements of historical facts, are forward-looking statements including statements regarding our strategy, future operations, future financial position, future revenue, projected costs, prospects, plans, objectives of management and expected market growth. These statements involve known and unknown risks, uncertainties and other important factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements.
The words “anticipate”, “believe”, “estimate”, “expect”, “intend”, “may”, “plan”, “predict”, “project”, “target”, “potential”, “will”, “would”, “could”, “should”, “continue” and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. These forward-looking statements include, among other things, statements about:
|
• |
our ability to continue as a going concern in the near term is dependent upon us successfully raising additional equity or debt financing to fund our operations; |
|
• |
the success of our future clinical trials; |
|
• |
we currently have no source of product sales revenue; |
|
• |
competition from existing products or new products that may emerge; |
|
• |
if we fail to comply with U.S. and foreign regulatory requirements, regulatory authorities could limit or withdraw any marketing or commercialization approvals we may receive and subject us to other penalties that could materially harm our business; |
|
• |
we may be unable to obtain U.S. or foreign regulatory approval and, as a result, unable to commercialize our product candidates; |
|
• |
the implementation of our business model and strategic plans for our business, technologies and product candidates; |
|
• |
potential product liability claims; |
|
• |
our dependency on third-party supply and manufacturing partners to supply the materials and components for, and manufacture, our research and development, preclinical and clinical trial devices; |
|
• |
our ability to establish or maintain collaborations, licensing or other arrangements and retain commercial rights for our product candidates subject to collaborations; |
|
• |
our ability and third parties’ abilities to protect intellectual property rights and our ability to operate our business without infringing the intellectual property rights of others; |
|
• |
our ability to adequately support future growth; |
|
• |
our estimates of our expenses, ongoing losses, future revenue and capital requirements; |
|
• |
our ability to attract and retain key management personnel and technical personnel to manage our business effectively; |
|
• |
risks associated with our identification of material weaknesses in our control over financial reporting; |
|
• |
our use of net proceeds received by us from any subsequent private placement or public financing; |
|
• |
natural disasters affecting us, our primary manufacturer or our suppliers; |
|
• |
our ability to establish relationships with health care professionals and organizations; |
|
• |
general economic uncertainty that adversely affects spending on medical procedures; |
|
• |
volatility in the market price of our stock; and |
|
• |
potential dilution to current stockholders from the issuance of equity awards. |
These forward-looking statements are only predictions and we may not actually achieve the plans, intentions or expectations disclosed in our forward-looking statements, so you should not place undue reliance on our forward-looking statements. Actual results or events could differ materially from the plans, intentions and expectations disclosed in the forward-looking statements we make. We have based these forward-looking statements largely on our current expectations and projections about future events and trends that we believe may affect our business, financial condition and operating results. We have included important factors in the cautionary statements included in this prospectus that could cause actual future results or events to differ materially from the forward-looking statements that we make. Our forward-looking statements do not reflect the potential impact of any future acquisitions, mergers, dispositions, joint ventures or investments we may make.
You should read this prospectus with the understanding that our actual future results may be materially different from what we expect. We do not assume any obligation to update any forward-looking statements whether as a result of new information, future events or otherwise, except as required by applicable law.
USE OF PROCEEDS
We estimate that the net proceeds from the offering will be approximately $9.0 million, assuming we complete the maximum offering pursuant to this prospectus, after deducting underwriting discounts and commissions and estimated offering expenses payable by us, assuming no exercise of the underwriter’s over-allotment option. These estimates exclude the proceeds, if any, from the exercise of Common Warrants and Pre-Funded Warrants, if any, sold in this offering. We will receive nominal proceeds, if any, upon exercise of the Pre-Funded Warrants.
We intend to use the net proceeds for (i) our clinical trial; (ii) other research and development; (iii) development of intellectual property and (iv) working capital.
We will require significant additional financing even if we complete the offering hereunder to complete our clinical trials and to commercial our product candidate. We have no commitments for such additional needed financing, and will likely be required to raise such financing through the sale of additional equity securities, which may occur at prices lower than the offering price of our common stock in this offering.
As of the date of this prospectus, we cannot specify with certainty all of the particular uses for the net proceeds to us from this offering. Accordingly, our management will have broad discretion in the application of these proceeds. Net offering proceeds not immediately applied to the uses summarized above will be invested in short-term investments such as money market funds, commercial paper, U.S. treasury bills and similar securities investments pending their use.
DIVIDEND POLICY
To date, we have not paid any dividends on our common stock and do not anticipate paying any dividends in the foreseeable future. The declaration and payment of dividends on the common stock is at the discretion of our Board of Directors and will depend on, among other things, our operating results, financial condition, capital requirements, contractual restrictions or such other factors as our Board of Directors may deem relevant. We currently expect to use all available funds to finance the future development and expansion of our business and do not anticipate paying dividends on our common stock in the foreseeable future.
DILUTION
If you invest in our securities in this offering, your interest will be diluted immediately to the extent of the difference between the public offering price paid by the purchasers of the Common Stock Units or PFW Units sold in this offering and the as adjusted net tangible book value per shares of common stock after this offering.
As of June 30, 2024, our net tangible book value was $5.3 million, or $5.50 per share of common stock. Net tangible book value per share represents our total tangible assets, less our total liabilities, divided by the number of outstanding shares of our common stock.
Our pro forma net tangible book value as of June 30, 2024 was $5.3 million, or $4.59 per share of common stock. Pro forma net tangible book value per share gives effect to: (i) the issuance of 12,500 shares of common stock in connection with the RFI license agreement in July 2024; and (ii) the issuance of 177,246 shares of common stock upon the exercise of warrants on a cashless basis subsequent to June 30, 2024.
Dilution represents the difference between the amount per Unit paid by purchasers in this offering and the pro forma as adjusted net tangible book value per share of common stock after the offering. After giving effect to the pro forma adjustments above and the sale of 698,812 Units of common stock in this offering at an assumed offering price of $14.31 per Unit, which was the closing price of our common stock as reported on Nasdaq on October 31, 2024, and after deducting underwriting discounts and commissions and estimated offering expenses payable by us, but excluding the proceeds to us, if any, from the exercise of the Common Warrants and Pre-Funded Warrants issued pursuant to this offering and without adjusting for any other change in our net tangible book value subsequent to June 30, 2024, our pro forma as adjusted net tangible book value would have been $7.71 per share. This represents an immediate increase in net tangible book value of $3.12 per share to our existing stockholders on a pro forma basis and immediate dilution of $6.60 per share to new investors purchasing securities at the proposed public offering price. The following table illustrates the dilution in net tangible book value per share to new investors as of June 30, 2024:
|
Assumed public offering price per Unit |
$ | 14.31 | ||||||
|
Historical net tangible book value per share at June 30, 2024 |
$ | 5.50 | ||||||
|
Pro forma net tangible book value per share as of June 30, 2024 |
$ | 4.59 | ||||||
|
Increase in net tangible book value per share to the existing stockholders attributable to this offering on a pro forma basis. |
$ | 3.12 | ||||||
|
Pro forma as adjusted net tangible book value per share after this offering |
$ | 7.71 | ||||||
|
Dilution in net tangible book value per share to new investors |
$ | 6.60 |
Each $1.00 increase (decrease) in the assumed public offering price of $14.31 per share, would increase (decrease) our as adjusted net tangible book value per share to existing investors by $0.35, and would increase (decrease) dilution per share to new investors in this offering by $0.65, assuming that the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same, and after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us. We may also increase or decrease the number of securities to be issued in this offering. Each increase (decrease) of 100,000 shares offered by us would increase (decrease) our as adjusted net tangible book value per share by $0.71 and the dilution per share to new investors purchasing securities in this offering by $0.71 assuming that the assumed public offering price remains the same, and after deducting underwriting discounts and commissions and estimated offering expenses payable by us. The information discussed above is illustrative only and will be adjusted based on the actual public offering price and other terms of this offering as determined between us and the underwriters at pricing.
The number of shares of common stock to be outstanding after this offering is based on 962,374 shares outstanding as of June 30, 2024 and excludes:
|
● |
292,234 shares of common stock underlying outstanding warrants at a weighted average exercise price of $3.30 per share; |
|
● |
216,483 shares of common stock underlying outstanding options with a weighted average exercise price of $36.43 per share; |
|
● |
33,250 shares of common stock underlying outstanding convertible notes at a weighted average conversion price of $40.00 per share; |
|
● |
75,633 shares available for future issuance under the Autonomix Medical, Inc. 2023 Stock Plan; |
| ● |
698,812 shares of common stock underlying the Common Warrants issuable in this offering; and |
|
● |
41,928 shares of common stock underlying the Representative Warrants issuable to the underwriter in connection with this offering. |
We may choose to raise additional capital due to market conditions or strategic considerations even if we believe we have sufficient funds for our current or future operating plans. To the extent that additional capital is raised through the sale of equity or convertible debt securities, the issuance of these securities could result in further dilution to our stockholders.
CAPITALIZATION
The following table sets forth our cash and cash equivalents and capitalization as of June 30, 2024:
|
• |
on an actual basis; |
|
• |
on a pro forma basis to give effect to (i) the issuance of 12,500 shares of common stock in connection with the RFI license agreement in July 2024; and (ii) the issuance of 177,246 shares of common stock upon the exercise of warrants on a cashless basis subsequent to June 30, 2024; |
|
• |
on a pro forma adjusted basis to give further effect to (i) the issuance and sale of 698,812 Units in this offering at an assumed offering price of $14.31 per Unit, which was the closing price of our common stock as reported on Nasdaq on October 31, 2024, after deducting the underwriting discounts and commissions and estimated offering expenses payable by us. |
Our capitalization following the closing of this offering will be adjusted based on the actual public offering price and other terms of this offering determined at pricing. You should read this table in conjunction with “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our financial statements and related notes appearing elsewhere in this prospectus.
|
Adjusted |
||||||||||||
|
Actual |
Pro Forma |
Pro Forma |
||||||||||
|
(in thousands, except par value and share data) |
||||||||||||
|
Cash and cash equivalents |
$ | 6,751 | $ | 6,751 | $ | 15,731 | ||||||
|
Notes Payable |
$ | 1,043 | $ | 1,043 | $ | 1,043 | ||||||
|
Stockholders’ equity: |
||||||||||||
|
Common stock, par value $0.001 per share: 500,000,000 shares authorized as of June 30, 2024; 962,374 shares issued and outstanding as of June 30, 2024; 1,152,120 shares issued and outstanding pro forma; and 1,850,932 shares issued and outstanding adjusted pro forma |
$ | 1 | $ | 1 | $ | 2 | ||||||
|
Additional paid-in capital |
$ | 46,956 | $ | 47,056 | $ | 56,036 | ||||||
|
Accumulated deficit |
$ | (41,668 | ) | $ | (41,768 | ) | $ | (41,768) | ||||
|
Total stockholders’ equity |
$ | 5,289 | $ | 5,289 | $ | 14,269 | ||||||
|
Total capitalization |
$ | 6,332 | $ | 6,332 | $ | 15,312 | ||||||
|
(1) |
An $1.00 increase or decrease in the assumed public offering price of $14.31 per Unit, which was the closing price of our common stock as reported on NASDAQ on October 31, 2024, would increase or decrease, respectively, our pro forma as adjusted cash and cash equivalents, additional paid-in capital, total stockholders’ equity, and total capitalization by approximately $642,907 assuming the number of securities offered by us, as set forth on the cover page of this prospectus, remains the same, after deducting underwriting discounts and commissions and estimated offering expenses payable by us. We may also increase or decrease the number of securities to be issued in this offering. An increase or decrease of 100,000 in the number of Units offered by us would increase or decrease, respectively, our pro forma as adjusted cash and cash equivalents, additional paid-in capital, total stockholders’ equity, and total capitalization by $1,316,520 assuming that the assumed public offering price remains the same, after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us. The information discussed above is illustrative only and will be adjusted based on the actual public offering price and other terms of this offering as determined between us and the underwriters. |
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND
RESULTS OF OPERATIONS
You should read the following discussion and analysis of our financial condition and results of operations in conjunction with the financial statements and the related notes appearing elsewhere in this prospectus. This discussion contains forward-looking statements reflecting our current expectations that involve risks and uncertainties, including those set forth under "Cautionary Statement About Forward-Looking Statements." Actual results and the timing of events could differ materially from those discussed and other expectations expressed in our forward-looking statements as a result of many factors, including but not limited to those discussed herein and in the "Risk Factors” section above.
Overview
We are a development stage medical device development company focused on advancing innovative technologies for sensing and treating disorders relating to the nervous system. Our first-in-class technology platform includes a catheter-based microchip-enabled sensing array that can detect and differentiate neural signals with a high degree of sensitivity as demonstrated in animal studies. We calculate sensitivity in units of minimum signal detection voltage in micro volts (uV) time area of the electrode (square millimeters). It is a combined measure that is related to the signal resolving power and spatial resolution of the system. For the BSC Orion, the nearest device on the market, the metrics are 10uV for signal detection levels, and roughly 0.4mm by 0.5mm for the electrode dimensions. For the Autonomix device, the metrics are <1uV for signal detection levels and roughly 0.02mm by 0.03mm for the electrode dimensions. The differences in these metrics result in a calculation of 3,000 times greater sensitivity for the Autonomix device. We believe, if we can recreate these results in clinical trials, this will enable a method of transvascular targeting, treating, and confirming treatment of diseases involving the nervous system throughout the body that is not currently available and may be capable of filling a wide range of unmet medical needs.
We are initially developing our technology for patients with pancreatic cancer, a condition that can cause debilitating pain and need a more effective solution. However, we believe our technology constitutes a platform with the potential to address dozens of indications in a range of areas including chronic pain management from all causes, hypertension, cardiovascular disease and a wide range of other nerve-related disorders.
Our development efforts can be divided into to two sub parts: sensing and treatment, where sensing is focused on identifying neuronal activity that may be associated with a disorder with enough precision to enable targeted therapy with ablation. While the treatment may vary depending on the disorder, in our initial indications this will involve energy-based ablation (deliberate tissue damage, also referred to as denervation) intended to stop unwanted neuronal activity.
Our sensing catheter has already been developed sufficiently to demonstrate in animal models successful identification of a signal from a specific nerve before ablation and confirmation of termination of the signal from the treated nerve after ablation. We are now in the process of improving the assembly of this catheter to meet the standards required for human use and developing an RF ablation catheter designed specifically for treatment in the vessels of the pancreatic region. In parallel with this effort, we are currently conducting a first-in-human demonstration of transvascular ablation (without the use of our sensing technology) to relieve pain associated with pancreatic cancer. Once these two efforts are completed, we plan to bring sensing and treatment together in a pivotal clinical trial to enable the regulatory clearance and commercial launch of our technology. As stated above, we are a development stage company and there is no guarantee that the results of any trials will produce positive results or that the results will support our claims.
Recent Developments
On July 10, 2024, we entered into a license agreement (the “Agreement”) with RF Innovations, Inc. (“RFI”), a privately held medical technology company, to license products utilizing RFI’s intellectual property related to its Apex 6 Radiofrequency Generator (the “Licensed Products”). The Apex 6 Generator is a United States Food and Drug Administration (“FDA”) cleared ablation technology designed to lesion neural tissue for pain management in the peripheral nervous system. Pursuant to the Agreement, RFI granted us a perpetual non-exclusive worldwide royalty free fully paid license related to the Licensed Products, provided that the license did not include the right to sell certain products to customers for the treatment of spine pain. In connection with the Agreement, we issued RFI 12,500 unregistered shares of our common stock as consideration for the license. The Agreement provides RFI the right to terminate the license if we breach any representation, warranty or covenant contained in the Agreement, subject to any relevant cure periods, or if we are subject to a bankruptcy or insolvency event.
On January 26, 2024, we consummated our IPO. In the IPO, we sold a total of 111,962 shares of common stock at a purchase price of $100.00 per share for gross proceeds of $11.2 million and net proceeds of $9.8 million. On May 13, 2024, we cancelled 53 shares represented in the IPO for payment disputes. In connection with the closing of the IPO, a portion of our convertible notes were converted into 16,750 shares of our common stock. Upon the closing of the IPO, certain notes were to be automatically converted according to their terms into our common stock to the extent and provided that certain holders of these notes are not permitted to convert such notes to the extent that the holders or any of its affiliates would beneficially own in excess of 4.99% of our common stock after such conversion. Due to this 4.99% limitation, principal representing $1.3 million, or 33,250 shares, of these notes remains outstanding.
On January 26, 2024, as part of our IPO, we issued a warrant to purchase 2,989 shares pursuant to the agreement with the selling agent in our IPO. These warrants equaled approximately 2.675% of the 111,962 shares sold in our IPO.
On January 29, 2024, we issued a warrant to purchase 80,000 shares (the “Warrant”) pursuant to the Termination Agreement noted in Note 6 – Related Party Transactions. The shares underlying the Warrant are subject to a lockup agreement for a period of six months after the closing of the IPO with respect to 12.5% of the shares issued and twelve months after the closing of the IPO for the remainder of the shares. In connection with the Termination Agreement, the Company agreed to register the resale of the shares of common stock underlying the Warrant.
Results of Operations for the Three Months Ended June 30, 2024 Compared to the Three Months Ended June 30, 2023
Below is a summary of the results of operations (in thousands):
|
Three Months Ended June 30, |
||||||||||||||||
|
Change |
Change |
|||||||||||||||
|
2024 |
2023 |
( $ ) |
( % ) |
|||||||||||||
|
Operating expenses: |
||||||||||||||||
|
General and administrative |
$ | 1,799 | $ | 503 | $ | 1,296 | 258 | % | ||||||||
|
Research and development |
954 | 368 | 586 | 159 | % | |||||||||||
|
Total operating expenses |
$ | 2,753 | $ | 871 | $ | 1,882 | 216 | % | ||||||||
General and Administrative Expense
General and administrative expense was $1.8 million for the three months ended June 30, 2024 compared to $0.5 million for the same period in 2023. This $1.3 million increase was driven primarily by increases in officer and employee compensation and benefits of $0.6 million, as we expanded our management team, stock-based compensation of $0.3 million, legal and professional fees of $0.2 million, insurance expense of $0.1 million, State of Delaware franchise tax of $0.1 million and other expenses of $0.1 million, offset by a decrease in advertising of $0.1 million
Research and Development Expense
Research and development expense was $1.0 million for the three months ended June 30, 2024 compared to $0.4 million for the same period in 2023. The increase in research and development expenses during the current year was mainly attributed to clinical trial planning and development cost. We expect to incur increased research and development costs in the future as we continue our clinical trial.
Interest expense
For the three months ended June 30, 2024, we had interest expense of less than $0.1 million, related to the amortization of debt discount. Interest expense was $0 during the three months ended June 30, 2023 as there was no comparable instrument or expense in the prior period.
Interest income
For the three months ended June 30, 2024, we had interest income of $0.1 million. Interest income for the three months ended June 30, 2023 was less than $0.1 million due to relatively lower cash balances.
Results of Operations for the Year Ended March 31, 2024 Compared to the Year Ended March 31, 2023
Below is a summary of the results of operations (in thousands):
|
Year Ended March 31, |
||||||||||||||||
|
Change |
Change |
|||||||||||||||
|
2024 |
2023 |
( $ ) |
( % ) |
|||||||||||||
|
Operating expenses: |
||||||||||||||||
|
General and administrative |
$ | 5,249 | $ | 1,245 | $ | 4,004 | 322 | % | ||||||||
|
Research and development |
2,225 | 745 | 1,480 | 199 | % | |||||||||||
|
Warrant expense - termination agreement |
4,556 | — | 4,556 | — | ||||||||||||
|
Total operating expenses |
$ | 12,030 | $ | 1,990 | $ | 10,040 | 505 | % | ||||||||
General and Administrative (G&A). G&A expenses increased by $4.0 million compared to the same period in 2023, primarily due to increases in advertising of $1.7 million related to our IPO, officer and employee compensation and benefits of $0.7 million, stock-based compensation of $0.6 million, professional fees of $0.6 million, legal fees of $0.2 million, insurance expense of $0.1 million and travel expense of $0.1 million.
Research and Development (R&D). R&D expenses increased by $1.5 million compared to the same period in 2023, primarily due to clinical trial execution and product development cost. We expect to incur increased research and development costs in the future as we continue our clinical trial and product development efforts.
Warrant expense – termination agreement
We had warrant expense of $4.6 million related to a license termination agreement. See Note 2 - Warrant Liability and Fair Value of Financial Instruments to the financial statements for additional information. Warrant Expense – termination agreement was $0 during the same period in 2023 as there was no comparable instrument.
Warrant liability mark-to-market
We had expense for mark-to-market adjustments of warrants of $3.4 million. Warrant Liability - mark-to market adjustment was $0 during the same period in 2023 as there was no comparable instrument.
Interest expense
We had interest expense of less than $0.1 million, related to the amortization of debt discounts. Interest expense was $0 during the same period in 2023 as there was no comparable instrument.
Interest income
We had interest income of $0.1 million. Interest income for the same period in 2023 was $0.
Liquidity and Capital Resources
On June 30 2024, we had cash of $6.8 million and working capital of $6.3 million. We have historically funded our operations from proceeds from debt and equity sales. In June 2023, we completed a financing with several accredited investors for the sale of 71,001 shares of common stock with gross proceeds of $2.8 million. Additionally, the Company received proceeds of $2.0 million in unsecured, non-interest bearing convertible promissory notes (the “Notes”) and accompanying warrants (the “Bridge Financing Warrants”) (collectively, the “Bridge Offering”) that will mature on December 31, 2025. On January 26, 2024, we completed our IPO of common stock. In the IPO, we sold a total of 111,962 shares of common stock at a purchase price of $100.00 per share for gross proceeds of $11.2 million and net proceeds of $9.8 million. On May 13, 2024, we cancelled 53 shares represented in the IPO for payment disputes. We estimate our current cash resources are sufficient to fund our operations into but not beyond the second calendar quarter of 2025.
Our plan of operations is primarily focused on developing our product candidate, with the product candidate in the proof-of-concept stage at this time. We are initially focusing on the treatment of pain associated with pancreatic cancer and we have designed our commercialization efforts around this as our first proposed indication for use.
We will need to raise additional capital to meet our obligations and execute our business plan. We estimate that we will require additional financing of approximately $40 million to fund our operations through clinical phase. The timing and costs of clinical trials are difficult to predict and trial plans may change in response to evolving circumstances and as such the foregoing estimates may prove to be inaccurate. If we are unable to raise sufficient funds, we will be required to develop and implement an alternative plan to further extend payables, reduce overhead or scale back our business plan until sufficient additional capital is raised to support further operations. There can be no assurance that such a plan will be successful. The Company recognizes it will need to raise additional capital to continue to execute its business plan, including obtaining regulatory clearance for its products currently under development and commercializing and generating revenues from products under development. There is no assurance that additional financing will be available when needed or that management will be able to obtain financing on terms acceptable to the Company. A failure to raise sufficient capital, generate sufficient product revenues, control expenditures and regulatory matters, among other factors, will adversely impact the Company’s ability to meet its financial obligations as they become due and payable and to achieve its intended business objectives. If the Company is unable to raise sufficient additional funds, it will have to scale back its operations.
Summary of Cash Flows for the Three Months Ended June 30, 2024 and 2023
Cash used in operating activities
Net cash used in operating activities was $1.9 million during the three months ended June 30, 2024, consisting of a net loss of $2.7 million and a decrease in operating assets and liabilities of $0.5 million. The change in operating assets and liabilities included sources of cash from a decrease in other current assets of $0.5 million and an increase in accrued expenses of $0.2 million offset by a use of cash decrease for accounts payable of $0.2 million. The decrease in other current assets was driven primarily by the receipt of funds from our marketing partner that were a holdback from our IPO and the amortization of prepaid insurance costs. The increase in accrued expenses and the decrease in accounts payable are offsetting and are driven primarily by the timing of receipt of vendor invoices. Non-cash items consisted of stock-based compensation of $0.4 million.
Cash used in investing activities
Net cash used in investing activities was $5 thousand for the three months ended June 30, 2024 related to the purchase of computer hardware and software.
Cash provided by financing activities
Net cash provided by financing activities was zero for the three months ended June 30, 2024.
Net cash provided by financing activities was $2.7 million for the three months ended June 30, 2023 consisting of $2.8 million from the sale of common stock. We also paid $0.1 million in offering costs related to our IPO.
Summary of Cash Flows for the Years Ended March 31, 2024 and 2023
Cash used in operating activities
Net cash used in operating activities was $6.6 million during the year ended March 31, 2024, consisting of a net loss of $15.4 million and a change in operating assets and liabilities of $0.1 million. The change in operating assets and liabilities included sources of cash from an increase in accounts payable of $0.3 million and accrued expenses of $0.3 million offset by a use of cash for other current assets of $0.5 million. The increases in accounts payable and accrued expenses were driven primarily by increased research and development costs for the development of our medical devices, general and administrative costs consisting of professional fees, officer compensation and legal expenses. The increase in other current assets was driven primarily by prepaid insurance costs. Non-cash items consisted of $4.6 million for warrant expense – termination agreement, $3.4 million for warrant liability – mark-to-market adjustment, stock-based compensation of $0.6 million and depreciation and amortization of $0.1 million.
Net cash used in operating activities was $1.9 million during the year ended March 31, 2023, consisting of a net loss of $2.0 million and an increase in operating assets and liabilities of $0.1 million, which primarily consisted of an increase in accounts payable.
Cash used in investing activities
Net cash used in investing activities was $19 thousand for the year ended March 31, 2024, related to the purchase of computer hardware and software.
Net cash used in investing activities was $0 for the year ended March 31, 2023.
Cash provided by financing activities
Net cash provided by financing activities was $14.4 million for the year ended March 31, 2024 consisting of $10.9 million of gross proceeds from the sale of common stock related to our IPO, $2.8 million from the sale of common stock and $2.0 million of cash proceeds from convertible notes. We also paid $1.3 million in issuance costs related to our IPO.
Net cash provided by financing activities was $0.7 million for the year ended March 31, 2023, comprised of $0.7 million from the sale of common stock.
Contractual Obligations and Commitments
None.
Employment Arrangements
We have agreements with key employees to provide certain benefits, including salary and other wage-related benefits, in the event of termination. In addition, we have adopted a severance policy for certain key members of executive management to provide certain benefits, including salary and other wage-related benefits, in the event of termination without cause. In total, these benefits would amount to a range of $1.1 million to $1.6 million using the rate of compensation in effect at June 30, 2024.
Off-balance Sheet Arrangements
As of June 30, 2024 and March 31, 2024, we did not have any relationships with unconsolidated entities or financial partnerships, such as entities often referred to as structured finance or special purpose entities, established for the purpose of facilitating off-balance sheet arrangements or other contractually narrow or limited purposes.
Critical Accounting Estimates
The financial statements in this prospectus have been prepared in accordance with generally accepted accounting principles in the United States of America (“GAAP”). The preparation of financial statements in conformity with GAAP requires management to make estimates, assumptions and judgments that affect the amounts reported in the financial statements, including the notes thereto. We consider critical accounting policies to be those that require more significant judgments and estimates in the preparation of our financial statements, including the following: research and development expenses, warrants, and stock-based compensation. Management relies on historical experience and other assumptions believed to be reasonable in making its judgments and estimates. Actual results could differ materially from those estimates.
Management believes its application of accounting policies, and the estimates inherently required therein, are reasonable. These accounting policies and estimates are periodically reevaluated, and adjustments are made when facts and circumstances dictate a change.
Our accounting policies are more fully described under the heading “Description of the Business, Basis of Presentation and Summary of Significant Accounting Policies” in Note 1 to our Financial Statements included in this prospectus.
We believe that the following accounting policies are the most critical to aid in fully understanding and evaluating our reported financial results, and they require our most difficult, subjective or complex judgments, resulting from the need to make estimates about the effect of matters that are inherently uncertain.
Components of our Results of Operations and Financial Condition
Operating expenses
We classify our operating expenses into three categories: (i) research and development, (ii) general and administrative and (iii) warrant expense – termination agreement.
Research and development.
Research and development expenses consist primarily of:
|
• |
costs incurred to conduct research, such as animal research; |
|
|
• |
costs related to the design and development of our technology, including fees paid to contract engineering firms and contract manufacturers; |
|
|
• |
salaries and expenses, including stock-based compensation, related to our employees primarily engaged in research and development activities; |
|
|
• |
fees paid to clinical consultants, clinical trial sites and vendors, including clinical research organizations, in preparation for clinical trials and our applications with the FDA; |
|
|
• |
costs to develop our intellectual property; and |
|
|
• |
costs related to compliance with regulatory requirements. |
We expect our research and development expenses to increase in the future as we advance our product into and through clinical trials, pursue additional regulatory approvals of our product in the United States, and continue commercial development of our device(s). The process of conducting the necessary clinical research to obtain regulatory approval is costly and time-consuming. The probability of success for our technology may be affected by a variety of factors including: the quality of our product, early clinical data, investment in our clinical program, competition, manufacturing capability and commercial viability. We may not succeed in achieving all necessary regulatory approvals for our product candidates. As a result of the uncertainties discussed above, we are unable to determine the duration and completion costs of our research and development process or when and to what extent, if any, we will generate revenue from the commercialization and sale of our device.
General and administrative
General and administrative expenses consist of personnel related costs, which include salaries, as well as the costs of professional services, such as accounting and legal, facilities, information technology, stock-based compensation for general and administrative personnel, insurance, travel costs and other administrative expenses and costs to defend our patents. We expect our general and administrative expenses to increase due to the IPO, the anticipated growth of our business and related infrastructure, as well as accounting, insurance, investor relations and other costs associated with being a public company.
Advertising
It is our policy to expense advertising costs as incurred. Advertising expenses are included within general and administrative expenses within the statement of operations. For the three months ended June 30, 2024 and 2023, the Company recorded less than $0.1 million and $0.1 million, respectively. For the years ended March 31, 2024 and 2023, the Company recorded $1.7 million and $0.1 million, respectively.
Stock-based compensation
Stock-based compensation transactions are recognized as compensation expense in the statements of operations based on their fair values on the date of the grant. The expense for equity awards expected to vest is recognized over the applicable vesting period of the stock award using either the straight-line method or the accelerated method, depending on the vesting structure, and is included in general and administrative. We estimate the fair value of options granted using the Black-Scholes option pricing model. This estimate uses assumptions regarding a number of inputs that require us to make significant estimates and judgments. The expected volatility assumption was based on industry peer information.
Accounting for Warrants
We issued warrants to purchase shares of common stock (i) in connection with the Bridge Offering, (ii) as part of selling agent compensation in 2024, and (iii) in connection with the Exclusive License Termination Agreement (the “Termination Agreement”). We accounted for such warrants in accordance with Accounting Standards Codification (“ASC”) Topic 480-10, Distinguishing Liabilities from Equity and ASC Topic 815-40, Derivatives and Hedging – Contracts in Entity’s Own Equity. Based on this guidance, we determined that warrants issued in connection with the Termination Agreement should be accounted for as a liability and the remaining warrants issued meet the requirements for equity classification. Liability classified warrants are subject to remeasurement at each balance sheet date, while equity classified warrants are valued at inception only.
Bridge Financing Warrants
The fair value of the Bridge Financing Warrants is estimated using a Monte Carlo simulation model with probability-weighted expected return method ("PWERM") based on the probabilities of different potential outcomes for the Notes issued with the Bridge Financing Warrants. The outcomes considered included (i) qualified financing as part of our planned IPO at various points in time and (ii) repayment in cash at maturity. Any increase in the amount of time expected until a qualified financing event and/or a reduction in the likelihood of a qualified financing event occurring during the term of the Notes would likely increase the fair value of the warrant, while the inverse of each scenario would have the opposite effect. The significant judgments and assumptions to the Monte Carlo simulation include the Company’s stock price, volatility based on a selection of publicly held peer companies, discount rate, and a discount for lack of marketability.
Common Stock Fair Value – The fair value of our common stock price was determined through a back solve, solving for the stock price that results in the average total value of the Notes and the warrants being equal to the cash proceeds received in the transaction it was issued at across one million iterations of the simulation.
Historical Volatility – We determine the expected volatility by weighing the historical average volatilities of publicly traded industry peers. Our intention is to consistently apply this methodology using the same or similar public companies until a sufficient amount of historical information regarding the volatility of our common stock becomes available. We will monitor our peer group for circumstances that may require a change to the composition or make-up of the entities and will identify if/when more suitable companies whose stock prices are publicly available would be utilized in the calculation.
Discount Rate - The rate is chosen based on private equity rates of return as described in the AICPA Practice Aid on Valuation of Privately-Held-Company Equity securities Issued as Compensation, choosing the rate at the lower end of the range.
Credit Rating – Our credit rating impacts the identification and calculation of the discount rate.
Discount for lack of marketability – Subsequent to the IPO, any shares issued pursuant to an exercise of the Bridge Financing Warrants, would be subject to a six-month lock-up. Consistent with AICPA’s Accounting and Valuation Guide: Valuation of Privately-Held-Company Equity Securities Issued as Compensation, the Finnerty model was used to estimate the discount for lack of marketability.
The fair value of the Notes and Bridge Financing Warrants is calculated such that they will combine to equal the cash purchase price of the Bridge Offering. Any changes in these assumptions will impact how the transaction price from the Bridge Offering is distributed between the Notes and the Bridge Financing Warrants.
Termination Agreement Warrants
The fair value of the Termination Agreement Warrants is estimated using a discounted cash flow model under various scenarios and used the probability-weighted expected return method (“PWERM”) comparing the probabilities of different outcomes. The outcomes considered included (i) qualified financing as part of our planned IPO at various points in time and (ii) possibility of default whereby the investor receives nothing. Any increase in the amount of time expected until a qualified financing event and/or a reduction in the likelihood of a qualified financing event occurring during the term of the warrant would decrease the fair value of the warrant, while the inverse of each scenario would have the opposite effect.
Additional significant assumptions and judgments used in preparing the discounted cash flow model include:
Discount Rate - The rate is chosen based on private equity rates of return as described in the AICPA Practice Aid on Valuation of Privately-Held-Company Equity Securities Issued as Compensation, choosing the rate at the lower end of the range.
Credit Rating – Our credit rating impacts the identification and calculation of the discount rate.
Any ongoing improvements in our credit rating would have the effect of driving down the discount rate used in the periodic re-measurement of the Termination Agreement warrants. Reductions in the Company’s discount rate would increase the fair value of the Termination Agreement warrants, while an increase in this factor will have an opposite effect.
Other Warrants
The fair value of equity-based warrants issued is estimated using the Black-Scholes option pricing model. The significant judgments and assumptions used in applying the Black-Scholes option pricing model include the underlying common stock at the measurement dates, the expected term, expected dividend yield and historical volatility of comparable companies’ stock.
Common Stock Fair Value – Prior to our IPO, we periodically sold shares of our common stock for cash in an arms-length transaction. We consider these transactions as indicative of the fair value of our common stock when applying the Black-Scholes option pricing model. Subsequent to our IPO, we base the value of our shares on observable share data.
Expected Term – The estimate of the expected term of awards was determined in accordance with the contractual term of the arrangement.
Expected Dividend Yield – We have not declared or paid any cash dividends and do not presently intend to pay any in the foreseeable future. We have no plans or expectations that this assumption will change in the foreseeable future.
Historical Volatility – We determine the expected volatility by weighing the historical average volatilities of publicly traded industry peers. Our intention is to consistently apply this methodology using the same or similar public companies until a sufficient amount of historical information regarding the volatility of our common stock becomes available. We will monitor our peer group for circumstances that may require a change to the composition or make-up of the entities and will identify if/when more suitable companies whose stock prices are publicly available would be utilized in the calculation.
A decrease in volatility and expected term will decrease the estimated fair value of the warrant, while an increase in these factors will have an opposite effect.
BUSINESS
Overview
We are a development stage medical device development company focused on advancing innovative technologies for sensing and treating disorders relating to the nervous system. Our first-in-class technology platform includes a catheter-based microchip-enabled sensing array that can detect and differentiate neural signals with a high degree of sensitivity as demonstrated in animal studies. We are initially developing our technology for patients with pancreatic cancer, a condition that can cause debilitating pain and needs a more effective solution. However, we believe our technology constitutes a platform with the potential to address dozens of indications in a range of areas including chronic pain management from all causes, hypertension, cardiovascular disease and a wide range of other nerve-related disorders.
We calculate sensitivity in units of minimum signal detection voltage in micro volts (uV) time area of the electrode (square millimeters). It is a combined measure that is related to the signal resolving power and spatial resolution of the system. For the BSC Orion, the nearest device on the market, the metrics are 10uV for signal detection levels, and roughly 0.4mm by 0.5mm for the electrode dimensions. For the Autonomix device, the metrics are <1uV for signal detection levels and roughly 0.02mm by 0.03mm for the electrode dimensions. The differences in these metrics result in a calculation of 3,000 times greater sensitivity for the Autonomix device. We believe, if we can recreate these results in clinical trials, this will enable a method of transvascular targeting, treating, and confirming treatment of diseases involving the nervous system throughout the body that is not currently available and may be capable of filling a wide range of unmet medical needs.
Our development efforts can be divided into to two sub parts: diagnostic and therapeutic, where diagnostic is focused on sensing and identifying neuronal activity that may be associated with a disorder with enough precision to enable targeted therapy with ablation. Our sensing catheter has already been developed sufficiently to demonstrate in animal models successful identification of a signal from a specific nerve bundle before ablation and confirmation of termination of that signal from the treated nerves after ablation. We are now in the process of improving the design of this catheter to meet the standards required for human use. In parallel with this effort, we are conducting a first-in-human demonstration of transvascular ablation to relieve pain associated with pancreatic cancer, with the intent to bring sensing and treatment together in a future pivotal clinical trial to enable the commercial launch of our technology. We are a development stage company and there is no guarantee that the results of any trials will produce positive results or that the results will support our claims.
We believe one of the most demanding aspects of our commercialization plan will be scaling up from our existing sensing prototype to a robust commercial version. Today, our sensing device is hand built and includes a combination of hand-crafted and 3D printed parts. We have not yet assembled or tested what will be the commercial version of our proposed device. Even if our proposed device is cleared for commercial use, there is no assurance that we will be able to successfully build such device on a commercial scale.
As of June 30, 2024, we had an accumulated deficit of $41.7 million, negative cash flows from operating activities of $1.9 million and working capital of $6.3 million, which raises substantial doubt about our ability to continue as a going concern. Further, we have incurred and expect to continue to incur significant costs in pursuit of our business plans. We cannot assure you that we will be successful in raising additional funds. These factors, among others, raise substantial doubt about our ability to continue as a going concern.
Our Technology
Targeting the Peripheral Nervous System
The peripheral nervous system comprises a vast network of nerve fibers extending throughout the human body and interacting with every organ. Peripheral nerves can be further classified as autonomic (supplying sympathetic and parasympathetic nerve signals from the brain to tissue and organs, i.e., fear inducing production of adrenaline) and somatosensory (supplying signals to the brain from tissue and organs, i.e., the sensation of pain). Whether as a root cause or a manifestation of resulting symptoms, these nerves play a role in virtually all diseases.
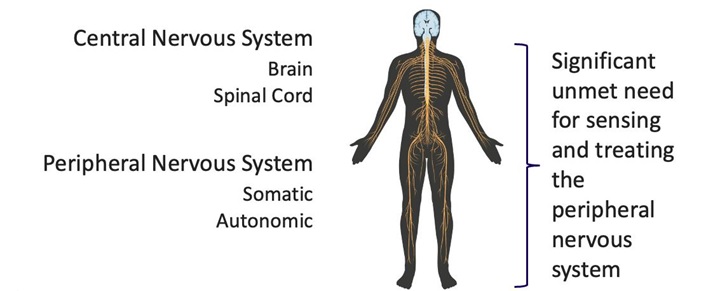
Unfortunately, we believe that very few tools currently exist for the sensing and targeting of nerve fibers within the peripheral nervous system. At Autonomix, our primary goal is to provide a breakthrough in sensing technology that will become an indispensable tool for diagnosing, targeting, and treating disorders relating to the peripheral nervous system. And, although our Company’s name hails from the autonomic subgroup of the peripheral nervous system, our technology is intended for both the autonomic and somatosensory systems and could eventually find uses within the central nervous system.
Exploiting the Vascular Superhighway
The Autonomix system we are developing is primarily catheter based, meaning that our sensing equipment will be delivered to its targeted location via a lumen within the body. While this could include oral, urethral, and other natural openings of the body, our primary focus is using the vasculature, most often arteries, to reach our target. Fortunately, nature has endowed us with “superhighway” access in the form of our arterial structure, as most of the peripheral nerves travel along our arteries. As can be seen in this cross-sectional view of the kidney and renal artery, the web of peripheral nerve fibers (shown in yellow) parallels the renal artery, and this form of nerve pathway development is typical throughout the body.
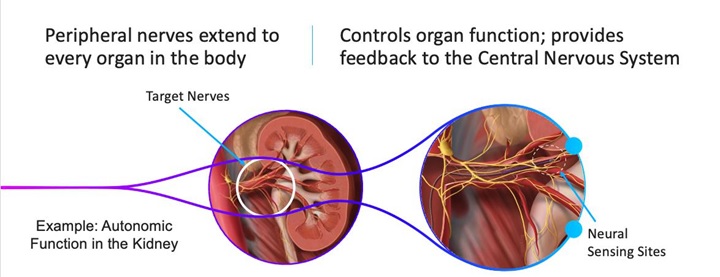
Our sensing catheter has been designed to be introduced by a small incision into an artery (such as the femoral artery) and with a conventional guide wire or sheath be directed to any organ in the body where it will be close enough to the nerve fibers servicing that organ to sense, target and treat the nerves associated with that targeted disorder, and to confirm that the intended treatment was successful.
The Sensing Problem
Although this vascular superhighway has long been utilized for certain catheter-based evaluation and intervention, we believe its use throughout the body has been limited by the lack of adequate sophistication of catheter systems. According to a Markets and Markets report, titled “Electrophysiology Market Global Forecast to 2027” published in February 2023, the global electrophysiology market in terms of revenue was estimated to be $6.8 billion in 2021 and is expected to reach $11.6 billion by 2027. The report cites as a driver for such market the increased incidence of cardiovascular disease and the use of catheters to deliver corrective ablation in the field of cardiology. Most commonly, radio frequency (RF) energy is emitted from inside the walls of the heart or arteries sufficient to ablate (destroy) a cardiomyocyte or nerve within its path. This “transvascular” use of ablation forms the basis for treating atrial fibrillation, for example.
More recently, companies like Medtronic have successfully used transvascular ablation of the nerves surrounding the renal artery to treat refractory hypertension (high blood pressure that has been resistant to standard drug therapy). One of the challenges they face however is that the nerves they are targeting operate at much lower voltage levels than, say, the level emitted by a cardiomyocyte. In cardiology, there are sensing systems capable of sensing down to a level of about 10 to 15 microvolts. That’s more than enough sensitivity to detect (and target) a cardiomyocyte that is emitting 100 microvolts per pulse, but the nerves around the renal artery (and around most peripheral nerve targets throughout the body) are operating at around 1 to 2 microvolts; much too low to be detected by existing sensing technology.
What this means is that the ablation of nerves from within the renal artery is essentially conducted “blind.” Without a sensing system capable of detecting and targeting signals from nerves within the nervous system, clinicians cannot see the nerves causing hypertension in the patient. As a result, they are forced to hypothesize and treat one small area at a time, hoping they hit the desired target without hitting an unintended target. Over-treating the area could relegate the patient to life in a wheelchair by destroying their ability to regulate blood pressure.
The Autonomix Solution
We believe the reason no one has commercialized a sensing system capable of solving this problem is that the physics involved demanded a major technological breakthrough. By their very nature, electrical signals from the body are analog and even though a 10-microvolt signal can be detected and transmitted down the roughly 2 meters of wire required to travel along the catheter, outside the patient, and into the necessary processing equipment shown in this picture below of a typical catheter lab, this isn’t feasible with the 1 to 2 microvolt signal from a typical peripheral nerve. Given the cacophony of other signals emitted throughout the body and by other equipment in the lab as well as degradation of the signal due to the distance traveled along the catheter, these faint signals become lost or are rendered meaningless.
We are seeking to solve this problem through our design, which is still in development, of a proprietary microchip comprised of multiple key components. Each antenna is comprised of two small electrodes that can detect the presence of voltage down to as little as 0.5 microvolts giving us sufficient sensitivity to register the impulse of a nearby nerve bundle that might typically be generating 1.5 to 2.0 microvolts with each impulse. Our current design connects 8 antennae to our proprietary chipset (which is designed to handle up to 16 antennae) where an onboard amplifier and analog to digital converter convert each signal into a robust digital form. The chipset also includes a multiplexer intended to enable the transmission of data from each of the antennae simultaneously down the catheter body to the catheter handle. The Wi-Fi handpiece then transmits this data to a nearby laptop for viewing and analysis by the clinician.
In a typical catheter lab, these signal conversion functions are often carried out by “briefcase” sized devices processing the raw analog signals that must travel the full length of the catheter, outside the patient’s body and then from the patient to the equipment. While this is feasible for higher voltage signals from the heart, the signals from peripheral nerve bundles are often too faint to travel all this distance without loss or corruption and yet the typical catheter lab equipment is far too big to fit inside a catheter. The patented Autonomix solution shrinks these processes down to a microchip small enough to place immediately adjacent to the antennae detecting the signals, greatly reducing the distance the signals must travel. The picture of the “proprietary chipset” below is our actual chipset and is not a rendering.
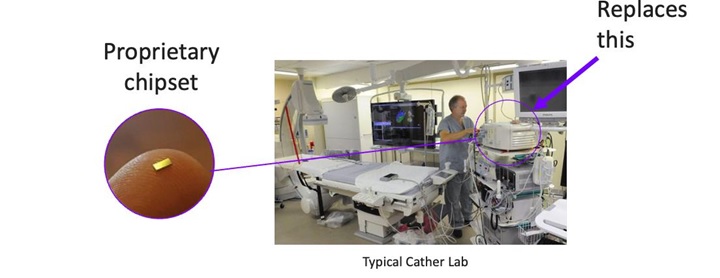
As shown in the diagram below, this basket antenna array is built from a micro-thin, laser-cut flexible circuit board. We believe the special arrangement of the antennae will make it possible to effectively geolocate the nerve in 3-dimensional space for targeting treatment.
 |
|
Sense, Ablate, Confirm
This sensing system is currently being developed to be deployed alongside a separate radio frequency ablation catheter system for a combined diagnostic and therapeutic solution which will require the use of two separate catheters that will be used during the procedure. However, our longer-term design is intended to combine the two catheters into one device so that the combined system could be capable of sensing, treating (ablating) and confirming successful treatment all with one relatively simple and minimally invasive procedure.
Focus on Pancreatic Cancer Patients
We believe the Autonomix sensing technology has the potential to provide a level of detail and resolution in navigating the peripheral nervous system that until now has simply not been possible. As such, we believe this platform, if shown to be effective, could be applied to a wide range of disorders throughout the body. With that said, our experience tells us that the best way to develop a new technology like this is to narrowly focus on a proof of concept that we think will reflect the capabilities of our system while providing the most expeditious pathway to regulatory clearance, commercialization and revenue generation.
For this reason, we are initially focusing on the treatment of pain associated with pancreatic cancer and we have designed our commercialization efforts around this as our first proposed indication for use.
We believe this is a good choice for several reasons:
Significant Unmet Need
According to a report by The Oncologist, titled “Pancreas Cancer-Associated Pain Management” first published April 22, 2021, “[p]ain is highly prevalent in patients with pancreas cancer,” “90% of these patients reported discussing pain with their health care provider,” and “50% of the respondents reported visits to the emergency room for symptoms related to pain.” One of the tragedies of this condition is that most pancreatic cancer patients have a short time to live and the debilitating pain resulting from the tumor can significantly reduce the quality of that remaining time. Moreover, we believe that prolonged pain can diminish a patient’s will to live, making that remaining time even shorter.
The standard of care treatment usually begins with opioids, but patients often become resistant, and the side effects of chronic opioid use can eventually outweigh the benefits.
The most common alternative method of treatment is a neurolytic celiac plexus blockade (“NCPB”), which is a percutaneous (via needle through the skin) ethanol injection guided by CT scan to attempt to direct the ethanol (which will destroy neural tissue on contact) to the area of the pancreatic tumor and related peripheral nerves. Regardless of this initial targeting, the varied structure of the abdominal cavity leads to the potential for ethanol to either miss the intended target or migrate to unintended areas creating unwanted side effects.
Furthermore, according to a study titled “Neurolytic Celiac Plexus Block for Pain Control in Unresectable Pancreatic Cancer” published by the American Journal of Gastroenterology, 2007, Vol.102 (2), p.430-438, Article 430, meta-analysis from multiple randomized controlled trials suggests that patient benefits from NCPB are only marginally better than opioids and may not be outweighed by the potential risks. The most common side effects are diarrhea, transient hypotension, constipation, nausea and vomiting, and lethargy while rare major adverse events reported in the literature include infectious complications, bowel perforation, intraabdominal hemorrhage, fistula formation, stomach paralysis, partial paralysis of the lower limbs or loss of other motor function, chronic diarrhea, arterial damage, water on the lung, and death.
In contrast, we believe the Autonomix procedure has the potential to represent a safer and more reliable treatment. This has the potential to significantly increase remaining quality of life for pancreatic cancer patients, and in so doing, even potentially extend overall survival.
To begin with, our entire approach is via arterial catheter, inserted in most cases via the femoral or brachial artery. We believe this method of access that should significantly reduce the potential for complications as compared with NCPB. We believe our sensing technology has the potential to identify and target the nerves that are responsible for the pain signal and with the ability to focus the ablative energy on that target, we should have a much greater degree of accuracy, control and reliability as compared with NCPB.
When comparing to the use of opioids, we believe the potential benefits are even more obvious. The Autonomix procedure we are developing is, by design, targeted directly to the nerves responsible for the pain being treated and offers the potential for “one and done” durability, whereas opioids are systemic treatments subjecting the entire body to unnecessary exposure, requiring constant dosing, and inducing debilitating chronic systemic side effects as a consequence.
Beneficial Clinical Trial Dynamics/Expedited Regulatory Process
Despite the significant unmet need, there are very few clinical trials worldwide focused on improving pain management for pancreatic cancer patients and currently none that we are aware of in the proposed location of our planned first-in-human proof of concept study. We believe this means there is limited competition for such patients, making it theoretically easier to recruit for our trial.
At the same time, because we are focusing on palliative care for patients whose lives are being limited by a rare cancer, we believe regulatory authorities are willing to consider lower preclinical hurdles and smaller and simpler trial designs to help encourage trial sponsors to seek improved treatment options. However, these decisions are under the exclusive control of regulatory authorities and there is no guarantee that our trial designs will be approved. If regulatory authorities are willing to consider lower preclinical hurdles and smaller and simpler trial designs, this would translate into lower preclinical and clinical trial cost, as well as shorter completion times. Furthermore, study duration is also shortened by the very nature of the indication and primary efficacy endpoint: reduction of pain associated with pancreatic cancer.
Specifically, we intend for each patient to need only one treatment and we expect we will be able to immediately determine if there is any reduction of pain from our procedure such that an initial indication of pain reduction will likely be provided by patients upon conclusion of that treatment. Although follow up visits will be required to assess continuing safety and durability of efficacy over a span of several months, an initial indication of efficacy will be available almost as quickly as patients are treated . For this reason, we are hopeful that the overall duration of this first trial will be measured in months rather than years, as is often the case for clinical trials with longer treatment durations or where a clinically significant response takes more time to be produced.
Meaningful Commercial Market
Although pancreatic cancer is considered a rare disease, according to the American Cancer Society “Key Statistics for Pancreatic Cancer” (https://www.cancer.org/cancer/types/pancreatic-cancer/about/key-statistics.html), the American Cancer Society estimates in 2023 that approximately 64,000 will be diagnosed with pancreatic cancer in the U.S. on an annual basis and an article in the International Journal of Cancer (Int. J. Cancer. 2021;149:993–1001) indicates that annual new cases in the European Union reached 109,000 in 2019 and are expected to grow. A market analysis published by Precedence Research (https://www.precedenceresearch.com/ pancreatic-cancer-market) reported that the global market for treatment of pancreatic cancer in 2022 was estimated to be $2.2 billion. Published research by The Oncologist, titled “Pancreas Cancer-Associated Pain Management” stated that “90% of patients [with pancreatic cancer] reported discussing pain with their health care provider”. As a point of reference, a one-month course of Abraxane (a commonly prescribed drug for the treatment of pancreatic cancer) has a retail price of more than $10,000. While this should not be considered an indicator of how an Autonomix procedure will ultimately be priced, we believe it reflects the magnitude of potential market size and helps form the basis for expecting a significant revenue opportunity from this indication.
The incidence of pancreatitis, a non-cancerous condition that can also result in chronic pain, is estimated to be as much as three times that of pancreatic cancer. We believe that, if our procedure is cleared for use in treating pancreatic cancer pain, we should be able to eventually expand that clearance to include pain resulting from pancreatitis.
The Potential to Impact Cancer
Recent independent research has indicated that neural pathways may play an insidious role in cancer progression. An article published in Metastatic Cancer: Clinical and Biological Perspectives, titled “Sympathetic Nervous System Regulation of Metastasis” demonstrates that as pancreatic tumors progress to invade the liver (a common occurrence in patients with pancreatic cancer and a significant driver of morbidity) they do so by traveling along local neural pathways. Our development team speculated that disruption of these pathways might have the potential to slow or stop the progression of the primary tumor.
In collaboration with a specialist in pancreatic cancer, we conducted a study in mice to see if ablation (in this study, ethanol ablation was used, similar to its use with NCPB in humans) of the nerve fibers around the pancreas might have an effect on tumor progression. As can be seen in this study summary, there was a reduction in tumor progression in this model. This was a small study, and we can’t be certain that these results are indicative of the potential for impacting tumor progression in humans, but we do see this as encouraging further study and may represent a future opportunity beyond pain management.
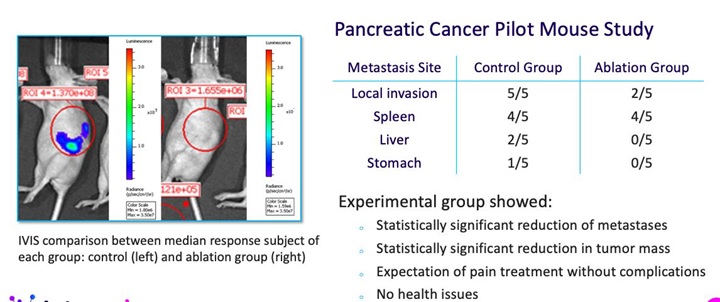
Indicative of Additional Market Potential
We believe pancreatic cancer pain management is a “proof of concept,” and we believe success here will be indicative of the potential of our system in a wide range of disorders where the peripheral nervous system is involved.
Examples of future potential additional uses include renal denervation for treating hypertension, addressing other sources of pain including lower back and other joint locations, Complex Regional Pain Syndrome (“CRPS”), other tumor related pain, and pelvic pain, pulmonary disorders such as chronic obstructive pulmonary disease, and urinary tract and digestive disorders, and enabling more targeted treatments in cardiology, just to name a few.
A Markets and Markets report, titled “Electrophysiology Market Global Forecast to 2027” published in February 2023 describes the global electrophysiology (EP) market as representing approximately $6.8 billion in 2021 in annual global revenue, and is expected to reach $11.6 billion by 2027. The vast majority of this market today is represented by cardiology related diagnosis and intervention. Our vision for the Autonomix technology is to help expand electrophysiology well beyond cardiology to include nearly all reaches of the peripheral nervous system and we believe doing so will ultimately result in a market opportunity much greater than the current projections for the EP.
We believe enabling targeted transvascular treatment of pain will enable us to access the $78 billion pain management market, as cited in the Mordor Intelligence Pain Management Market Industry Report (https://www.mordorintelligence.com/industry-reports/pain-management-market). Additionally, if we are able to facilitate a safer, more targeted method for renal denervation we may be able to access the $25 billion hypertension market, as indicated by Polaris Market Research (https://www.polarismarketresearch.com/industry-analysis/global-hypertension-drug-market). When additional indications such as COPD, irritable bowl syndrome, and overactive bladder are included, we believe the Autonomix platform has the potential to address more than $100 billion in market opportunities.
Commercialization Plan
Regulatory Pathway
We believe, the most likely approval pathway for our technology is referred to as “de novo” premarket notification. This is differentiated from the more common “510(k)” pathway, which is only applicable when there is a clear “predicate” device already on the market (doing the same thing in substantially the same way) and from the lengthier “PMA” process when there is no precedent at all for a technology. In our case, both sensing and ablation have established precedence, just not at this level of sensitivity or in our targeted indications.
Whether in the United States or EU, we must demonstrate that our technology is safe and effective. The safety standard is ultimately met through a combination of animal studies, independent laboratory testing, a design history file documenting compliance with established standards and, ultimately, human clinical trials. Many of these requirements are staged such that not all must be met on the front end of development. In addition, efficacy must be based on a sound scientific rationale and ultimately demonstrated in a human clinical trial.
Human trials are often designed to begin with a Proof of Concept (“PoC”); the US Food and Drug Administration (“FDA”) sometimes refers to these as Early Feasibility Studies (“EFS”) and then progress to a “Pivotal” or approval, trial. The design and endpoints of pivotal trials are often negotiated with the relevant regulatory authority (i.e., FDA in the United States, EMA or country-specific Competent Authority (“CA”) in Europe). Our regulatory package for authorization to conduct our first-in-human clinical trial was approved by the Ethics Committee (“EC”) at our intended clinical site hospital outside the United States. This approval allowed the clinical trial to begin The regulatory package submitted included not only a detailed clinical protocol for conducting the study, but also an extensive Investigator’s Brochure (“IB”) setting forth details about the equipment to be used, historical safety of human procedures conducted with this equipment and details of our animal studies using this equipment for the first time in the area of the pancreas.
We plan to present the relevant data from this study to the FDA in a pre-submission meeting to request “Breakthrough Status” in an effort to minimize the clinical requirements for clearance in the United States. The first trial is not designed to replace the trial that will be required by the FDA to support our submissions for clearance in the United States, but rather to potentially impact the size of that required trial. There is no assurance that the FDA will accept the results of the data we obtain from our clinical trials performed outside of the United States.
According to an article by Applied Clinical Trials, titled “Medical Device Development: U.S. and EU Differences” published August 1, 2006 “[t]he way in which devices are regulated in the EU is very different from the way they are regulated in the United States,… [which] has introduced significant differences in time-to-market approval for the United States versus the EU, particularly in the case of high-risk Class III and Class IIb implantable devices.” While this is changing based on the advent of a new EU regulation called Medical Device Regulation (“MDR”) that is expected to make the EU process more like the FDA process, MDR is being rolled out country by country. We believe the approval process in some EU countries for utilizing CE marked devices off label is less demanding than in the US. As a result, we have decided to conduct the PoC in Europe instead of the US.
Once the PoC is established, however, there are compelling reasons to focus the approval process first and foremost in the US. One reason is that therapeutic procedures in the US will usually command higher prices and once those prices are set, EU pricing authorities will often index off of the US price. Another is that product launches are usually easier in the US where a single sales force can serve the entire region (as opposed to country-specific distribution teams in the EU) and where one regulatory standard applies across the board.
The development of the PoC data forms the basis for two FDA-related processes. First is the request of a “Pre-Submission” meeting to discuss the overall regulatory strategy, the primary focus of which is to agree on a pivotal testing protocol. Assuming promising PoC data, the second is to submit a request for “Breakthrough” status based on the significant unmet need. Breakthrough status affords us expedited access to FDA and a higher level of proactive interaction that may support faster approval.
Upon completion of the Pivotal Clinical Trial, we believe we will then be in a position to submit our de novo application to the FDA, the review of which is expected to require approximately 150 days. If we able to receive FDA clearance (applications like this are not technically “approved” but rather “allowed” or “cleared”), of which there is no assurance, we currently estimate such clearance would occur in the first half of 2027. The foregoing timeline is not a guarantee and is subject to many of the risks and uncertainties disclosed in this prospectus and is subject to change.
Technology Development
Commercialization of our technology can be thought of in three distinct phases: (1) sensing, (2) ablation, and (3) the combination of these two technologies into an integrated device. We believe that commercial success can be achieved with either of the two technologies and is not dependent upon successful integration (meaning two distinct systems, one for sensing and one for ablation could also be viable, even if not optimal).
Similarly, our clinical development plan reflects the fact that the sensing and ablation systems are at different stages of development. Extensive testing in pigs (whose abdominal structure is considered similar to humans) has demonstrated that our sensing technology is capable of locating and targeting individual nerves around the renal artery. Considering the similarity in anatomy, both between pigs and humans and between the renal artery (supplying the kidneys) and the celiac and related arteries supplying the pancreas, we believe our sensing system may also be effective in humans and that the primary remaining risk relating to the sensing system is commercial execution.
Specifically, we believe one of the most demanding aspects of our commercialization plan will be scaling up from our existing sensing prototype to a robust commercial version. Today, our sensing device is hand built and includes a combination of hand-crafted and 3D printed parts, but we are actively engaged in the development of a more robust version which will meet requirements to be used in human clinical trials. We will divide this device into two subsets: (a) an electronics package (subassembly) that relies on semi-automated production of printed flexible circuit boards and electrical leads that is supplied to (b) a qualified catheter production process that will be contracted to a catheter production facility already experienced and certified in the “art” of catheter assembly. We expect the human clinical version to be completed by mid-2025 and the commercial scale up process to be completed by mid-2027, although there is no assurance that we will be able to meet such timeline.
Regarding the ablation system, safe and reliable off-the-shelf RF systems are currently available for use in cardiology and other electrophysiology indications. We are currently using one of these existing systems “off label” (in an area of the body for which the system is not yet approved by the relevant regulatory agency) in our proof-of-concept study to ablate the nerves near the pancreas of pancreatic cancer patients to demonstrate, for the first time ever, that transvascular ablation of those nerves may reduce pain. The regulatory package submitted includes not only a detailed clinical protocol for conducting the study, but also an extensive Investigator’s Brochure (IB) setting forth details about the equipment to be used, historical safety of human procedures conducted with this equipment and details of our animal studies using this equipment for the first time in the area of the pancreas. To be clear, this first PoC trial is being conducted without the benefit of our sensing system because it has not yet been cleared by the FDA for use in humans.
The fact that this first PoC is essentially being performed “blind” (as all denervation procedures are currently done) simply means that it will not be as accurate as it could be. However, we believe if we successfully demonstrate that transvascular ablation is capable of mitigating pancreatic cancer pain, this would be a medical “first” and would represent an important breakthrough for the electrophysiology community. Likewise, we believe it will help support a request to the FDA to be granted “Breakthrough” status, which could accelerate our future commercialization efforts.
Also, what we are learning in the trial, about how to optimize ablation catheters for use outside the cardiology space, is being incorporated into the design of a customized RF catheter design for use in our own therapeutic device.
It is possible to receive clearance from the FDA (and EMA) based on a prototype system that is not optimized for manufacturing and for the necessary design for manufacturability process to be running in the background with the goal of having the commercial version ready for launch shortly after the prototype platform is FDA cleared. We would then use our existing de novo cleared device as our own predicate for 510(k) clearance of the commercial version.
Our plan is then to launch the commercial version of our system, most likely to a controlled region or list of KOLs (Key Opinion Leaders) to debug and optimize the commercial strategy before committing to a national/global launch.
Accordingly, our planning considers both the possibility of a stand-alone commercial launch or a licensing arrangement with a larger player. As we get closer to an actual FDA device clearance, our strategy approach will be reviewed and adjusted for practicality. The Autonomix management team, however, has experience with both approaches.
The following graphic sets forth our planned development timeline, however, these timeframes are not guarantees and this timeline is subject to many of the risks and uncertainties disclosed in this prospectus and is subject to change:
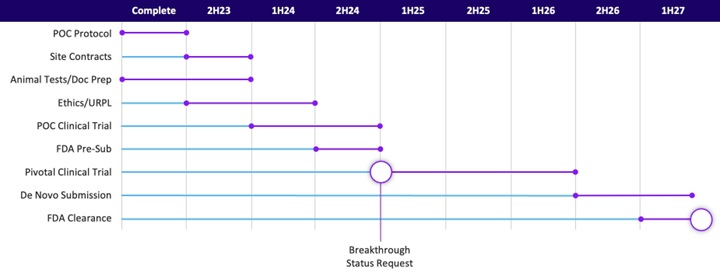
Revenue Model
We envision our device revenue model on several levels: (a) purely as a targeted therapy for disorders associated with pancreatic tumors (and pancreatitis), (b) as a standalone sensing technology for broad based diagnostics use and/or use in conjunction with other ablation systems or (c) as a combined sensing and ablation system targeting a wide array of disorders throughout the body.
There are four key elements of our device that will be provided to the user (hospital) to facilitate our technology: (1) a disposable sensing catheter with a handpiece (that connects to the catheter and could be partially or entirely disposable), (2) an RF catheter designed to reach the target nerves in the peripheral nervous system, (3) an RF energy source to power our custom RF ablation catheter, and (4) a user interface comprised primarily of software (translating data received from the catheter microchip and activating ablative energy in integrated systems) that could reside on existing hospital personal computer, or PC, systems or a dedicated PC system.
In general, we believe that hospitals place a high premium on disposability (maximizes patient safety, avoids complications of on-site sterilization) and that, if we can avoid the need for a significant outlay in “capital equipment” we should, since high-dollar capital equipment authorizations often involve additional bureaucracies within the hospital and complicate the sell-in process.
An important benefit of the Autonomix platform will be that it will rely on catheter systems and techniques that are familiar to interventional radiologists (our expected primary users) and should require a minimum of training for successful use.
Our selection of radio frequency as an energy source was made based upon what we believe is the well-understood status of the technology within the industry and the FDA. Additionally, we have found in pre-clinical testing that this energy source appears to provide a very effective method of ablating neural tissue. The software required in our device’s user interface will likely be provided at no or a nominal charge.
For the reasons described above, we believe the primary revenue model for Autonomix will be the sale of single-use disposable catheters to existing hospital catheter labs and that revenue volume will likely be a direct function of the number of procedures performed.
Intellectual Property
Patents and Pending Patent Applications
The Company has 18 patent families (representing various inventions relating to different aspects of its technology) comprising 87 issued patents (37 in the US thus far) and 42 pending patent applications. All patents and patent applications were originated by our co-founders, Mr. Landy Toth and/or Dr. Robert Schwartz, with filing dates ranging from 2012 through 2024.
The following table shows our material patents and the expiration dates (assuming maintenance/annuity fees are paid as required) as of October 31, 2024:
Issued Patents
|
Patent No. |
Jurisdiction |
Title |
Patent Expiration Date |
|
2013211951 |
Australia |
Controlled Sympathectomy and Micro-Ablation Systems and Methods |
1/25/2033 |
|
2,862,862 |
Canada |
Controlled Sympathectomy and Micro-Ablation Systems and Methods |
1/25/2033 |
| 3,151,885 | Canada | Controlled Sympathectomy and Micro-Ablation Systems and Methods | 1/25/2033 |
|
ZL201380016637.3 |
China |
Controlled Sympathectomy and Micro-Ablation Systems and Methods |
1/25/2033 |
|
ZL201710440397.X |
China |
Controlled Sympathectomy and Micro-Ablation Systems and Methods |
1/25/2033 |
|
2804527 |
European Patent Ratified in France, Germany, Ireland, Netherlands, UK |
Controlled Sympathectomy and Micro-Ablation Systems and Methods |
1/25/2033 |
|
497881 |
India |
Controlled Sympathectomy and Micro-Ablation Systems and Methods |
1/25/2033 |
|
6,552,824 |
Japan |
Controlled Sympathectomy and Micro-Ablation Systems and Methods |
1/25/2033 |
|
347625 |
Mexico |
Controlled Sympathectomy and Micro-Ablation Systems and Methods |
1/25/2033 |
|
372926 |
Mexico |
Controlled Sympathectomy and Micro-Ablation Systems and Methods |
1/25/2033 |
|
11201406006X |
Singapore |
Controlled Sympathectomy and Micro-Ablation Systems and Methods |
1/25/2033 |
|
10,470,684 |
United States of America |
Controlled Sympathectomy and Micro-Ablation Systems and Methods |
11/10/2034 |
|
9,649,064 |
United States of America |
Controlled Sympathectomy and Micro-Ablation Systems and Methods |
4/28/2033 |
|
10,022,085 |
United States of America |
Controlled Sympathectomy and Micro-Ablation Systems and Methods |
1/25/2033 |
|
11,013,459 |
United States of America |
Controlled Sympathectomy and Micro-Ablation Systems and Methods |
6/30/2033 |
|
2013267672 |
Australia |
Endoscopic Sympathectomy Systems and Methods |
5/28/2033 |
|
2,874,620 |
Canada |
Endoscopic Sympathectomy Systems and Methods |
5/28/2033 |
|
2852339 |
European Patent Ratified in France; Germany; Ireland: Netherlands; UK |
Endoscopic Sympathectomy Systems and Methods |
5/28/2033 |
|
436507 |
India |
Endoscopic Sympathectomy Systems and Methods |
5/28/2033 |
|
504735 |
India |
Endoscopic Sympathectomy Systems and Methods |
5/28/2033 |
|
11201407873R |
Singapore |
Endoscopic Sympathectomy Systems and Methods |
5/28/2033 |
|
9,968,790 |
United States of America |
Endoscopic Sympathectomy Systems and Methods |
7/14/2033 |
|
10,226,633 |
United States of America |
Endoscopic Sympathectomy Systems and Methods |
5/28/2033 |
|
11,344,731 |
United States of America |
Endoscopic Sympathectomy Systems and Methods |
5/28/2033 |
| 12,053,634 | United States of America | Endoscopic Sympathectomy Systems and Methods | 5/28/2033 |
|
2013274158 |
Australia |
Devices, Systems, and Methods for Diagnosis and Treatment of Overactive Bladder |
6/13/2033 |
|
2,876,080 |
Canada |
Devices, Systems, and Methods for Diagnosis and Treatment of Overactive Bladder |
6/13/2033 |
|
2,861,145 |
European Patent Ratified in France; Germany; Ireland; Netherlands; UK |
Devices, Systems, and Methods for Diagnosis and Treatment of Overactive Bladder |
6/13/2033 |
|
532368 |
India |
Devices, Systems, and Methods for Diagnosis and Treatment of Overactive Bladder |
6/13/2023 |
|
6,335,888 |
Japan |
Devices, Systems, and Methods for Diagnosis and Treatment of Overactive Bladder |
6/13/2033 |
|
6,672,370 |
Japan |
Devices, Systems, and Methods for Diagnosis and Treatment of Overactive Bladder |
6/13/2033 |
|
11201408219T |
Singapore |
Devices, Systems, and Methods for Diagnosis and Treatment of Overactive Bladder |
6/13/2033 |
|
10,206,616 |
United States of America |
Devices, Systems, and Methods for Diagnosis and Treatment of Overactive Bladder |
9/10/2034 |
|
11,564,616 |
United States of America |
Devices, Systems, and Methods for Diagnosis and Treatment of Overactive Bladder |
8/18/2033 |
|
2013337879 |
Australia |
Systems and Methods for Treatment of Tissues Within and/or Through a Lumen Wall |
10/31/2033 |
|
2,889,674 |
Canada |
Systems and Methods for Treatment of Tissues Within and/or Through a Lumen Wall |
10/31/2033 |
|
2,914,334 |
European Patent Ratified in France; Germany; Ireland; Netherlands; UK |
Systems and Methods for Treatment of Tissues Within and/or Through a Lumen Wall |
10/31/2033 |
|
406600 |
India |
Systems and Methods for Treatment of Tissues Within and/or Through a Lumen Wall |
10/31/2033 |
|
11201503472P |
Singapore |
Systems and Methods for Treatment of Tissues Within and/or Through a Lumen Wall |
10/31/2033 |
|
9,956,034 |
United States of America |
Systems and Methods for Treatment of Tissues Within and/or Through a Lumen Wall |
10/18/2034 |
|
10,905,495 |
United States of America |
Systems and Methods for Treatment of Tissues Within and/or Through a Lumen Wall |
11/6/2033 |
|
12,0111,214 |
United States of America |
Systems and Methods for Treatment of Tissues Within and/or Through a Lumen Wall |
5/13/2034 |
|
2013354932 |
Australia |
Systems and Methods for Regulating Organ and/or Tumor Growth Rates, Function, and/or Development |
12/9/2033 |
|
2,892,449 |
Canada |
Systems and Methods for Regulating Organ and/or Tumor Growth Rates, Function, and/or Development |
12/9/2033 |
|
388850 |
India |
Systems and Methods for Regulating Organ and/or Tumor Growth Rates, Function, and/or Development |
12/9/2033 |
|
11201504119V |
Singapore |
Systems and Methods for Regulating Organ and/or Tumor Growth Rates, Function, and/or Development |
12/9/2033 |
|
10,674,963 |
United States of America |
Systems and Methods for Regulating Organ and/or Tumor Growth Rates, Function, and/or Development |
6/1/2036 |
|
10,363,359 |
United States of America |
Systems and Methods for Regulating Organ and/or Tumor Growth Rates, Function, and/or Development |
2/25/2036 |
|
11,478,582 |
United States of America |
Systems and Methods for Regulating Organ and/or Tumor Growth Rates, Function, and/or Development |
8/2/2034 |
|
2014241205 |
Australia |
Neurological Traffic and Receptor Evaluation and Modification: Systems and Methods |
3/27/2034 |
|
2,907,625 |
Canada |
Systems and Methods for Neurological Traffic and/or Receptor Functional Evaluation and/or Modification |
3/27/2034 |
|
2,978,372 |
European Patent Ratified in Belgium; France; Germany; Ireland; Italy; Netherlands; Spain; UK |
Systems and Methods for Neurological Traffic and/or Receptor Functional Evaluation and/or Modification |
3/27/2034 |
|
480497 |
India |
System for Neurological Traffic and Receptor Evaluation and Modification |
3/27/2034 |
|
11201507936U |
Singapore |
Neurological Traffic and Receptor Functional Evaluation and Modification |
3/27/2034 |
|
10,004,458 |
United States of America |
Systems and Methods for Neurological Traffic and/or Receptor Functional Evaluation and/or Modification |
1/16/2035 |
|
10,765,370 |
United States of America |
Systems and Methods for Neurological Traffic and/or Receptor Functional Evaluation and/or Modification |
4/10/2034 |
|
11,589,820 |
United States of America |
Systems and Methods for Neurological Traffic and/or Receptor Functional Evaluation and/or Modification |
12/4/2034 |
|
2014337552 |
Australia |
Systems and Methods for Treating Cancer and/or Augmenting Organ Function |
10/14/2034 |
|
2,926,088 |
Canada |
Systems and Methods for Treating Cancer and/or Augmenting Organ Function |
10/14/2034 |
|
443928 |
India |
Systems and Methods for Treating Cancer and/or Augmenting Organ Function |
10/14/2034 |
|
364705 |
Mexico |
Systems and Methods for Treating Cancer and/or Augmenting Organ Function |
10/14/2034 |
|
10,143,419 |
United States of America |
Systems and Methods for Treating Cancer and/or Augmenting Organ Function |
2/6/2035 |
|
11,272,877 |
United States of America |
Systems and Methods for Treating Cancer and/or Augmenting Organ Function |
2/6/2035 |
| 12,064,256 | United States of America | Systems and Methods for Treating Cancer and/or Augmenting Organ Function | 3/6/2035 |
|
10,136,944 |
United States of America |
Systems and Methods for Treating Cancer and/or Augmenting Organ Function |
5/24/2035 |
|
11,382,687 |
United States of America |
Systems and Methods for Treating Cancer and/or Augmenting Organ Function |
4/3/2035 |
|
2015302050 |
Australia |
ANS Assessment Systems, Kits, and Methods |
8/7/2035 |
|
2,957,766 |
Canada |
ANS Assessment Systems, Kits, and Methods |
8/7/2035 |
|
3,177,201 |
European Patent Ratified as Unitary Patent and in Ireland and UK |
ANS Assessment Systems, Kits, and Methods |
8/7/2035 |
|
369552 |
India |
ANS Assessment Systems, Kits, and Methods |
8/7/2035 |
|
11201701018P |
Singapore |
ANS Assessment Systems, Kits, and Methods |
8/7/2035 |
|
10,791,924 |
United States of America |
ANS Assessment Systems, Kits, and Methods |
9/2/2037 |
|
11,883,103 |
United States of America |
ANS Assessment Systems, Kits, and Methods |
10/13/2036 |
|
ZL201680068094.3 |
China |
Controlled and Precise Treatment of Cardiac Tissues |
10/20/2036 |
|
3,364,869 |
European Patent Ratified as Unitary Patent and in Ireland and UK |
Controlled and Precise Treatment of Cardiac Tissues |
10/20/2036 |
|
11,154,239 |
United States of America |
Controlled and Precise Treatment of Cardiac Tissues |
5/28/2038 |
|
11,521,738 |
United States of America |
Medical Devices with Circuitry for Capturing and Processing Physiological Signals |
4/14/2039 |
|
11,848,078 |
United States of America |
Medical Devices with Circuitry for Capturing and Processing Physiological Signals |
10/17/2038 |
|
9,730,639 |
United States of America |
Elongated Conductors and Methods of Making and Using the Same |
5/19/2036 |
|
10,092,241 |
United States of America |
Elongated Conductors and Methods of Making and Using the Same |
5/19/2036 |
|
10,238,340 |
United States of America |
Elongated Conductors and Methods of Making and Using the Same |
5/19/2036 |
|
10,485,484 |
United States of America |
Elongated Conductors and Methods of Making and Using the Same |
5/19/2036 |
|
10,869,635 |
United States of America |
Elongated Conductors and Methods of Making and Using the Same |
5/19/2036 |
|
11,445,979 |
United States of America |
Elongated Conductors and Methods of Making and Using the Same |
5/19/2036 |
| 12,070,334 | United States of America | Elongated Conductors and Methods of Making and Using the Same | 5/19/2036 |
|
107924736 |
China |
Elongated Conductors and Methods of Making and Using the Same |
5/19/2036 |
|
10,874,830 |
United States of America |
Smart Torquer and Methods of Using the Same |
6/14/2037 |
The following table shows our material pending patent applications as of October 31, 2024 (note that no expiration dates are specified for the pending patent applications since, in certain jurisdictions, e.g., United States of America, the patent expiration date is calculated at the time of issue/grant):
Pending Patent Applications
|
Application No. |
Filing Date |
Jurisdiction |
Title |
|
3239581 |
5/24/2024 |
Canada |
Controlled Sympathectomy and Micro-Ablation Systems and Methods |
|
21168169.7 |
5/13/2021 |
European Patent Office |
Controlled Sympathectomy and Micro-Ablation Systems and Methods |
|
16/591,126 |
10/2/2019 |
United States of America |
Controlled Sympathectomy and Micro-Ablation Systems and Methods |
|
17/325,833 |
5/20/2021 |
United States of America |
Controlled Sympathectomy and Micro-Ablation Systems and Methods |
|
3158197 |
5/4/2022 |
Canada |
Endoscopic Sympathectomy Systems and Methods |
|
18/771,099 |
7/12/2024 |
United States of America |
Endoscopic Sympathectomy Systems and Methods |
|
3184524 |
12/15/2022 |
Canada |
Devices, Systems, and Methods for Diagnosis and Treatment of Overactive Bladder |
|
202448028613 |
4/8/2024 |
India |
Devices, Systems, and Methods for Diagnosis and Treatment of Overactive Bladder |
|
18/079,361 |
12/12/2022 |
United States of America |
Devices, Systems, and Methods for Diagnosis and Treatment of Overactive Bladder |
|
3183802 |
12/9/2022 |
Canada |
Systems and Methods for Treatment of Tissues Within and/or Through a Lumen Wall |
|
18/656,031 |
5/6/2024 |
United States of America |
Systems and Methods for Treatment of Tissues Within and/or Through a Lumen Wall |
|
3151434 |
3/9/2022 |
Canada |
Systems and Methods for Regulating Organ and/or Tumor Growth Rates, Function, and/or Development |
|
EP24170108.5 |
4/12/2024 |
European Patent Office |
Systems and Methods for Regulating Organ and/or Tumor Growth Rates, Function, and/or Development |
|
16/855,080 |
4/22/2020 |
United States of America |
Systems and Methods for Regulating Organ and/or Tumor Growth Rates, Function, and/or Development |
|
17/971,202 |
10/21/2022 |
United States of America |
Systems and Methods for Regulating Organ and/or Tumor Growth Rates, Function, and/or Development |
|
3,205,904 |
7/10/2023 |
Canada |
Systems and Methods for Neurological Traffic and/or Receptor Functional Evaluation and/or Modification |
|
19159405 |
2/26/2019 |
European Patent Office |
Systems and Methods for Neurological Traffic and/or Receptor Functional Evaluation and/or Modification |
|
202348081808 |
12/1/2023 |
India |
Systems and Methods for Neurological Traffic and/or Receptor Functional Evaluation and/or Modification |
|
18/104,460 |
2/1/2023 |
United States of America |
Systems and Methods for Neurological Traffic and/or Receptor Functional Evaluation and/or Modification |
|
3182302 |
11/28/2022 |
Canada |
Systems and Methods for Treating Cancer and/or Augmenting Organ Function |
|
22175617.4 |
5/26/2022 |
European Patent Office |
Systems and Methods for Treating Cancer and/or Augmenting Organ Function |
|
18/772/819 |
7/15/2024 |
United States of America |
Systems and Methods for Treating Cancer and/or Augmenting Organ Function |
|
17/833,964 |
6/7/2022 |
United States of America |
Systems and Methods for Treating Cancer and/or Augmenting Organ Function |
|
23182480.6 |
6/29/2023 |
European Patent Office |
ANS Assessment Systems, Kits, and Methods |
|
3,210,898 |
9/1/2023 |
Canada |
ANS Assessment Systems, Kits, and Methods |
|
10202300654X |
3/10/2023 |
Singapore |
ANS Assessment Systems, Kits, and Methods |
|
18/543,538 |
12/18/2023 |
United States of America |
ANS Assessment Systems, Kits, and Methods |
|
23171646.5 |
5/4/2023 |
European Patent Office |
Systems and Methods for Treating Cancer and/or Augmenting Organ Function |
|
15864478.1 |
7/4/2017 |
European Patent Office |
Systems and Methods for Regulating Organ and/or Tumor Growth Rates, Function, and/or Development |
|
202210497355 |
5/9/2022 |
China |
Controlled and Precise Treatment of Cardiac Tissues |
|
23170175.6 |
4/26/2023 |
European Patent Office |
Controlled and Precise Treatment of Cardiac Tissues |
|
17/501,502 |
10/14/2021 |
United States of America |
Controlled and Precise Treatment of Cardiac Tissues |
|
18868117.5 |
4/17/2020 |
European Patent Office |
Medical Devices with Circuitry for Capturing and Processing Signals |
|
18/387,585 |
11/7/2023 |
United States of America |
Medical Devices with Circuitry for Capturing and Processing Signals |
|
PCT/US2023/029193 |
8/1/2023 |
Patent Cooperation Treaty (PCT)/ United States of America, Receiving Office |
Medical Devices Configured for Therapeutic Electroporation of Biologic Tissues |
|
18/779,387 |
7/22/2024 |
United States of America |
Elongated Conductors and Methods of Making and Using the Same |
|
16803986.5 |
11/29/2017 |
European Patent Office |
Elongated Conductors and Methods of Making and Using the Same |
|
16/951,317 |
11/18/2020 |
United States of America |
Smart Torquer and Methods of Using the Same |
|
16854068 |
4/9/2018 |
European Patent Office |
Smart Torquer and Methods of Using the Same |
|
63/636,467 |
4/19/2024 |
United States of America |
Medical Devices Configured for Temperature-Controlled Therapeutic Electroporation |
|
63/636,471 |
4/19/2024 |
United States of America |
Medical Devices Configured for Therapeutic Electroporation with Adjustable Sections of an Elongate Member |
|
63/636,477 |
4/19/2024 |
United States of America |
Medical Device Guide Sheaths with Return Electrodes for Bipolar Therapeutic Electroporation |
Trademarks
The Company has one trademark for the mark “AUTONOMIX” in the United States, Australia, China, the European Union, India, Japan, Mexico, and Singapore. The registrations of these trademarks are effective for varying periods of time and may be renewed periodically provided we comply with all applicable renewal requirements, including, where necessary, the continued use of the trademarks in the applicable jurisdictions in connection with certain goods and services.
License Agreement
In December 2021, we granted a company affiliated with certain early investors in the Company a exclusive worldwide license to our technology for use in the diagnosis and treatment of cardiovascular conditions and/or hypertension through renal denervation-based methods. During the term of the license agreement, we did not receive any fees from the licensee, and did not generate any revenue under the license agreement. On July 7, 2023, we entered into an Exclusive License Termination Agreement (the “Termination Agreement”) with the Licensee in exchange for the issuance, upon the closing of our IPO within one year of the agreement’s execution, of a warrant to purchase shares of the Company for a variable number of shares based on a value of $8.0 million. Upon the closing of our IPO on January 29, 2024, we issued the licensee a warrant to purchase 80,000 shares which were issued at an assumed valuation of $100.00 per share for a fixed value of $8.0 million. The warrants are exercisable at a price of $0.02 per share and may be exercised any time after the issuance date, subject to a beneficial ownership limitation, and expire five years from the original issuance. The warrants provide dividend rights. The shares underlying the warrant are subject to a lockup agreement for a period of six months after the closing of the IPO with respect to 12.5% of the shares issued and twelve months after the closing of the IPO for the remainder of the shares. One of our directors, David Robins, holds a 19.5% interest in the company receiving the warrant.
Competition
The electrophysiology market is served by a number of very large players including, for example, Medtronic and Boston Scientific, all of whom have substantially greater resources than Autonomix.
While the current standards of care for treating pancreatic cancer pain are comprised largely of generic drugs and injections, we are not aware of any person or company working on a transvascular method for treating this indication. Although competitors like Medtronic are expanding the use of transvascular ablation techniques, we are not aware of anyone developing a sensing technology similar in capability to our technology.
Recent tests using Endoscopic Ultrasound-Guided Ablation to treat pancreatic cancer pain have claimed outcomes that may be better than NCPB, however this is still a percutaneous technique that we believe involves similar inherent risks of infection and internal damage as does NCPB. Conversely, we believe these risks are significantly reduced using a transvascular approach such as the Autonomix technology.
Description of Property
The Company leases office space as its headquarters in Texas and has access to a research and development facility in Pennsylvania. We believe our facilities are sufficient to meet our current needs and that suitable space will be available as and when needed.
Employees
As of October 1, 2024, we have 10 individuals providing services to the Company, six on a full-time basis and four on a part-time basis. Accordingly, a high percentage of the work performed for our development projects is conducted by qualified part-time staff and independent contractors.
Legal Proceedings
From time to time, we may become involved in litigation matters relating to claims arising from the ordinary course of business. While the results of such claims and legal actions cannot be predicted with certainty, our management does not believe that there are claims or actions, pending or threatened against us, the ultimate disposition of which would have a material adverse effect on our business, results of operations, financial condition, or cash flows.
Regulation of Our Business
Our product candidate and operations are subject to extensive and rigorous regulation by the U.S. Food and Drug Administration (“FDA”), under the Federal Food, Drug, and Cosmetic Act (“FDCA”), and it’s implementing regulations, guidance documentation, and standards. Our sensing device and catheters are regulated by the FDA as medical devices. The FDA regulates the design, development, research, testing, manufacturing, safety, labeling, storage, record keeping, promotion, distribution, sale and advertising of medical devices in the United States to ensure that medical products distributed domestically are safe and effective for their intended uses. The FDA also regulates the export of medical devices manufactured in the United States to international markets. Any violations of these laws and regulations could result in a material adverse effect on our business, financial condition and results of operations. In addition, if there is a change in law, regulation or judicial interpretation, we may be required to change our business practices, which could have a material adverse effect on our business, financial condition and results of operations.
Unless an exemption applies, before we can commercially distribute medical devices in the United States, we must obtain, depending on the type of device, either prior premarket clearance or premarket approval (PMA), from the FDA. The FDA classifies medical devices into one of three classes:
|
• |
Class I devices, which are subject to only general controls (e.g., labeling, medical devices reporting, and prohibitions against adulteration and misbranding) and, in some cases, to the premarket clearance requirements; |
|
• |
Class II devices, generally requiring premarket clearance before they may be commercially marketed in the United States; and |
|
• |
Class III devices, consisting of devices deemed by the FDA to pose the greatest risk, such as life-sustaining, life-supporting or implantable devices, or devices deemed not substantially equivalent to a predicate device, generally requiring submission of a PMA supported by clinical trial data. |
Our current product candidates are all class II devices and will require submission of a premarket notification.
510(k) Clearance Pathway
When a 510(k) clearance is required, we must submit a premarket notification demonstrating that our proposed device is substantially equivalent to a previously cleared 510(k) device or a device that was in commercial distribution before May 28, 1976 for which the FDA has not yet called for the submission of PMAs. By regulation, the FDA is required to clear or deny a 510(k) premarket notification within 90 days of submission of the application. As a practical matter, clearance may take longer. The FDA may require further information, including clinical data, to make a determination regarding substantial equivalence.
Any modification to a 510(k)-cleared device that would constitute a major change in its intended use, or any change that could significantly affect the safety or effectiveness of the device, requires a new 510(k) clearance and may even, in some circumstances, require a PMA, if the change raises complex or novel scientific issues or the product has a new intended use. The FDA requires every manufacturer to make the determination regarding the need for a new 510(k) submission in the first instance, but the FDA may review any manufacturer's decision.
PMA Pathway
A PMA must be submitted to the FDA if the device cannot be cleared through the 510(k) process. A PMA must be supported by extensive data, including but not limited to, technical, preclinical, clinical trials, manufacturing and labeling to demonstrate to the FDA's satisfaction the safety and effectiveness of the device for its intended use. During the review period, the FDA will typically request additional information or clarification of the information already provided. Also, an advisory panel of experts from outside the FDA may be convened to review and evaluate the application and provide recommendations to the FDA as to the approvability of the device. The FDA may or may not accept the panel's recommendation. In addition, the FDA will generally conduct a pre-approval inspection of the manufacturing facility or facilities to ensure compliance with the QSRs.
New PMAs or PMA supplements are required for modifications that affect the safety or effectiveness of the device, including, for example, certain types of modifications to the device's indication for use, manufacturing process, labeling and design. PMA supplements often require submission of the same type of information as a PMA, except that the supplement is limited to information needed to support any changes from the device covered by the original PMA and may not require as extensive clinical data or the convening of an advisory panel.
de novo Classification
Medical device types that the FDA has not previously classified as Class I, II or III are automatically classified into Class III regardless of the level of risk they pose. The FDA Modernization Act of 1997 established a new route to market for low to moderate risk medical devices that are automatically placed into Class III due to the absence of a predicate device, called the “Request for Evaluation of Automatic Class III Designation,” or the de novo classification procedure. This procedure allows a manufacturer whose novel device is automatically classified into Class III to request down-classification of its medical device into Class I or Class II on the basis that the device presents low or moderate risk, rather than requiring the submission and approval of a PMA application. Prior to the enactment of the FDA Safety and Innovation Act of 2012, or the FDASIA, a medical device could only be eligible for de novo classification if the manufacturer first submitted a 510(k) premarket notification and received a determination from the FDA that the device was not substantially equivalent. FDASIA streamlined the de novo classification pathway by permitting manufacturers to request de novo classification directly without first submitting a 510(k) premarket notification to the FDA and receiving a not substantially equivalent determination. Under FDASIA, the FDA is required to classify the device within 120 days following receipt of the de novo application. If the manufacturer seeks reclassification into Class II, the manufacturer must include a draft proposal for special controls that are necessary to provide a reasonable assurance of the safety and effectiveness of the medical device. In addition, the FDA may reject the reclassification petition if it identifies a legally marketed predicate device that would be appropriate for a 510(k) or determines that the device is not low to moderate risk or that general controls would be inadequate to control the risks and special controls cannot be developed.
Clinical Trials
Clinical trials are generally required to support a PMA application and are sometimes required for 510(k) or de novo clearance. Such trials generally require an investigational device exemption application, or IDE, approved in advance by the FDA for a specified number of patients and study sites, unless the product is deemed a nonsignificant risk device eligible for more abbreviated IDE requirements. Clinical trials are subject to extensive monitoring, record keeping and reporting requirements. Clinical trials must be conducted under the oversight of an institutional review board, or IRB, for the relevant clinical trial sites and must comply with FDA regulations, including but not limited to those relating to good clinical practices. To conduct a clinical trial, we also are required to obtain the patients' informed consent in form and substance that complies with both FDA requirements and state and federal privacy and human subject protection regulations. We, the FDA or the IRB could suspend a clinical trial at any time for various reasons, including a belief that the risks to study subjects outweigh the anticipated benefits. Even if a trial is completed, the results of clinical testing may not adequately demonstrate the safety and efficacy of the device or may otherwise not be sufficient to obtain FDA approval to market the product in the U.S. Similarly, in Europe the clinical study must be approved by a local ethics committee (EC) and in some cases, including studies with high-risk devices, by the ministry of health in the applicable country.
Pervasive and Continuing Regulation
After a device is placed on the market, numerous regulatory requirements apply. These include:
|
• |
Product listing and establishment registration, which helps facilitate FDA inspections and other regulatory action; |
|
• |
Quality System Regulation, or QSR, which requires manufacturers, including third-party manufacturers, to follow stringent design, testing, control, documentation and other quality assurance procedures during all aspects of the manufacturing process; |
|
• |
labeling regulations and FDA prohibitions against the promotion of products for uncleared, unapproved or off-label use or indication; |
|
• |
clearance of product modifications that could significantly affect safety or efficacy or that would constitute a major change in intended use of one of our cleared devices; |
|
• |
approval of product modifications that affect the safety or effectiveness of one of our approved devices; |
|
• |
medical device reporting regulations, which require that manufacturers comply with FDA requirements to report if their device may have caused or contributed to a death or serious injury, or has malfunctioned in a way that would likely cause or contribute to a death or serious injury if the malfunction of the device or a similar device were to recur; |
|
• |
post-approval restrictions or conditions, including post-approval study commitments; |
|
• |
post-market surveillance regulations, which apply when necessary to protect the public health or to provide additional safety and effectiveness data for the device; |
|
• |
the FDA's recall authority, whereby it can ask, or under certain conditions order, device manufacturers to recall from the market a product that is in violation of governing laws and regulations; |
|
• |
regulations pertaining to voluntary recalls; and |
|
• |
notices of corrections or removals. |
Advertising and promotion of medical devices, in addition to being regulated by the FDA, are also regulated by the Federal Trade Commission and by state regulatory and enforcement authorities. Recently, promotional activities for FDA-regulated products of other companies have been the subject of enforcement action brought under healthcare reimbursement laws and consumer protection statutes. In addition, under the federal Lanham Act and similar state laws, competitors and others can initiate litigation relating to advertising claims. In addition, we are required to meet regulatory requirements in countries outside the U.S., which can change rapidly with relatively short notice. If the FDA determines that our promotional materials or training constitutes promotion of an unapproved use, it could request that we modify our training or promotional materials or subject us to regulatory or enforcement actions.
Furthermore, our products could be subject to voluntary recall if we or the FDA determine, for any reason, that our products pose a risk of injury or are otherwise defective. Moreover, the FDA can order a mandatory recall if there is a reasonable probability that our device would cause serious adverse health consequences or death.
The FDA has broad post-market and regulatory enforcement powers. Once we have a marketed product, we will be subject to unannounced inspections by the FDA to determine our compliance with the QSR and other regulations, and these inspections may include the manufacturing facilities of some of our subcontractors. Failure by us or by our suppliers to comply with applicable regulatory requirements can result in enforcement action by the FDA or other regulatory authorities, which may result in sanctions including, but not limited to:
|
• |
untitled letters, warning letters, fines, injunctions, consent decrees and civil penalties; |
|
• |
unanticipated expenditures to address or defend such actions |
|
• |
customer notifications for repair, replacement, refunds; |
|
• |
recall, detention or seizure of our products; |
|
• |
operating restrictions or partial suspension or total shutdown of production; |
|
• |
refusing or delaying our requests for premarket clearance or premarket approval of new products or modified products; |
|
• |
operating restrictions; |
|
• |
withdrawing premarket clearances or PMA approvals that have already been granted; |
|
• |
refusal to grant export approval for our products; or |
|
• |
criminal prosecution. |
Glossary
Unless we otherwise indicate, or unless the context requires otherwise, any references in this prospectus to the following medical terms have the respective meanings set forth below:
ablation: the removal or destruction of a body part or tissue or its function. This is often conducted with energy-based devices utilizing radio frequency or pulsed electrical field energy.
cardiomyocyte: the cell responsible for the contraction of the heart.
catheter: a thin tube made from medical grade materials serving a broad range of functions; catheters are medical devices that can be inserted in the body to treat diseases or perform a surgical procedure.
celiac plexus: also known as the solar plexus because of its radiating nerve fibers, is a complex network of nerves located in the abdomen, near where the celiac trunk, and other arteries branch from the abdominal aorta.
electrophysiology: the branch of physiology that deals with the electrical phenomena associated with nervous and other bodily activity.
hypertension: high blood pressure.
microvolt: one millionth of a volt.
palliative care: specialized medical care for people living with a serious illness focused on providing relief from the symptoms and stress of serious illness.
renal artery: the main blood vessel that supplies blood to a kidney.
transvascular: across the wall of a blood vessel (or similar vessel).
unresectable: unable to be removed with surgery.
Available Information
Our Internet address is www.autonomix.com. On this website, we post the following filings as soon as reasonably practicable after they are electronically filed with or furnished to the SEC: our Annual Reports on Form 10-K; our Quarterly Reports on Form 10-Q; our Current Reports on Form 8-K; our proxy statements related to our annual stockholders’ meetings; and any amendments to those reports or statements. All such filings are available on our website free of charge. The charters of our audit, nominating and governance and compensation committees and our Code of Business Conduct and Ethics Policy are also available on our website and in print to any stockholder who requests them. The content on our website is not incorporated by reference into this prospectus.
MANAGEMENT
The following table sets forth the names and ages of all of our directors and executive officers as of October 1, 2024. Our officers are appointed by, and serve at the pleasure of, the Board of Directors.
|
Name |
Age |
Position |
||
|
Brad Hauser |
47 |
Chief Executive Officer |
||
|
Lori Bisson |
54 |
Vice Chair and Director |
||
|
Dr. Robert Schwartz |
73 |
Chief Medical Officer |
||
|
Landy Toth |
47 |
Chief Technology Officer |
||
|
Trent Smith |
55 |
Chief Financial Officer |
||
|
Walter V. Klemp |
65 |
Executive Chairman |
||
|
Jonathan P. Foster |
60 |
Director |
||
|
David Robins |
55 |
Director |
||
|
Christopher Capelli |
64 |
Director |
Set forth below is biographical information about each of the individuals named in the table above:
Bradley Hauser, Chief Executive Officer. Mr. Hauser became our President and Chief Executive Officer effective June 17, 2024. Prior to his appointment as President and Chief Executive Officer, Mr. Hauser served as the Chief Operating Officer at Beauty Health from January 2023 to June 2024. Mr. Hauser previously served as the President and Chief Executive Officer of Soliton, Inc., a medical device company focused on developing new technology for use in aesthetics, from November 2020 to December 2021. Soliton was purchased by AbbVie in 2021 and he continued to provide transition support post-acquisition as the President and Chief Executive Officer of Soliton with Allergan Aesthetics, an AbbVie company, until July 2022. Mr. Hauser previously served as Vice President, Research and Development and General Manager for CoolSculpting at Allergan Pharmaceuticals since ZELTIQ Aesthetics, Inc. was acquired by Allergan in April 2017. Previously, he served as the Senior Vice President of Research and Development at ZELTIQ Aesthetics, Inc. from January 2017 to April 2017 and as its Vice President of Research and Development from July 2015 to January 2017. Mr. Hauser joined ZELTIQ in December 2013 as Vice President of Product and Clinical Strategy. Prior to joining ZELTIQ, he held multiple roles in the aesthetic industry, including Executive Vice President of Commercial Operations for Cutera, Director of Research and Development at Medicis and Managing Director of Product and Clinical Marketing at Solta Medical. Mr. Hauser received his B.A. degree in Human Biology from Stanford University.
Lori Bisson, Vice Chair and Director. Ms. Bisson joined us in July 2023 as a director and served as our Chief Executive Officer from July 2023 until June 2024 and as Vice Chair since June 2024. From January 2015 until June 2022, Ms. Bisson served as Chief Financial Officer of Soliton, Inc., a medical device company focused on developing new technology for use in aesthetics. Soliton was purchased by AbbVie in 2021 and she continued to provide transition support post-acquisition. Prior to joining Soliton, Ms. Bisson worked as a financial and business development consultant as a shareholder in Condon & Company, PC, from 2009 through December 2014, where she advised a number of life science companies. From 2005 to 2009, Ms. Bisson served as the Chief Financial Officer and Vice President of Operations for Zeno Corporation, a medical device company focused on new technology in the aesthetics area. Ms. Bisson previously served as the Chief Financial Officer of Gulfstream Trading, Ltd., an international oil trading organization from 2001 to 2005. From 1995 to 2001, Ms. Bisson held various positions with Drypers Corporation, a publicly traded multinational consumer products company, where she ultimately held the title of Vice President of Integrated Solutions and oversaw accounting, information technology, and logistics for the U.S. operation. Ms. Bisson began her career at Arthur Andersen, LLP as an auditor focused on consumer products companies. Ms. Bisson also serves as an advisor to Moleculin Biotech, Inc., a clinical stage pharmaceutical company focused on the development of oncology drug candidates. Ms. Bisson is a Certified Public Accountant and holds a B.A. degree in Accounting from Baylor University. We believe that Ms. Bisson’s extensive experience in the medical device field provides her with the qualifications to serve as a director.
Dr. Robert Schwartz, Chief Medical Officer. Dr. Schwartz was a co-founder of our company, has served as our Chief Medical Officer since June 2023, and served as our Chief Executive Officer from February 2022 until June 2023. Dr. Schwartz is President of the Jon DeHaan Center for Medical Innovation. He was previously Director at the Center for Applied Vascular Biology and Interventions, Mayo Foundation. Dr. Schwartz has also served as Professor and Associate Professor at the Mayo Medical School. He holds Board Certifications on the National Board of Medical Examiners, American Board of Internal Medicine, and the American Board of Internal Medicine, Cardiovascular Diseases. He has published numerous articles in peer review journals dealing with various topics in interventional cardiology. Dr. Schwartz is the recipient of various awards including the Andreas Gruentzig Award for Basic Research in Coronary Restenosis from the Thoraxcenter/European Society of Cardiology. He holds professional memberships as Fellow of the American College of Cardiology, American Heart Association, and The Society for Cardiac Angiography and Interventions. Dr. Schwartz is also a member of the Society of Atherosclerosis Imaging. Dr. Schwartz received a Master’s Degree in Electrical Engineering, and his doctorate from the University of Colorado-Denver and continued his internship, residency, and Fellowship at the Mayo Graduate School of Medicine. Dr. Schwartz has agreed to provide services to us on a part-time basis of 25% of his working time.
Landy Toth, Chief Technology Officer. Mr. Toth is a key inventor and is responsible for Autonomix’s development efforts to date. Mr. Toth founded Tricord Holdings, LLC in 2012 and Autonomix in August 2014 and has been the Chief Technology Officer since that time. In addition to these efforts, he founded and has filled the role of Chief Technology Officer of LifeLens Technologies, Inc. since September 2016. Over the past 20 years, Mr. Toth has successfully commercialized medical device technologies within startup environments. His focus has been the development and commercialization of wearable and interventional diagnostic medical technologies. He presently has 647 publications across 56 patent families. His portfolio has a grant rate greater than 70% after 2 years. Mr. Toth holds a MASc from the University of Toronto and a BASc from the University of Waterloo. Mr. Toth has also been an employee of Davos Chemical Corporation since January 2011. Mr. Toth has agreed to provide services to us on a part-time basis of 25% of his working time.
Trent Smith, Chief Financial Officer. Mr. Smith joined us in July 2023 as our Chief Financial Officer. Mr. Smith has extensive management, accounting, financial and international experience. From June 2018 to September 2022, Mr. Smith served as the Corporate Controller and Vice-President of Soliton, Inc., a medical device company focused on developing new technology for use in aesthetics that was acquired by AbbVie, Inc. in December 2021 and he continued to provide transition support post acquisition. From 2011 to 2018, Mr. Smith held various positions with InfuSystem Holdings, Inc., a national provider of infusion pumps and related services to the healthcare industry, where he served as Corporate Controller and Vice-President before a promotion to Chief Accounting Officer and Executive Vice President. From 2010 to 2011, Mr. Smith served as the Director of External Reporting of Syncreon Holdings, Inc., an international leader in global supply chain management, where he was responsible for creating the external reporting department to be compliant with SEC type reporting. From 2006 to 2010, Mr. Smith served as the Director of Accounting and Financial Reporting of Champion Homes, Inc., a leader in the manufactured housing industry and one of the largest modular homebuilders in North America. From 2005 to 2006, Mr. Smith served as the Director of External Financial Reporting for Dura Automotive Systems, a Tier 1 international designer and manufacturer of automotive components headquartered in Auburn Hills, MI. From 1999 to 2006, Mr. Smith served in various roles and divisions for Valeo, Inc., an international Tier 1 automotive supplier, including as Financial Controller and Treasurer of Valeo Distribution North America and Director of Accounting and Internal Controls for Valeo Wiper Systems, Valoe’s largest division in North America. Mr. Smith began his professional career as an auditor with Deloitte & Touche, LLP in 1995. Mr. Smith served on active duty in the United States Navy from 1987 through 1991 and the reserves until 1993. Mr. Smith is a Certified Public Accountant and a graduate of the University of Illinois where he earned a Bachelor of Science in Accounting.
Walter V. Klemp, Executive Chairman. Mr. Klemp joined us in January 2022 as our Executive Chairman. Mr. Klemp is a co-founder of Moleculin Biotech, Inc., a clinical-stage pharmaceutical company, and has served as its Chairman of the Board and Chief Executive Officer since July 2015 and as president since August 2017. From July 2018 until December 2021, Mr. Klemp served as Executive Chairman on the Board of Directors of Soliton, Inc., a medical device company focused on developing new technology for use in aesthetics. In December 2021 Soliton, Inc. was acquired by AbbVie, Inc. From November 2011 to July 2018, Mr. Klemp served as Chief Executive Officer of Soliton. Mr. Klemp served as President and Chief Executive Officer of Zeno Corporation from 2004 to April 2011, where he developed and marketed dermatology devices and drugs from concept through FDA approval and market launch. From 1987 to 2000, Mr. Klemp served as Chief Executive Officer and Chairman of Drypers Corporation, a publicly traded multinational consumer products company that was listed as #1 on the INC 500 List of America’s Fastest Growing Companies. We believe that Mr. Klemp’s extensive experience in the medical device field provide him with the qualifications to serve as a Chairman of the Board.
Jonathan P. Foster, Director. Mr. Foster joined us in January 2022 as a director. Mr. Foster has served as the Chief Financial Officer and Executive Vice President of Moleculin Biotech, Inc., a clinical-stage pharmaceutical company, since August 2016. Mr. Foster brings more than 30 years in financial experience holding a variety of executive and senior financial positions with public, private, start-up to large corporate and international companies. From February 2012 to August 2016, Mr. Foster served as Chief Financial Officer and Executive Vice President of InfuSystem Holdings, Inc., a national provider of infusion pumps and related services to the healthcare industry. From May 2011 to January 2012, Mr. Foster served as a consultant to the Chief Financial Officer of LSG Sky Chefs, USA, Inc., a subsidiary of Deutsche Lufthansa AG. Prior to that Mr. Foster served in various C-suite capacities with public and private companies with his start beginning as Manager at Deloitte & Touche, LLP. Mr. Foster served on the Board of Financial Institutions for the State of South Carolina from 2006 to 2012 and from June 2018 until December 2021 served on the Board of Directors of Soliton, Inc., a medical device company focused on developing new technology for use in aesthetics, where he was the Chairman of the Audit and Compensation Committees and the past Chairman of the Nominating & Governance Committee. In December 2021 Soliton, Inc. was acquired by AbbVie, Inc. Since June 2021, Mr. Foster has served on the Board of Directors of Volcon, Inc. where he is the past Chairman and current member of the Audit Committee, a member of the Nominating & Governance Committee and is the Chairman of the Compensation Committee. Mr. Foster is a Certified Public Accountant (South Carolina) and holds the designation of Chartered Global Management Accountant from the American Institute of Certified Public Accountants. He received his BS in Accounting from Clemson University in 1985. We believe that Mr. Foster’s extensive experience in the medical field provide him with the qualifications to serve as a director.
David Robins, Director. Mr. Robins was a co-founder of our company and has served as a director since February 2022. Mr. Robins is also a founder and board member of LifeLens that is currently developing on the body sensing devices. Since 2013, Mr. Robins has served as co-CEO of DavosPharma, a privately held corporation that focuses on supporting biotechnology and medical device companies with manufacturing their products for clinical trials and commercialization. He received his BS in Engineering Chemistry from Queen’s University in Kingston, Ontario and a MS in Chemical Engineering from Syracuse University. Mr. Robins has a significant background in taking medical devices and drugs from clinical trials to commercialization and FDA approvals. We believe that Mr. Robins’ extensive experience in medical device development provide him with the qualifications to serve as a director.
Christopher Capelli, Director. Dr. Capelli currently works at AbbVie as the Scientific Officer & Medical Device Advisor/Soliton for Allergan Aesthetics R&D Surgical Devices. Prior to being acquired by AbbVie in December 2021, he was the Vice Chairman of the Board, Chief Science Officer and Co-founder for Soliton, Inc., a medical device company that was commercializing RESONIC™ for the dermatologic esthetics marketplace based on its Rapid Acoustic Pulse (“RAP”) technology. Dr. Capelli is the lead inventor in Soliton’s RAP technology. A graduate of Massachusetts Institute of Technology (“MIT”) with a Bachelor of Science degree in Mechanical Engineering, Dr. Capelli earned his MD from the University of Wisconsin Medical School and maintains a medical license in the State of Wisconsin. As a result of his scientific work since graduating from MIT, Dr. Capelli holds over 100 issued patents and patent applications worldwide. These patents served as the basis for the creation of five companies having a total market capitalization greater than $36 billion. As a businessman, Dr. Capelli has been directly involved in the start-up of numerous venture-capital backed biomedical company ventures. We believe that Dr. Capelli’s extensive experience in medical device development provide him with the qualifications to serve as a director.
No director is related to any other director or executive officer of our company or our subsidiaries, and, there are no arrangements or understandings between a director and any other person pursuant to which such person was elected as director.
EXECUTIVE AND DIRECTOR COMPENSATION
Director Independence
The rules of the Nasdaq Stock Market, or the Nasdaq Rules, require a majority of a listed company’s board of directors to be composed of independent directors. In addition, the Nasdaq Rules require that, subject to specified exceptions, each member of a listed company’s audit, compensation and nominating and governance committees be independent. Under the Nasdaq Rules, a director will only qualify as an independent director if, in the opinion of our Board of Directors, that person does not have a relationship that would interfere with the exercise of independent judgment in carrying out the responsibilities of a director. The Nasdaq Rules also require that audit committee members satisfy independence criteria set forth in Rule 10A-3 under the Securities Exchange Act of 1934, as amended, or the Exchange Act. In order to be considered independent for purposes of Rule 10A-3, a member of an audit committee of a listed company may not, other than in his or her capacity as a member of the audit committee, the board of directors, or any other board committee, accept, directly or indirectly, any consulting, advisory, or other compensatory fee from the listed company or any of its subsidiaries or otherwise be an affiliated person of the listed company or any of its subsidiaries. In considering the independence of compensation committee members, the Nasdaq Rules require that our board of directors must consider additional factors relevant to the duties of a compensation committee member, including the source of any compensation we pay to the director and any affiliations with our company.
Our board of directors undertook a review of the composition of our board of directors and its committees and the independence of each director. Based upon information requested from and provided by each director concerning his or her background, employment and affiliations, including family relationships, our board of directors has determined that each of our directors, with the exception of Ms. Bisson and Mr. Klemp, are independent as defined under the Nasdaq Rules.
Executive Officer Compensation
Summary Compensation Table – 2024
|
Name and Principal Position |
Year |
Salary |
Non-Equity Incentive Plan Compensation |
Option Awards |
All Other Compensation |
Total |
||||||||||||||||
|
Lori Bisson - Chief Executive Officer (3) |
2024 |
$ | 225,000 | $ | 150,000 | $ | 1,823,991 | $ | 17,505 | $ | 2,216,496 | |||||||||||
|
Dr. Robert Schwartz, Former Chief Executive Officer and current Chief Medical Officer |
2024 |
$ | 100,000 | $ | 35,000 | $ | 247,861 | $ | - | $ | 382,861 | |||||||||||
|
2023 |
$ | 100,000 | $ | - | $ | - | $ | - | $ | 100,000 | ||||||||||||
|
Landy Toth - Chief Technology Officer |
2024 |
$ | 187,500 | $ | - | $ | 165,241 | $ | - | $ | 352,741 | |||||||||||
|
2023 |
$ | 156,250 | $ | - | $ | - | $ | 156,250 | ||||||||||||||
|
Trent Smith - Chief Financial Officer |
2024 |
$ | 159,375 | $ | 74,250 | $ | 888,595 | $ | 18,114 | $ | 1,140,334 | |||||||||||
|
Walter Klemp - Executive Chair |
2024 |
$ | 200,000 | $ | - | $ | 165,241 | $ | - | $ | 365,241 | |||||||||||
|
2023 |
$ | 200,000 | $ | - | $ | - | $ | - | $ | 200,000 | ||||||||||||
|
(1) |
Represents the full grant date fair value of the options granted calculated in accordance with FASB ASC Topic 718. These amounts do not necessarily correspond to the actual value that may be realized by the named executive officer. Included in the award amount for Ms. Bisson and Mr. Smith were $1,468,725 and $673,783 for shares earned in fiscal 2024 awarded on June 30, 2023 and July 24, 2023, respectively. Also included in the award amount for Ms. Bisson, Dr. Schwartz, Mr. Toth, Mr. Smith and Mr. Klemp were $355,266, $247,861, $165,241, $214,812, and $165,241 for shares earned in fiscal 2024 but awarded on June 21, 2024. For a summary of the assumptions made in the valuation of the awards, please see Note 4 to our financial statements as of and for the period ended March 31, 2024 included in this prospectus.
|
|
(2) |
Other compensation amount represents health care insurance costs the Company currently pays for all employees, including $12,634 each for Ms. Bisson and Mr. Smith. In addition, the Company reimbursed Ms. Bisson $4,871 and Mr. Smith $5,480 each for COBRA reimbursements. |
|
(3) |
Ms. Bisson joined the Company on July 1, 2023. Ms. Bisson’s annual base salary was $300,000. |
|
(4) |
Mr. Smith joined the Company on July 24, 2023. Mr. Smith’s annual base salary was $225,000. |
Employment Agreements
Brad Hauser Employment Agreement
On June 17, 2024, we entered into an employment agreement with Brad Hauser pursuant to which Mr. Hauser agreed to serve as our chief executive officer and president for an initial three-year period, which may be extended on a year-to-year basis. Mr. Hauser’s agreement provides for an initial annual base salary of $450,000 (subject to an annual review and increase at the discretion of our Compensation Committee) and a target annual bonus of 60% of his base salary. Pursuant to the agreement, Mr. Hauser was granted a ten-year option (the “Inducement Options”) to purchase 45,000 shares of common stock at an exercise price equal to the closing price of our common stock on the date of the employment agreement. The option vests in four equal annual installments (or 11,250 shares each installment) on each of the succeeding four anniversary dates of the execution of the employment agreement, provided Mr. Hauser is employed by us on each vesting date. In the event of a “change of control” or the termination of the agreement by us without “cause” or by Mr. Hauser for “good reason,” all of the unvested options shall immediately vest. The Inducement Options were granted outside of our 2023 Stock Plan as an inducement material to Mr. Hauser’s entering into employment with us in accordance with Nasdaq Stock Market Listing Rule 5635(c)(4). Commencing with the year ending March 31, 2025, Mr. Hauser will be eligible to receive annual option grants as determined by the Compensation Committee of the Board of Directors, based on criteria established by the Compensation Committee. The number of shares underlying the target annual option grant will be equal to $1,000,000 divided by the Black-Scholes value per share of our common stock on the date of grant.
If Mr. Hauser’s employment is terminated at our election without “cause,” or by Mr. Hauser for “good reason,” Mr. Hauser shall be entitled to receive severance payments equal to twelve months of Mr. Hauser’s base salary and 100% of the target bonus for the year in which such termination occurs; provided that such amounts shall be increased by 50% if Mr. Hauser’s agreement is terminated without “cause” or by Mr. Hauser for “good reason” within three months prior to or twelve months after a “change of control.” In the event that any payments or benefits provided to Mr. Hauser would trigger the excise tax under Section 4999 of the Internal Revenue Code or any similar provision, the Company agreed to provide Mr. Hauser with a gross-up payment to ensure that, after payment of all taxes (including the excise tax, federal, state, and local income taxes, and employment taxes) imposed on the gross-up payment, Mr. Hauser receives a net amount equal to the payments or benefits Mr. Hauser would have received if the excise tax didn't apply.
Lori Bisson – Vice Chair (former Chief Executive Officer)
On June 17, 2024, we entered into an employment agreement with Lori Bisson pursuant to which Ms. Bisson agreed to serve as our Executive Vice Chair and Strategic Adviser to the Chief Executive Officer (“Vice Chair”) for a two-year period. Ms. Bisson’s agreement provides for an initial annual base salary of $150,000 (subject to an annual review and increase at the discretion of our Compensation Committee) and a target annual bonus of 50% of her base salary. Pursuant to the agreement, Ms. Bisson continued to vest in the option grants issued to Ms. Bisson in her role as chief executive officer and president in accordance with the vesting schedule set out in her initial employment agreement. In the event of a “change of control” or the termination of the agreement by us without “cause” or by Ms. Bisson for “good reason,” all of the unvested options shall immediately vest. Ms. Bisson is entitled to receive any compensation, including incentive compensation, for the fiscal year ended March 31, 2024 that has not been paid as of the date of the agreement. Commencing with the year ending March 31, 2025, Ms. Bisson will be eligible to receive annual option grants as determined by the Compensation Committee of the Board of Directors, based on criteria established by the Compensation Committee. Ms. Bisson agreed to waive any severance payments due to her in connection with the termination of the prior employment agreement that we entered into with her on June 30, 2023.
On June 30, 2023, we entered into an employment agreement (the “prior agreement”) with Lori Bisson pursuant to which Ms. Bisson agreed to serve as our Chief Executive Officer commencing July 1, 2023 for an initial term of three years, which would have been automatically renewed for additional one-year terms unless either party chose not to renew the prior agreement. The prior agreement provided for an initial annual base salary of $300,000. Ms. Bisson was eligible to receive an annual bonus of up to 50% of her base salary, provided that the final determination on the amount of the annual bonus, if any, would be made by the Compensation Committee of the Board of Directors, based on criteria established by the Compensation Committee.
Pursuant to the prior agreement, Ms. Bisson was granted a ten-year option to purchase 46,680 shares of our common stock at an exercise price of $40.00 per share. The option vests in 48 equal installments (or approximately 973 shares each installment) on each of the succeeding forty-eight monthly anniversary dates of the execution of the prior agreement, provided Ms. Bisson is either CEO or a member of the Board of Directors on such vesting date. In the event of a “change of control” or the termination of the prior agreement by us without “cause” or by Ms. Bisson for “good reason,” all of the unvested options shall immediately vest. Commencing with the first full compensation year subsequent to the completion of this offering, Ms. Bisson was eligible to receive annual option grants as determined by the Compensation Committee of the Board of Directors, based on criteria established by the Compensation Committee. The number of shares underlying the target annual option grant for the initial grant after the completion of the IPO was to be equal to $300,000 divided by the Black-Scholes value per share of our common stock on the date of grant. In the event Ms. Bisson’s prior agreement was terminated without “cause” or by Ms. Bisson for “good reason” within three months prior to or twelve months after of a “change of control”, all of the unvested options shall immediately vest.
If Ms. Bisson’s employment was terminated at our election without “cause”, which required 90 days advance notice, or by Ms. Bisson for “good reason,” Ms. Bisson was entitled to receive severance payments equal to twelve months of Ms. Bisson’s base salary and 100% of the target bonus for the year in which such termination occurs; provided that such amounts would have been increased by 50% if Ms. Bisson’s agreement was terminated without “cause” or by Ms. Bisson for “good reason” within three months prior to or twelve months after of a “change of control.” Ms. Bisson agreed not to compete with us until twelve months after the termination of her employment.
Trent Smith – Chief Financial Officer
On July 24, 2023, we entered into an employment agreement with Trent Smith pursuant to which Mr. Smith agreed to serve as our Chief Financial Officer for an initial term of three years, which will be automatically renewed for additional one-year terms unless either party provides 90 days written notice to the other party of its decision not to renew the agreement. The agreement provided for an initial annual base salary of $225,000. Mr. Smith is eligible to receive an annual bonus of up to 33% of his base salary, provided that the final determination on the amount of the annual bonus, if any, will be made by the Compensation Committee of the Board of Directors, based on criteria established by the Compensation Committee.
Pursuant to the agreement, Mr. Smith was granted a ten-year option to purchase 21,250 shares of our common stock at an exercise price of $40.00 per share. The option vests vest in four equal annual installments (or approximately 5,313 shares each installment) on each of the succeeding four anniversary dates of the execution of the employment agreement, provided Mr. Smith is CFO on such vesting date. In the event of a “change of control” or the termination of the agreement by us without “cause” or by Mr. Smith for “good reason,” all of the unvested options shall immediately vest. Commencing with the fiscal year ended March 31, 2025, Mr. Smith will be eligible to receive annual option grants as determined by the Compensation Committee of the Board of Directors, based on criteria established by the Compensation Committee.
If Mr. Smith’s employment is terminated upon his disability or death, at our election without “cause”, which requires 90 days advance notice, or by Mr. Smith for “good reason,” which requires 30 days advance notice, Mr. Smith shall be entitled to receive severance payments equal to nine months of Mr. Smith’s base salary and 100% of the target bonus for the year in which such termination occurs; provided that such amounts shall be increased to thirteen and one-half months of Mr. Smith’s base salary and 125% of the target bonus for the year in which such termination occurs if Mr. Smith’s agreement is terminated without “cause” or by Mr. Smith for “good reason” within three months prior to or twelve months after a “change of control.” Mr. Smith agreed not to compete with us until twelve months after the termination of his employment.
Other Agreements
In January 2022, we entered into an at-will employment letter with Dr. Schwartz to serve as our acting Chief Executive Officer on a part-time basis comprising 25% of his working time until we identified a full-time Chief Executive Officer, at which time Dr. Schwartz would become our Chief Medical Officer. Pursuant to the letter, we agreed to pay Dr. Schwartz a base salary of $100,000 per year, and to pay Dr. Schwartz an annual incentive cash bonus of up to 35% of his base salary subject to the achievement of agreed upon goals and objectives and the approved of our Board of Directors or Compensation Committee. In connection with the employment letter, we issued Dr. Schwartz a grant of 20,000 shares of our common stock. Upon the appointment of Ms. Bisson as Chief Executive Officer, Dr. Schwartz agreed to serve as our Chief Medical Officer on a part-time basis comprising 25% of his working time.
Effective January 2022, we entered into an amended and restated consulting agreement with Mr. Toth to provide services to us on a part-time basis comprising 25% of his working time. Pursuant to the consulting agreement, we agreed to pay Mr. Toth a base salary of $187,500 per year commencing in June 2022. In connection with the consulting agreement, we issued Mr. Toth a stock grant of 77,500 shares of common stock. The consulting agreement may be terminated by either party on 30 days’ notice.
In January 2022, we entered into a director offer letter with Mr. Klemp to serve as our Executive Chairman. Pursuant to the letter, we agreed to pay Mr. Klemp annual board fees of $200,000 per year. In connection with Mr. Klemp’s appointment to the Board, we issued Mr. Klemp a stock grant of 144,500 shares of common stock.
Narrative Disclosure to Summary Compensation Table
We have established for compensation purposes a compensation year that matches our fiscal year that ends March 31. Subsequent to the end of the fiscal year, our Compensation Committee completes its annual review of executive compensation and determines, after researching comparable companies, the compensation arrangements for the next compensation year.
We review compensation annually for all employees, including our executives. In setting executive base salaries and bonuses and granting equity incentive awards, we consider compensation for comparable positions in the market, the individual executive’s performance as compared to our expectations and objectives, our desire to motivate our employees to achieve short and long-term results that are in the best interests of our stockholders and a long-term commitment to our company. We do not target a specific competitive position or a specific mix of compensation among base salary, bonus or long-term incentives. Our Compensation Committee typically reviews and discusses management’s proposed compensation with the Chief Executive Officer for all executives other than the Chief Executive Officer. Based on those discussions and its discretion, the Compensation Committee then determines the compensation for each executive officer. Our Compensation Committee, without members of management present, discusses and ultimately approves the compensation of our executive officers.
Annual Base Salary
For the 2024 fiscal year, the annual base salaries for Ms. Bisson, Dr. Schwartz, Mr. Toth, Mr. Smith and Mr. Klemp were $300,000, $100,000, $187,500, $225,000 and $200,000, respectively. For the 2025 fiscal year, the base salaries for Ms. Bisson, Dr. Schwartz, Mr. Toth, Mr. Smith, Mr. Klemp and Mr. Hauser will be $375,000, $100,000, $137,500, $285,000, $150,000, and $450,000, respectively. Upon Ms. Bisson’s change in responsibilities in June 2024, her annual base salary was reduced to $150,000.
Annual Bonus and Non-Equity Incentive Plan Compensation.
We seek to motivate and reward our executives for achievements relative to our corporate goals and objectives, and with respect to their respective individual goals, for each fiscal year. For the last fiscal year, the target bonus for Ms. Bisson, Dr. Schwartz, Mr. Toth, Mr. Smith and Mr. Klemp were 50%, 35%, 0%, 33% and 0%, respectively, of their base salary. For the 2025 fiscal year, the target bonus each year for Ms. Bisson, Dr. Schwartz, Mr. Toth, Mr. Smith, Mr. Klemp and Mr. Hauser are 50%, 35%, 0%, 40%, 0%, and 60%, respectively, of their base salary.
The actual performance-based annual bonus paid is calculated by multiplying the executive’s annual base salary, target bonus percentage, the percentage attainment of the corporate goals established by the Board for such year, which represents the total potential bonus payable to our named executive officers, and the percentage attainment of the individual goals approved by our Compensation Committee with respect to our other executive officers. However, the Compensation Committee is not required to calculate bonuses in this manner and retains discretion in the amounts it awards and the factors it takes into consideration in determining bonus amounts. At the end of the fiscal year, the Compensation Committee reviews our performance against our goals and objectives and approves the extent to which we achieved each of our corporate and individual goals and objectives, and, for each named executive officer, the amount of the bonus awarded.
For the last fiscal year, bonuses were awarded based on our achievement of specified corporate goals, including a successful IPO, setting up and beginning our initial proof-of-concept trial, and searching and establishing new relationships with vendors to further the development of our sensing and ablation products, and individual goals, as applicable. Based on the level of achievement, our Compensation Committee awarded Ms. Bisson, Dr. Schwartz, Mr. Smith and Mr. Klemp 100%, respectively, of their potential bonuses for the year. These actual bonus amounts are reflected in the "Non-Equity Incentive Plan Compensation" column of the Summary Compensation Table above.
For the 2025 fiscal year, bonuses will be awarded based on our achievement of specified corporate goals, including securing strategic partnerships, progress on our pivotal trial, completion of financings, and completion of the ongoing clinical proof of concept trial. These corporate goals account for 100% of our base line bonuses. In addition, our Compensation Committee specified a series of “stretch goals,” that, if achieved, would result in an additional 20%.
Long-Term Incentives
Our 2023 Stock Plan provides for the grant of stock options, stock awards, stock unit awards and stock appreciation rights to key employees, non-employee directors and consultants.
Each year our Compensation Committee establishes a value for the expected equity grant issuable to each of our named executive officers. For options, we typically set the option exercise price and grant date fair value based on the closing price of our common stock on Nasdaq on the date of grant, however, there may be instances where we may use an average closing price, up to five days, to set the option exercise price. The shares underlying options typically vest in four equal annual installments. For other equity awards, the grant date fair value is based on the closing price of our common stock on Nasdaq on the date of grant.
For the 2025 fiscal year, the fair value of the equity grants for Ms. Bisson, Mr. Smith and Mr. Klemp were established at $300,000, $302,900, and $233,000, respectively, although the final determination for any equity grants remain at the discretion of the Compensation Committee.
Equity Awards
The following table sets forth certain information concerning our outstanding equity awards for our named executive officers at March 31, 2024.
Outstanding Equity Awards At Fiscal Year-End
|
Option Awards |
||||||||||||||||||||
|
Name and Principal Position |
Grant Date of Equity Award |
Number of Securities Underlying Unexercised Options |
Number of Securities Underlying Unexercised Options |
Option Exercise Price |
Option Expiration |
|||||||||||||||
|
Lori Bisson - Chief Executive Officer |
06/30/23 |
8,753 | 37,927 | $ | 40.00 |
06/30/33 |
||||||||||||||
|
Dr. Robert Schwartz, Former Chief Executive Officer and current Chief Medical Officer |
- | - | - | $ | - | - | ||||||||||||||
|
Trent Smith - Chief Financial Officer |
07/24/23 |
- | 21,250 | $ | 40.00 |
07/24/33 |
||||||||||||||
|
Landy Toth - Chief Technology Officer |
- | - | - | $ | - | - | ||||||||||||||
|
Walter Klemp - Executive Chairman |
- | - | - | $ | - | - | ||||||||||||||
|
(1) |
The shares underlying the options granted on June 30, 2023 for Ms. Bisson vest in equal monthly installments over a four-year period (i.e., 1/48th of each grant vests each month on the anniversary of the grant date). The shares underlying the options granted on July 24, 2023 for Mr. Smith vest in annual installments over a four-year period (i.e., one-quarter of each grant vests on the first, second, third and fourth anniversary of the grant date), subject to continued service with us through each applicable vesting date. |
Director Compensation
In January 2022, we entered into a director offer letter with Mr. Klemp to serve as our Executive Chairman. Pursuant to the letter, we agreed to pay Mr. Klemp annual board fees of $200,000 per year commencing April 1, 2022. In connection with Mr. Klemp’s appointment to the Board, we issued Mr. Klemp a stock grant of 144,500 shares of common stock.
In January 2022, we entered into a director offer letter with Mr. Foster to serve as a director. Pursuant to the letter, we agreed to pay Mr. Foster annual board fees of $50,000 per year. In connection with Mr. Foster’s appointment to the Board, we issued Mr. Foster a stock grant of 35,000 shares of common stock.
In February 2022, we entered into a director offer letter with Mr. Robins to serve as a director. In connection with Mr. Robins’ appointment to the Board, we issued Mr. Robins a stock grant of 5,000 shares of common stock.
In September 2023, we entered into a director offer letter with Mr. Capelli to serve as a director. In connection with Mr. Capelli’s appointment to the Board, we issued Mr. Capelli a stock options to purchase up to 3,750 shares of common stock.
Commencing upon the closing of our IPO in January 2024, our non-employee directors began to receive annual compensation of $50,000.
In May 2024, the Board of Directors approved an updated non-employee director compensation plan, pursuant to which upon the initial appointment (or election) of an non-employee director to the Board, the non-employee director shall be issued a 10-year option to purchase 3,750 shares of the Company’s common stock, under the 2023 Stock Plan, that will vest in three equal annual installments over a three-year period. In addition, on the date of our annual meeting, each non-employee director that is re-elected at the Annual Shareholder Meeting will be issued a 10-year option to purchase 2,500 shares of the Company’s common stock, under the 2023 Stock Plan that will vest quarterly over a one-year period. In addition, each non-employee director will receive an annual compensation of $40,000 and special service pay amounts based on service for committee responsibilities as follows: Audit Committee Chair - $15,000; Compensation Committee Chair - $10,000; and Nominating and Governance Chair - $7,500. Each non-chair committee member will also received the following compensations: Audit Committee member - $7,500; Compensation Committee member - $5,000; and Nominating and Governance Committee member - $3,750.
The following table sets forth the total compensation earned by our non-employee directors in fiscal 2024. Mr. Klemp’s compensation is fully reflected in the “Summary Compensation Table” above:
| Name | Year |
Fees earned or paid in cash ($) |
Option awards ($)(1) |
Total ($) |
||||||||||
| Jonathan P. Foster | 2024 | $ | 50,000 | (2) | $ | - | $ | 50,000 | ||||||
| David Robins | 2024 | $ | 8,333 | (3) | $ | - | $ | 8,333 | ||||||
| Christopher Capelli | 2024 | $ | 8,333 | (3) | $ | 116,980 | $ | 125,313 | ||||||
|
(1) |
Represents the full grant date fair value of the option award our board approved and granted to each non-employee director, calculated in accordance with FASB ASC Topic 718. These amounts do not necessarily correspond to the actual value that may be realized by the director. For a summary of the assumptions made in the valuation of the awards, please see Note 4 to our audited financial statements as of and for the period ended March 31, 2024 included in this prospectus. As of March 31, 2024, the aggregate number of shares outstanding under all options to purchase our common stock held by our non-employee directors were: Mr. Capelli – 3,750 shares. |
|
(2) |
Annual cash Board fee commenced with agreement dated April 1, 2022. |
|
(3) |
Annual cash Board fee commenced with the completion of the Company’s IPO on January 29, 2024. |
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
In addition to the compensation arrangements, including employment, termination of employment and change in control arrangements, with our directors and executive officers, including those discussed in the sections titled “Management” and “Executive and Director Compensation,” the following is a description of each transaction since January 1, 2022 or any currently proposed transaction in which:
• we have been or are to be a party to;
• the amount involved exceeded or exceeds $120,000 or 1% of the average of our total assets as of the end of the last two completed fiscal years; and
• any of our directors, executive officers or holders of more than 5% of our outstanding capital stock, or any immediate family member of, or person sharing the household with, any of these individuals or entities, had or will have a direct or indirect material interest.
We utilize a consulting firm that is owned by Matthew Lourie, our former Chief Financial Officer, to provide accounting and financial reporting services and to pay certain expenses on behalf of the Company. During the years ended March 31, 2024 and 2023, we incurred fees of $59,450 and $49,056 for these services, respectively, excluding officer compensation. During the three months ended June 30, 2024 and 2023, the Company incurred fees of $0 and $23,063, respectively, for these services, excluding officer compensation. As of June 30, 2024 and March 31, 2024, we owed this consulting firm $0 and $609, respectively, for services and expenses.
As part of the March 2022 sale of our common stock for cash, a member of the Board of Directors purchased 2,500 shares of common stock for $100,000 of cash proceeds. As part of the March 2023 sale of common stock for cash, three members of the Board of Directors purchased, in the aggregate, 8,125 shares of common stock for $325,000 of cash proceeds.
In December 2021, we granted a company affiliated with certain early investors in the Company a license to our technology for use in the field of cardiology. In July 2023, we entered into a termination agreement for the license agreement in exchange for the issuance, upon the closing of our IPO, of a warrant to purchase 80,000 shares of our common stock at an exercise price of $0.02 per share. The shares underlying the warrant are subject to a lockup agreement for a period of six months after the closing of the IPO with respect to 12.5% of the shares and twelve months after the closing of the IPO for the remainder of the shares. We agreed to provide demand registration rights to the licensee with respect to the resale of the shares of common stock underlying the warrants. One of our directors, David Robins, holds a 19.5% interest in the company receiving the warrant.
During the year ended March 31, 2022, in connection with the sale of securities and the entrance of the license agreement discussed above, we paid a total of $175,000 to the licensee, which was recorded as general and administrative expense, and incurred an additional $26,282 of legal costs from the licensee related to the license, which were paid during the year ended March 31, 2023.
In September 2023, we commenced a private placement for up to $2.0 million in principal amount of convertible notes with a maturity date of December 31, 2025. For each dollar in principal amount of convertible notes purchased, we issued a warrant to purchase 0.0125 shares of our common stock, with an exercise price of $20.00 per share. On the closing of our IPO, the principal amount of the convertible notes converted into our common stock at a conversion price of $40.00 per share. Subsequent to September 30, 2023, members of the Company’s management, Board of Directors and an immediate family member of the Company’s management, collectively purchased $500,000 ($400,000 (for management and members of the Board of Directors) and $100,000 (for the immediate family member of a member of the Company’s management, respectively) in principal amount of convertible notes in the private placement.
Policies and Procedures for Related Party Transactions
Our audit committee charter provides that our audit committee is responsible for reviewing and approving in advance any related party transaction. This will cover, with certain exceptions set forth in Item 404 of Regulation S-K under the Securities Act, any transaction, arrangement or relationship, or any series of similar transactions, arrangements or relationships in which we were or will be a participant to, where the amount involved exceeds the lesser of $120,000 or one percent of the average of our total assets at year-end for the last two completed fiscal years, and a related person had or will have a direct or indirect material interest, including, without limitation, purchases of goods or services by or from the related person or entities in which the related person has a material interest, indebtedness, guarantees of indebtedness and employment by us of a related person. In determining whether to approve a proposed transaction, our audit committee will consider all relevant facts and circumstances including: (i) the materiality and character of the related party’s direct or indirect interest; (ii) the commercial reasonableness of the terms; (iii) the benefit or perceived benefit, or lack thereof, to us; (iv) the opportunity cost of alternate transactions; and (v) the actual or apparent conflict of interest of the related party.
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
The following table sets forth information, as of October 1, 2024, regarding beneficial ownership of our common stock by:
• each of our directors;
• each of our named executive officers;
• all directors and current executive officers as a group; and
• each person, or group of affiliated persons, known by us to beneficially own more than five percent of our shares of common stock.
The percentage of beneficial ownership before the offering is based on 1,152,120 shares outstanding as of October 1, 2024. Beneficial ownership is determined according to the rules of the SEC, and generally means that person has beneficial ownership of a security if he or she possesses sole or shared voting or investment power of that security and includes options that are currently exercisable or exercisable within 60 days. Each director or officer, as the case may be, has furnished us with information with respect to beneficial ownership. Except as otherwise indicated, we believe that the beneficial owners of common stock listed below, based on the information each of them has given to us, have sole investment and voting power with respect to their shares, except where community property laws may apply. Except as otherwise noted below, the address for each person or entity listed in the table is c/o Autonomix Medical, Inc., 21 Waterway Avenue, Suite 300, The Woodlands, TX 77380.
|
Shares beneficially owned |
Percent of Class |
|||||||
| Name of Beneficial Owner | ||||||||
| Executive officers and directors: | ||||||||
| Lori Bisson | 20,283 | (1) | 1.7 | % | ||||
| Dr. Robert Schwartz | 35,764 | 3.1 | % | |||||
| Landy Toth | 101,314 | 8.8 | % | |||||
| Walter Klemp | 150,750 | 13.1 | % | |||||
| Jonathan P. Foster | 36,563 | 3.2 | % | |||||
| Christopher Capelli | 3,750 | (1) | * | |||||
| David Robins | 26,581 | 2.3 | % | |||||
| Trent Smith | 9,063 | * | ||||||
| Brad Hauser | - | - | ||||||
| All current Executive Officers and Directors as a group (9 persons) | 384,068 | 32.5 | % | |||||
| 5% Shareholders: | ||||||||
| BioStar Ventures III, L.P. | 120,816 | (3) | 10.5 | % | ||||
* Less than 1%
(1) Consists of shares of common stock underlying options and convertible notes to purchase common stock that vest within 60 days of October 1, 2024.
(2) Includes a portion of 80,000 warrants to purchase shares of the Company’s common stock that was held by Impulse Medical, Inc., in which Mr. Robins owns 19.5% through Portsmouth Therapeutics, Inc., an entity in which Mr. Robins also owns a 33.33% interest. The shares underlying the warrants are subject to a lockup agreement for a period of six months after the closing of the Company’s IPO with respect to 12.5% of the shares issued and twelve months after the closing of the Company’s IPO for the remainder of the shares.
(3) The business address of BioStar Ventures III, L.P. is 206, Bridge Street, Charlevoix, MI 49720. General Partner, BioStar Ventures III, LLC, 206, Bridge Street, Charlevoix, MI 49720 has voting and dispositive power over the shares held by BioStar Ventures III, L.P. Louis A. Cannon serves as the Senior Managing Director of BioStar Ventures III, LLC.
DESCRIPTION OF CAPITAL STOCK
The following summary of the rights of our capital stock is not complete and is subject to and qualified in its entirety by reference to our certificate of incorporation and bylaws, copies of which are filed as exhibits to the registration statement of which this prospectus forms a part, which are incorporated by reference herein, and the applicable provisions of the Delaware General Corporation Law.
Common Stock
We are authorized to issue up to 500,000,000 shares of common stock. Shares of our common stock have the following rights, preferences and privileges:
Voting
Each holder of common stock is entitled to one vote for each share of common stock held on all matters submitted to a vote of stockholders. Any action at a meeting at which a quorum is present will be decided by a majority in voting power of the votes cast (excluding abstentions and broker non-votes), except in the case of any election of directors, which will be decided by a plurality of votes cast. There is no cumulative voting.
Dividends
Holders of our common stock are entitled to receive dividends when, as and if declared by our board of directors out of funds legally available for payment, subject to the rights of holders, if any, of any class of stock having preference over the common stock. Any decision to pay dividends on our common stock will be at the discretion of our board of directors. Our board of directors may or may not determine to declare dividends in the future. The board’s determination to issue dividends will depend upon our profitability and financial condition any contractual restrictions, restrictions imposed by applicable law and the SEC, and other factors that our board of directors deems relevant.
Liquidation Rights
In the event of a voluntary or involuntary liquidation, dissolution or winding up of the company, the holders of our common stock will be entitled to share ratably on the basis of the number of shares held in any of the assets available for distribution after we have paid in full, or provided for payment of, all of our debts and after the holders of all outstanding series of any class of stock have preference over the common stock, if any, have received their liquidation preferences in full.
Number of Holders
There are approximately 4,800 holders of our common stock as of October 1, 2024.
Other
Our issued and outstanding shares of common stock are fully paid and nonassessable. Holders of shares of our common stock are not entitled to preemptive rights. Shares of our common stock are not convertible into shares of any other class of capital stock, nor are they subject to any redemption or sinking fund provisions.
Preferred Stock
We are authorized to issue up to 10,000,000 shares of preferred stock. Our certificate of incorporation authorizes the board to issue these shares in one or more series, to determine the designations and the powers, preferences and relative, participating, optional or other special rights and the qualifications, limitations and restrictions thereof, including the dividend rights, conversion or exchange rights, voting rights (including the number of votes per share), redemption rights and terms, liquidation preferences, sinking fund provisions and the number of shares constituting the series. Our board of directors could, without stockholder approval, issue preferred stock with voting and other rights that could adversely affect the voting power and other rights of the holders of common stock and which could have the effect of making it more difficult for a third party to acquire, or of discouraging a third party from attempting to acquire, a majority of our outstanding voting stock.
Convertible Notes Payable
On September 9, 2023, our Board of Directors authorized an offering up to $2.0 million in unsecured, non-interest bearing convertible promissory notes (the “Notes”) and accompanying warrants (the “Bridge Financing Warrants”) (collectively, the “Bridge Offering”) that will mature on December 31, 2025. The Notes provide that, on the closing date of the IPO, the outstanding principal would be automatically converted into common stock at the conversion price of $40.00, provided that certain holders of the Notes are not permitted to convert such Notes to the extent that the holders or any of its affiliates would beneficially own in excess of 4.99% (the “Beneficial Ownership Limit”) of our common stock after such conversion. Each dollar in principal amount of the convertible notes purchased were accompanied by a five-year Bridge Financing Warrant to purchase approximately 0.0125 shares of common stock with an exercise price of $20.00 per share as discussed in “Warrants – Pre-IPO Warrants” below.
As of June 30, 2024, due to the Beneficial Ownership Limit, we have outstanding Notes in the aggregate amount of $1,330,000 that convert into 33,250 shares of our common stock.
Warrants
License Termination Warrants
As of the date hereof, we have outstanding warrants to purchase 80,000 shares of our common stock at an exercise price of $0.02 per share expiring January 2029. The shares underlying the warrant are subject to a lockup agreement for a period of six months after the closing of our IPO with respect to 12.5% of the shares issued and twelve months after the closing of our IPO for the remainder of the shares. We agreed to provide demand registration rights to the licensee with respect to the resale of the shares of common stock underlying the warrants.
Pre-IPO Warrants
As of October 1, 2024, we have outstanding warrants to purchase 4,542 shares of our common stock at a weighted average exercise price of $11.99 per share expiring through March 2032 and Bridge Financing Warrants to purchase an aggregate of 25,003 shares of our common stock at an exercise price of $20.00 per share expiring through October 2028. The foregoing warrants provide that no holder of these warrants will be permitted to exercise such warrants to the extent that the holder or any of its affiliates would beneficially own in excess of 4.99% of our common stock after such exercise.
Selling Agent Warrants
In connection with our IPO, we issued approximately 2,989 shares of our common stock issuable upon exercise of warrants issued to the selling agent in our IPO (the “Selling Agent’s Warrants”). The Selling Agent’s Warrants are exercisable commencing six months after the date of commencement of sales in our IPO and will be exercisable until the fifth anniversary of such date. The exercise price for the Selling Agent’s Warrants is $125.00 per share. The Selling Agent’s Warrants are not redeemable.
Limitations on Liability and Indemnification of Officers and Directors
Our certificate of incorporation and bylaws limit the liability of our officers and directors and provide that we will indemnify our officers and directors, in each case, to the fullest extent permitted by the Delaware General Corporation Law.
We have entered into separate indemnification agreements with each of our directors and executive officers. Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors or executive officers, we have been informed that in the opinion of the Commission such indemnification is against public policy and is therefore unenforceable.
Certificate of Incorporation and Bylaw Provisions
Our certificate of incorporation and bylaws include a number of anti-takeover provisions that may have the effect of encouraging persons considering unsolicited tender offers or other unilateral takeover proposals to negotiate with our board of directors rather than pursue non-negotiated takeover attempts. These provisions include:
Advance Notice Requirements. Our bylaws establish advance notice procedures with regard to stockholder proposals relating to the nomination of candidates for election as directors or new business to be brought before meetings of stockholders. These procedures provide that notice of stockholder proposals must be timely and given in writing to our corporate Secretary. Generally, to be timely, notice must be received at our principal executive offices not less than 90 days nor more than 120 days prior to the one-year anniversary of the preceding year’s annual meeting. The notice must contain the information required by the bylaws, including information regarding the proposal and the proponent.
Special Meetings of Stockholders. Our certificate of incorporation provides that special meetings of stockholders may be called at any time by, or at the direction of, only the Chairman of the Board, the Chief Executive Officer, the President or the board of directors.
No Written Consent of Stockholders. Our certificate of incorporation provides that any action required or permitted to be taken by stockholders must be effected at a duly called annual or special meeting of stockholders and may not be effected by any consent in writing by such stockholders.
Exclusive Forum Provision. Our certificate of incorporation provides that the Court of Chancery of the State of Delaware shall be the sole and exclusive forum for (i) any derivative action or proceeding brought on our behalf, (ii) any action asserting a claim of breach of a fiduciary duty owed by any of our directors or officers to us or our stockholders, (iii) any action asserting a claim against us arising pursuant to any provision of the Delaware General Corporation Law (the “DGCL”), or our certificate of incorporation or the bylaws, and (iv) any action asserting a claim against us governed by the internal affairs doctrine. This provision would not apply to suits brought to enforce a duty or liability created by the Exchange Act or Securities Act.
This choice of forum provision may limit a stockholder’s ability to bring a claim in a judicial forum that it finds favorable for disputes with us or our directors, officers or other employees, which may discourage such lawsuits against us and our directors, officers and employees. In addition, this forum selection provision may impose additional litigation costs on stockholders in pursuing the claims identified above, particularly if the stockholders do not reside in or near the State of Delaware. Alternatively, a court could find these provisions of our certificate of incorporation to be inapplicable or unenforceable in respect of one or more of the specified types of actions or proceedings, which may require us to incur additional costs associated with resolving such matters in other jurisdictions, which could adversely affect our business and financial condition.
Amendment of Bylaws. Our stockholders may amend any provisions of our bylaws by obtaining the affirmative vote of the holders of at least a majority of the voting power of all the then-outstanding shares of our voting stock with the power to vote generally in an election of directors, voting together as a single class.
Preferred Stock. Our certificate of incorporation authorizes our board of directors to create and issue rights entitling our stockholders to purchase shares of our stock or other securities. The ability of our board to establish the rights and issue substantial amounts of preferred stock without the need for stockholder approval may delay or deter a change in control of us. See “Preferred Stock” above.
Delaware Takeover Statute
We are subject to Section 203 of the DGCL which, subject to certain exceptions, prohibits a Delaware corporation from engaging in any “business combination” (as defined below) with any interested stockholder for a period of three years following the date that such stockholder became an interested stockholder, unless: (1) prior to such date, the board of directors of the corporation approved either the business combination or the transaction that resulted in the stockholder becoming an interested stockholder; (2) on consummation of the transaction that resulted in the stockholder becoming an interested stockholder, the interested stockholder owned at least 85% of the voting stock of the corporation outstanding at the time the transaction commenced, excluding for purposes of determining the voting stock outstanding those shares owned (a) by persons who are directors and also officers and (b) by employee stock plans in which employee participants do not have the right to determine confidentially whether shares held subject to this plan will be tendered in a tender or exchange offer; or (3) on or subsequent to such date, the business combination is approved by the board of directors and authorized at an annual or special meeting of stockholders, and not by written consent, by the affirmative vote of at least 66 2⁄3% of the outstanding voting stock that is not owned by the interested stockholder.
Section 203 of the DGCL defines generally “business combination” to include: (1) any merger or consolidation involving the corporation and the interested stockholder; (2) any sale, transfer, pledge or other disposition of 10% or more of the assets of the corporation involving the interested stockholder; (3) subject to certain exceptions, any transaction that results in the issuance or transfer by the corporation of any stock of the corporation to the interested stockholder; (4) any transaction involving the corporation that has the effect of increasing the proportionate share of the stock of any class or series of the corporation beneficially owned by the interested stockholder; or (5) the receipt by the interested stockholder of the benefit of any loans, advances, guarantees, pledges or other financial benefits provided by or through the corporation. In general, Section 203 defines an “interested stockholder” as any entity or person beneficially owning 15% or more of the outstanding voting stock of the corporation and any entity or person affiliated with or controlling or controlled by such entity or person.
Transfer Agent
The transfer agent for our common stock is Equity Stock Transfer, LLC.
DESCRIPTION OF SECURITIES WE ARE OFFERING
Units
We are offering up to 698,812 Common Stock Units, with each Common Stock Unit consisting of: (i) one share of our common stock and (ii) a Series A Warrant to purchase one share of our common stock.
We are also offering to each purchaser whose purchase of Common Stock Units in this offering would otherwise result in the purchaser, together with its affiliates and certain related parties, beneficially owning more than 4.99% (or, at the election of the purchaser, 9.99%) of our outstanding common stock immediately following the consummation of this offering, the opportunity to purchase, if the purchaser so chooses, PFW Units in lieu of Common Stock Units that would otherwise result in the purchaser’s beneficial ownership exceeding 4.99% (or, at the election of the purchaser, 9.99%) of our outstanding common stock. Each PFW Unit consists of: (i) one Pre-Funded Warrant and (ii) a Series A Warrant to purchase one share of our common stock. The Common Warrants included in the PFW Units are identical to the Common Warrants included in the Common Stock Units.
The shares of common stock in the Common Stock Units or the Pre-Funded Warrants in the PFW Units, as applicable, and the accompanying Common Warrants, can only be purchased together in this offering but will be issued separately and will be immediately separable upon issuance.
Pre-Funded Warrants
The following summary of certain terms and provisions of Pre-Funded Warrants that are being offered hereby is not complete and is subject to, and qualified in its entirety by, the provisions of the Pre-Funded Warrant, the form of which is filed as an exhibit to the registration statement of which this prospectus forms a part.
Form. The Pre-Funded Warrants will be issued as individual warrant agreements to the investors. You should review the form of Pre-Funded Warrant, filed as an exhibit to the registration statement of which this prospectus forms a part, for a complete description of the terms and conditions applicable to the Pre-Funded Warrants.
Exercisability. The Pre-Funded Warrants will be exercisable, at the option of each holder, in whole or in part, by delivering to us a duly executed exercise notice accompanied by payment in full in immediately available funds for the number of shares of our common stock purchased upon such exercise (except in the case of a cashless exercise as described below). A holder (together with its affiliates) may not exercise any portion of the Pre-Funded Warrant to the extent that the holder would own more than 4.99% (or, at the election of the holder, 9.99%) of the outstanding common stock immediately after exercise, except that upon at least 61 days’ prior notice from the holder to us, the holder may increase the amount of ownership of outstanding stock after exercising the holder’s Pre-Funded Warrants up to 9.99% of the number of shares of our common stock outstanding immediately after giving effect to the exercise, as such percentage ownership is determined in accordance with the terms of the Pre-Funded Warrants. Purchasers of Pre-Funded Warrants in this offering may also elect prior to the issuance of the Pre-Funded Warrants to have the initial exercise limitation set at 9.99% of our outstanding common stock. No fractional shares of common stock will be issued in connection with the exercise of a Pre-Funded Warrant. In lieu of fractional shares, we will pay the holder an amount in cash equal to the fractional amount multiplied by the exercise price.
Duration and Exercise Price. The exercise price per whole share of our common stock purchasable upon the exercise of the Pre-Funded Warrants is $0.001 per share of common stock. The Pre-Funded Warrants will be immediately exercisable and may be exercised at any time until the Pre-Funded Warrants are exercised in full. The exercise price of the Pre-Funded Warrants is subject to appropriate adjustment in the event of certain stock dividends and distributions, stock splits, stock combinations, reclassifications or similar events affecting our common stock and also upon any distributions of assets, including cash, stock or other property to our stockholders.
Cashless Exercise. In lieu of making the cash payment otherwise contemplated to be made to us upon the exercise of any Pre-Funded Warrants in payment of the aggregate exercise price, the holder may, at its option, instead receive upon such exercise (either in whole or in part) only the net number of shares of common stock determined according to a formula set forth in the Pre-Funded Warrants. Notwithstanding anything to the contrary, there are no circumstances that would require us to make any cash payments or net cash settle the Pre-Funded Warrants to the holders.
Transferability. Subject to applicable laws, the Pre-Funded Warrants may be offered for sale, sold, transferred or assigned at the option of the holder upon surrender of the Pre-Funded Warrant to us together with the appropriate instruments of transfer.
Exchange Listing. We do not plan on applying to list the Pre-Funded Warrants on Nasdaq, any other national securities exchange or any other nationally recognized trading system.
Fundamental Transactions. In the event of a fundamental transaction, as described in the Pre-Funded Warrants and generally including any reorganization, recapitalization or reclassification of our common stock, the sale, transfer or other disposition of all or substantially all of our properties or assets, our consolidation or merger with or into another person, the acquisition of more than 50% of our outstanding common stock, or any person or group becoming the beneficial owner of 50% of the voting power represented by our outstanding common stock, the holders of the Pre-Funded Warrants will be entitled to receive upon exercise of the Pre-Funded Warrants the kind and amount of securities, cash or other property that the holders would have received had they exercised the Pre-Funded Warrants immediately prior to such fundamental transaction.
Rights as a Stockholder. Except by virtue of such holder’s ownership of shares of our common stock, the holder of a Pre-Funded Warrant does not have the rights or privileges of a holder of our common stock, including any voting rights, until the holder exercises the Pre-Funded Warrant.
Series A Warrants
The following summary of certain terms and provisions of Series A Warrants that are being offered hereby is not complete and is subject to, and qualified in its entirety by, the provisions of the Series A Warrant, the form of which is filed as an exhibit to the registration statement of which this prospectus forms a part.
Duration and Exercise Price. Each Series A Warrant offered hereby has an assumed initial exercise price per share equal to $____ (representing ____), subject to adjustment. The Series A Warrants will be immediately exercisable. The exercise price and number of shares of common stock issuable upon exercise for each Series A Warrant is subject to appropriate adjustment in the event of stock dividends, stock splits, reorganizations or similar events affecting our shares of common stock and the exercise price. Equity Stock Transfer, LLC has agreed to serve as warrant agent with respect to the Series A Warrants pursuant to a warrant agency agreement to be entered into concurrently with the closing of this offering. The Series A Warrants will expire five years from the closing date of this offering.
Exercisability. The Series A Warrants will be exercisable, at the option of the holder, in whole or in part, by delivering to us a duly-executed exercise notice, with payment in full for the number of shares of common stock purchased upon such exercise (except in the case of a cashless exercise as discussed below) due prior to issuance of the common stock. A holder (together with its affiliates) may not exercise any portion of the Series A Warrant to the extent that the holder would own more than 4.99% (or, at the election of the holder, prior to issuance, 9.99%) of the outstanding shares of common stock immediately after exercise, referred to herein as the beneficial ownership limitation. However, upon notice from the holder to us, the holder may decrease or increase the beneficial ownership limitation, which may not exceed 9.99% of the number of shares of common stock outstanding immediately after giving effect to the exercise, as such percentage ownership is determined in accordance with the terms of the Series A Warrants, provided that any increase in the beneficial ownership limitation will not take effect until 61 days following notice to us. No fractional shares of common stock will be issued in connection with the exercise of a Series A Warrant. In lieu of fractional shares, we will either pay the holder an amount in cash equal to the fractional amount multiplied by the exercise price or round up to the next whole share.
Cashless Exercise. At any time, in lieu of making the cash payment otherwise contemplated to be made to us upon such exercise, the holder may elect instead to receive upon such exercise (either in whole or in part) the net number of the shares of common stock determined according to a formula set forth in the warrants.
Transferability. Subject to applicable laws, a Series A Warrant may be transferred at the option of the holder upon surrender of the Series A Warrant to us together with the appropriate instruments of transfer.
Exchange Listing. There is no trading market available for the Series A Warrants on any securities exchange or nationally recognized trading system. We do not intend to list the Series A Warrants on any securities exchange or nationally recognized trading system.
Right as a Stockholder. Except as otherwise provided in the Series A Warrants, including as described below under “Pro Rata Distributions,” or by virtue of such holder’s ownership of our shares of common stock, the holders of the Series A Warrants do not have the rights or privileges of holders of our shares of common stock, including any voting rights, until they exercise their Series A Warrants.
Subsequent Rights Offerings. In addition to in the event of stock dividends, stock splits, reorganizations or similar events affecting our shares of common stock and the exercise price, if at any time the Company grants, issues or sells any specified common stock equivalents or rights to purchase stock, warrants, securities or other property pro rata to the record holders of any class of shares of Common Stock (the “Purchase Rights”), then the holder of a Series A Warrant will be entitled to acquire, upon the terms applicable to such Purchase Rights, the aggregate Purchase Rights which the holder could have acquired if the holder had held the number of shares of common stock acquirable upon complete exercise of the holder’s Series A Warrant, without regard to any limitations on exercise hereof, including without limitation, the beneficial ownership limitation, immediately before the date on which a record is taken for the grant, issuance or sale of such Purchase Rights, or, if no such record is taken, the date as of which the record holders of shares of common stock are to be determined for the grant, issue or sale of such Purchase Rights (provided, however, that, to the extent that the holder’s right to participate in any such Purchase Right would result in the holder exceeding the beneficial ownership limitation, then the holder shall not be entitled to participate in such Purchase Right to such extent (or beneficial ownership of such shares of common stock as a result of such Purchase Right to such extent) and such Purchase Right to such extent shall be held in abeyance for the holder until such time, if ever, as its right thereto would not result in the holder exceeding the beneficial ownership limitation).
Pro Rata Distributions. During such time as a Series A Warrant is outstanding and has not reached its termination date, if the Company shall declare or make any dividend or other distribution of its assets (or rights to acquire its assets) to holders of shares of common stock, by way of return of capital or otherwise (including, without limitation, any distribution of cash, stock or other securities, property or options by way of a dividend, spin off, reclassification, corporate rearrangement, scheme of arrangement or other similar transaction) (a “Distribution”), at any time after the issuance of the Series A Warrants, then, in each such case, the holder of a Series A Warrant shall be entitled to participate in such Distribution to the same extent that the holder would have participated therein if the holder had held the number of shares of common stock acquirable upon complete exercise of the Series A Warrant (without regard to any limitations on exercise hereof, including without limitation, the beneficial ownership limitation) immediately before the date of which a record is taken for such Distribution, or, if no such record is taken, the date as of which the record holders of shares of common stock are to be determined for the participation in such Distribution (provided, however, that, to the extent that the holder’s right to participate in any such Distribution would result in the holder exceeding the beneficial ownership limitation, then the holder shall not be entitled to participate in such Distribution to such extent (or in the beneficial ownership of any shares of common stock as a result of such Distribution to such extent) and the portion of such Distribution shall be held in abeyance for the benefit of the holder until such time, if ever, as its right thereto would not result in such Holder exceeding the beneficial ownership limitation).
Fundamental Transaction. In the event of a fundamental transaction, as described in the Series A Warrants and generally including any reorganization, recapitalization or reclassification of our shares of common stock, the sale, transfer or other disposition of all or substantially all of our properties or assets, our consolidation or merger with or into another person, the acquisition of more than 50% of our outstanding shares of common stock, or any person or group becoming the beneficial owner of 50% of the voting power represented by our outstanding shares of common stock, the holders of the Series A Warrants will be entitled to receive upon exercise of the Series A Warrants the kind and amount of securities, cash or other property that the holders would have received had they exercised the Series A Warrants immediately prior to such fundamental transaction.
Change in Control. In the event of a Change in Control, as defined in the Series A Warrants, which generally includes any Fundamental Transaction other than any reorganization, recapitalization or reclassification of the Common Stock in which holders of the Company’s voting power immediately prior to such event continue after such event to be the holders of the voting power of the surviving entity, (ii) pursuant to a migratory merger effected solely for the purpose of changing the jurisdiction of incorporation of the Company or (iii) a merger in connection with a bona fide acquisition by the Company of any person in which (x) the gross consideration paid, directly or indirectly, by the Company in such acquisition is not greater than 50% of the Company’s market capitalization as calculated on the date of the consummation of such merger and (y) such merger does not contemplate a change to the identity of a majority of the board of directors of the Company, warrant holders may require the company to purchase the remaining unexercised portion of a Series A Warrant for an amount equal to the Black-Scholes Value (as defined in the relevant warrant) of that portion, as of the date of the Change of Control, unless the Change of Control is not within the Company’s control, as described in the warrant. In that event, holders will instead be entitled to receive the same type or form of consideration (and in the same proportion), at the Black-Scholes Value of the unexercised portion of the warrant, that is being offered and paid to the common shareholders.
Global Warrants. Series A Warrants shall be issued in book entry form (the “Global Warrants”). All of the Series A Warrants shall initially be represented by one or more Global Warrants deposited with the warrant agent and registered in the name of Cede & Co., a nominee of The Depository Trust Company (the “Depositary”), or as otherwise directed by the Depositary. Ownership of beneficial interests in the Series A Warrants shall be shown on, and the transfer of such ownership shall be effected through, records maintained by (i) the Depositary or its nominee for each Global Warrant or (ii) institutions that have accounts with the Depositary (such institution, with respect to a Series A Warrant in its account, a “Participant”). If the Depositary subsequently ceases to make its book-entry settlement system available for the Series A Warrants, the Company may instruct the warrant agent regarding other arrangements for book-entry settlement. In the event that the Series A Warrants are not eligible for, or it is no longer necessary to have the Series A Warrants available in, book-entry form, the warrant agent shall provide written instructions to the Depositary to deliver to the warrant agent for cancellation each Global Warrant, and the Company shall instruct the warrant agent to deliver to each holder of a Series A Warrant a Common Warrant certificate. A holder of a Series A Warrant has the right to elect at any time or from time to time a warrant exchange (as defined in the warrant agency agreement). Upon written notice by a holder to the warrant agent for the exchange of some or all of such holder’s Global Warrants for a Series A Warrant certificate evidencing the same number of Series A Warrants, which request shall be in the form specified in the warrant agency agreement, the warrant agent shall promptly effect the warrant exchange and shall promptly issue and deliver to the holder a warrant certificate for such number of Series A Warrants in the name set forth in the warrant certificate request notice. In the event a beneficial owner requests a warrant exchange, upon issuance of the paper warrant certificate, the Company shall act as warrant agent and the terms of the paper warrant certificate so issued shall exclusively govern in respect thereof.
MATERIAL UNITED STATES FEDERAL INCOME TAX CONSIDERATIONS
The following discussion is a summary of the material U.S. federal income tax consequences of the acquisition, ownership and disposition of the common stock, Pre-Funded Warrants and Common Warrants acquired in this offering and does not purport to be a complete analysis of all the potential tax considerations relating thereto. This discussion is based on the current provisions of the Internal Revenue Code of 1986, as amended, referred to as the Code, existing and proposed U.S. Treasury regulations promulgated thereunder, and administrative rulings and court decisions in effect as of the date hereof, all of which are subject to change at any time, possibly with retroactive effect, with the resulting U.S. federal income tax consequences being different from those set forth below. No ruling has been or will be sought from the Internal Revenue Service, or IRS, with respect to the matters discussed below, and there can be no assurance the IRS will not take a contrary position regarding the tax consequences of the acquisition, ownership or disposition of the common stock, Pre-Funded Warrants or Common Warrants, or that any such contrary position would not be sustained by a court.
We assume in this discussion that the shares of common stock, Pre-Funded Warrants and Common Warrants will be held as capital assets (generally, property held for investment). This discussion does not address all aspects of U.S. federal income taxes, does not discuss the potential application of the Medicare contribution tax or the alternative minimum tax and does not address state or local taxes or U.S. federal gift and estate tax laws, except as specifically provided below with respect to non-U.S. holders, or any non-U.S. tax consequences that may be relevant to holders in light of their particular circumstances. This discussion also does not address the special tax rules applicable to particular holders, such as:
|
● |
persons who acquired our common stock, Pre-Funded Warrants or Common Warrants as compensation for services; |
|
● |
traders in securities that elect to use a mark-to-market method of accounting for their securities holdings; |
|
● |
persons that own, or are deemed to own, more than 5% of our common stock (except to the extent specifically set forth below); |
|
● |
persons required for U.S. federal income tax purposes to conform the timing of income accruals to their financial statements under Section 451(b) of the Code (except to the extent specifically set forth below); |
|
● |
persons for whom our common stock constitutes “qualified small business stock” within the meaning of Section 1202 of the Code or “Section 1244 stock” for purposes of Section 1244 of the Code; |
|
● |
persons deemed to sell our common stock, Pre-Funded Warrants or Common Warrants under the constructive sale provisions of the Code; |
|
● |
banks or other financial institutions; |
|
● |
brokers or dealers in securities or currencies; |
|
● |
tax-exempt organizations or tax-qualified retirement plans; |
|
● |
pension plans; |
|
● |
regulated investment companies or real estate investment trusts; |
|
● |
persons that hold the common stock, Pre-Funded Warrants or Common Warrants as part of a straddle, hedge, conversion transaction, synthetic security or other integrated investment; |
|
● |
insurance companies; |
|
● |
controlled foreign corporations, passive foreign investment companies, or corporations that accumulate earnings to avoid U.S. federal income tax; and |
|
● |
certain U.S. expatriates, former citizens, or long-term residents of the United States. |
In addition, this discussion does not address the tax treatment of partnerships (including any entity or arrangement classified as a partnership for U.S. federal income tax purposes) or other pass-through entities or persons who hold shares of common stock, Pre-Funded Warrants or Common Warrants through such partnerships or other entities which are pass-through entities for U.S. federal income tax purposes. If such a partnership or other pass-through entity holds shares of common stock, Pre-Funded Warrants or Common Warrants, the treatment of a partner in such partnership or investor in such other pass-through entity generally will depend on the status of the partner or investor and upon the activities of the partnership or other pass-through entity. A partnership or other pass-through entity, a partner in such a partnership and an investor in such other pass-through entity that will hold shares of common stock, Pre-Funded Warrants or Common Warrants should consult his, her or its own tax advisor regarding the tax consequences of the ownership and disposition of shares of common stock, Pre-Funded Warrants or Common Warrants.
This discussion of U.S. federal income tax considerations is for general information purposes only and is not tax advice. Prospective investors should consult their own tax advisors regarding the U.S. federal, state, local and non-U.S. income and other tax considerations of acquiring, holding and disposing of our common stock, Pre-Funded Warrants and Common Warrants.
For the purposes of this discussion, a “U.S. Holder” means a beneficial owner of shares of common stock, Pre-Funded Warrants or Common Warrants that is for U.S. federal income tax purposes (a) an individual citizen or resident of the United States (b) a corporation (or other entity taxable as a corporation for U.S. federal income tax purposes), created or organized in or under the laws of the United States, any state thereof or the District of Columbia, (c) an estate the income of which is subject to U.S. federal income taxation regardless of its source, or (d) a trust if it (1) is subject to the primary supervision of a court within the United States and one or more U.S. persons (within the meaning of Section 7701(a)(30) of the Code) has the authority to control all substantial decisions of the trust or (2) has a valid election in effect under applicable U.S. Treasury regulations to be treated as a domestic trust. A “Non-U.S. Holder” is, for U.S. federal income tax purposes, a beneficial owner of shares of common stock, Pre-Funded Warrants or Common Warrants that is not a U.S. Holder or a partnership (or other entity treated as a partnership) for U.S. federal income tax purposes.
Treatment of Pre-Funded Warrants
Although it is not entirely free from doubt, we believe that a Pre-Funded Warrant should be treated as a share of common stock for U.S. federal income tax purposes and a holder of Pre-Funded Warrants should generally be taxed in the same manner as a holder of common stock, as described below. Accordingly, no gain or loss should be recognized upon the exercise of a Pre-Funded Warrant and, upon exercise, the holding period of a Pre-Funded Warrant should carry over to the share of common stock received. Similarly, we believe that the tax basis of the Pre-Funded Warrant should carry over to the share of common stock received upon exercise, increased by the exercise price of $0.001 per share.
Each holder should consult his, her or its own tax advisor regarding the risks associated with the acquisition of Pre-Funded Warrants pursuant to this offering (including potential alternative characterizations). The balance of this discussion generally assumes that the characterization described above is respected for U.S. federal income tax purposes.
Allocation of Purchase Price
For U.S. federal income tax purposes, each share of common stock (or, in lieu of common stock, each Pre-Funded Warrant) and the accompanying Common Warrants issued pursuant to this offering will be treated as an “investment unit” consisting of one share of common stock or one Pre-Funded Warrant (which, as described above, we believe should generally be treated as a share of common stock for U.S. federal income tax purposes), as applicable, and the accompanying Common Warrants, each to acquire one share of common stock. The purchase price for each investment unit will be allocated between these components in proportion to their relative fair market values at the time the unit is purchased by the holder. This allocation of the purchase price for each unit will establish the holder’s initial tax basis for U.S. federal income tax purposes in the share of common stock (or, in lieu of common stock, Pre-Funded Warrant) and the Common Warrant included in each unit.
We will not be providing holders with such allocation, and it is possible that different holders will reach different determinations regarding such allocation. A holder's allocation of purchase price between each share of common stock (or Pre-Funded Warrant) and the accompanying Common Warrants is not binding on the IRS or the courts, and no assurance can be given that the IRS or the courts will agree with a holder's allocation.
Each holder should consult his, her or its own tax advisor regarding the allocation of the purchase price between the common stock (or, in lieu of common stock, Pre-Funded Warrants) and the Common Warrants.
Tax Considerations Applicable to U.S. Holders
Exercise and Expiration of Common Warrants
Except as discussed below with respect to the cashless exercise of a Common Warrant, a U.S. Holder generally will not recognize gain or loss for U.S. federal income tax purposes upon exercise of a Common Warrant. The U.S. Holder will take a tax basis in the shares acquired on the exercise of a Common Warrant equal to the exercise price of the Common Warrant, increased by the U.S. Holder’s adjusted tax basis in the Common Warrant exercised (as determined pursuant to the rules discussed above). The U.S. Holder’s holding period in the shares of common stock acquired on the exercise of a Common Warrant will begin on the date of exercise or possibly the day after such exercise, and will not include the period for which the U.S. Holder held the Common Warrant.
The lapse or expiration of an unexercised Common Warrant will be treated as if the U.S. Holder sold or exchanged the Common Warrant and recognized a capital loss equal to the U.S. Holder’s tax basis in the Common Warrant. The deductibility of capital losses is subject to limitations.
The tax consequences of a cashless exercise of a Common Warrant are not clear under current U.S. tax law. A cashless exercise may be tax-free, either because the exercise is not a realization event or because the exercise is treated as a recapitalization for U.S. federal income tax purposes. In either tax-free situation, a U.S. Holder’s tax basis in the common stock received generally would equal the U.S. Holder’s tax basis in the Common Warrants. If the cashless exercise was not a realization event, it is unclear whether a U.S. Holder’s holding period for the common stock would be treated as commencing on the date of exercise of the Common Warrant or the day following the date of exercise of the Common Warrant. If the cashless exercise were treated as a recapitalization, the holding period of the common stock would include the holding period of the Common Warrants.
It is also possible that a cashless exercise could be treated as a taxable exchange in which gain or loss would be recognized. In such event, a U.S. Holder could be deemed to have surrendered Common Warrants having an aggregate fair market value equal to the exercise price for the total number of Common Warrants to be exercised. The U.S. Holder would recognize capital gain or loss in an amount equal to the difference between the fair market value of the common stock received in respect of the Common Warrants deemed surrendered and the U.S. Holder’s tax basis in such common warrants. Such gain or loss would be long-term or short-term, depending on the U.S. Holder’s holding period in the Common Warrants deemed surrendered. In this case, a U.S. Holder’s tax basis in the common stock received would equal the sum of the U.S. Holder’s initial investment in the exercised Common Warrants (i.e., the portion of the U.S. Holder’s purchase price for the investment unit that is allocated to the Common Warrants, as described above under “Allocation of Purchase Price”) and the exercise price of such Common Warrants. It is unclear whether a U.S. Holder’s holding period for the common stock would commence on the date of exercise of the Common Warrant or the day following the date of exercise of the Common Warrant. There may also be alternative characterizations of any such taxable exchange that would result in similar tax consequences, except that a U.S. Holder’s gain or loss would be short-term.
Due to the absence of authority on the U.S. federal income tax treatment of a cashless exercise, there can be no assurance which, if any, of the alternative tax consequences and holding periods described above would be adopted by the IRS or a court of law. Accordingly, U.S. Holders should consult their tax advisors regarding the tax consequences of a cashless exercise of the Common Warrants.
Distributions
As discussed above, we currently anticipate that we will retain future earnings, if any, to finance the growth and development of our business and do not intend to pay cash dividends in respect of shares of common stock in the foreseeable future. In the event that we do make distributions on our common stock to a U.S. Holder, those distributions generally will constitute dividends for U.S. tax purposes to the extent paid out of our current or accumulated earnings and profits (as determined under U.S. federal income tax principles). Distributions in excess of our current and accumulated earnings and profits will constitute a return of capital that is applied against and reduces, but not below zero, a U.S. Holder’s adjusted tax basis in our common stock. Any remaining excess will be treated as gain realized on the sale or exchange of shares of common stock as described below under the section titled “—Disposition of common stock, Pre-Funded Warrants or Common Warrants.”
Subject to applicable limitations, dividends paid to certain non-corporate U.S. Holders may be eligible for taxation as “qualified dividend income” and therefore may be taxable at rates applicable to long-term capital gains. U.S. Holders should consult their tax advisors regarding the availability of the reduced tax rate on dividends in their particular circumstances. Dividends received by a corporate U.S. Holder may be eligible for the dividends-received deduction if the U.S. Holder meets certain holding period and other applicable requirements.
Certain Adjustments to Pre-Funded Warrants or Common Warrants
The number of shares of common stock issued upon the exercise of the Pre-Funded Warrants or Common Warrants and the exercise price of Pre-Funded Warrants or Common Warrants are subject to adjustment in certain circumstances. Adjustments (or failure to make adjustments) that have the effect of increasing a U.S. Holder’s proportionate interest in our assets or earnings and profits may, in some circumstances, result in a constructive distribution to the U.S. Holder. Adjustments to the conversion rate made pursuant to a bona fide reasonable adjustment formula which has the effect of preventing the dilution of the interest of the holders of Pre-Funded Warrants or Common Warrants generally should not be deemed to result in a constructive distribution. If an adjustment is made that does not qualify as being made pursuant to a bona fide reasonable adjustment formula, a U.S. Holder of Pre-Funded Warrants or Common Warrants may be deemed to have received a constructive distribution from us, even though such U.S. Holder has not received any cash or property as a result of such adjustment. The tax consequences of the receipt of a distribution from us are described above under “Distributions.”
Disposition of Common Stock, Pre-Funded Warrants or Common Warrants
Upon a sale or other taxable disposition (other than a redemption treated as a distribution, which will be taxed as described above under “Distributions”) of shares of common stock, Pre-Funded Warrants or Common Warrants, a U.S. Holder generally will recognize capital gain or loss in an amount equal to the difference between the amount realized upon the disposition and the U.S. Holder’s adjusted tax basis in the common stock, Pre-Funded Warrants or Common Warrants sold. Capital gain or loss will constitute long-term capital gain or loss if the U.S. Holder’s holding period for the common stock, Pre-Funded Warrants or Common Warrants exceeds one year at the time of the disposition. The deductibility of capital losses is subject to certain limitations. U.S. Holders who recognize losses with respect to a disposition of shares of common stock, Pre-Funded Warrants or Common Warrants should consult their own tax advisors regarding the tax treatment of such losses.
Information Reporting and Backup Withholding
Information reporting requirements generally will apply to payments of distributions (including constructive distributions) on the common stock, Pre-Funded Warrants and Common Warrants and to the proceeds of a sale or other disposition of common stock, Pre-Funded Warrants and Common Warrants paid by us to a U.S. Holder unless such U.S. Holder is an exempt recipient, such as a corporation. Backup withholding will apply to those payments if the U.S. Holder fails to provide the holder’s taxpayer identification number, or certification of exempt status, or if the holder otherwise fails to comply with applicable requirements to establish an exemption.
Backup withholding is not an additional tax. Rather, any amounts withheld under the backup withholding rules will be allowed as a refund or a credit against the U.S. Holder’s U.S. federal income tax liability provided the required information is timely furnished to the IRS. U.S. Holders should consult their own tax advisors regarding their qualification for an exemption from information reporting and backup withholding and the procedure for obtaining such an exemption.
Tax Considerations Applicable to Non-U.S. Holders
Exercise and Expiration of Common Warrants
In general, a Non-U.S. Holder will not recognize gain or loss for U.S. federal income tax purposes upon the exercise of Common Warrants into shares of common stock, however, to the extent a cashless exercise results in a taxable exchange, the consequences would be similar to those described in the discussion below under “Disposition of Common Stock; Pre-Funded Warrants or Common Warrants”.
The expiration of a Common Warrant will be treated as if the Non-U.S. Holder sold or exchanged the Common Warrant and recognized a capital loss equal to the Non-U.S. Holder’s tax basis in the Common Warrant. However, a Non-U.S. Holder will not be able to utilize a loss recognized upon expiration of a Common Warrant against the Non-U.S. Holder’s U.S. federal income tax liability unless the loss is effectively connected with the Non-U.S. Holder’s conduct of a trade or business within the United States (and, if an income tax treaty applies, is attributable to a permanent establishment or fixed base in the United States) or is treated as a U.S.-source loss and the Non-U.S. Holder is present 183 days or more in the taxable year of disposition and certain other conditions are met.
Certain Adjustments to Warrants
As described under “—U.S. Holders—Certain Adjustments to Pre-Funded Warrants or Common Warrants,” an adjustment to the Pre-Funded Warrants or Common Warrants could result in a constructive distribution to a Non-U.S. Holder, which would be treated as described under “Distributions” below. Any resulting withholding tax attributable to deemed dividends would be collected from other amounts payable or distributable to the Non-U.S. Holder. Non-U.S. Holders should consult their tax advisors regarding the proper treatment of any adjustments to the Pre-Funded Warrants or Common Warrants.
In addition, regulations governing “dividend equivalents” under Section 871(m) of the Code may apply to the Pre-Funded Warrants. Under those regulations, an implicit or explicit payment under the Pre-Funded Warrants that references a dividend distribution on our common stock would possibly be taxable to a Non-U.S. Holder as described under “Distributions” below. Such dividend equivalent amount would be taxable and subject to withholding whether or not there is actual payment of cash or other property, and the Company may satisfy any withholding obligations it has in respect of the Pre-Funded Warrants by withholding from other amounts due to the Non-U.S. Holder. Non-U.S. Holders are encouraged to consult their own tax advisors regarding the application of Section 871(m) of the Code to the Pre-Funded Warrants.
Distributions
As discussed above, we currently anticipate that we will retain future earnings, if any, to finance the growth and development of our business and do not intend to pay cash dividends in respect of our common stock in the foreseeable future. In the event that we do make distributions on our common stock to a Non-U.S. Holder, those distributions generally will constitute dividends for U.S. federal income tax purposes as described in “—U.S. Holders—Distributions.” To the extent those distributions do not constitute dividends for U.S. federal income tax purposes (i.e., the amount of such distributions exceeds both our current and our accumulated earnings and profits), they will constitute a return of capital and will first reduce a Non-U.S. Holder’s basis in our common stock (determined separately with respect to each share of common stock), but not below zero, and then will be treated as gain from the sale of that common stock as described below under the section titled “—Disposition of Common Stock, Pre-Funded Warrants or Common Warrants.”
Any distribution (including constructive distributions) on shares of common stock that is treated as a dividend paid to a Non-U.S. Holder that is not effectively connected with the holder’s conduct of a trade or business in the United States will generally be subject to withholding tax at a 30% rate or such lower rate as may be specified by an applicable income tax treaty between the United States and the Non-U.S. Holder’s country of residence. To obtain a reduced rate of withholding under a treaty, a Non-U.S. Holder generally will be required to provide the applicable withholding agent with a properly executed IRS Form W-8BEN, IRS Form W-8BEN-E or other appropriate form, certifying the Non-U.S. Holder’s entitlement to benefits under that treaty. Such form must be provided prior to the payment of dividends and must be updated periodically. If a Non-U.S. Holder holds stock through a financial institution or other agent acting on the holder’s behalf, the holder will be required to provide appropriate documentation to such agent. The holder’s agent may then be required to provide certification to the applicable withholding agent, either directly or through other intermediaries. If you are eligible for a reduced rate of U.S. withholding tax under an income tax treaty, you should consult with your own tax advisor to determine if you are able to obtain a refund or credit of any excess amounts withheld by timely filing an appropriate claim for a refund with the IRS.
We generally are not required to withhold tax on dividends paid (or constructive dividends deemed paid) to a Non-U.S. Holder that are effectively connected with the holder’s conduct of a trade or business within the United States (and, if required by an applicable income tax treaty, are attributable to a permanent establishment or fixed base that the holder maintains in the United States) if a properly executed IRS Form W-8ECI, stating that the dividends are so connected, is furnished to us (or, if stock is held through a financial institution or other agent, to the applicable withholding agent). In general, such effectively connected dividends will be subject to U.S. federal income tax on a net income basis at the regular tax rates applicable to U.S. persons. A corporate Non-U.S. Holder receiving effectively connected dividends may also be subject to an additional “branch profits tax,” which is imposed, under certain circumstances, at a rate of 30% (or such lower rate as may be specified by an applicable treaty) on the corporate Non-U.S. Holder’s effectively connected earnings and profits, subject to certain adjustments.
See also the sections below titled “—Backup Withholding and Information Reporting” and “—Foreign Accounts” for additional withholding rules that may apply to dividends paid to certain foreign financial institutions or non-financial foreign entities.
Disposition of Common Stock, Pre-Funded Warrants or Common Warrants
Subject to the discussions below under the sections titled “—Backup Withholding and Information Reporting” and “—Foreign Accounts,” a Non-U.S. Holder generally will not be subject to U.S. federal income or withholding tax with respect to gain recognized on a sale or other disposition (other than a redemption treated as a distribution, which will be taxable as described above under “Distributions”) of shares of common stock, Pre-Funded Warrants or Common Warrants unless:
● the gain is effectively connected with the Non-U.S. Holder’s conduct of a trade or business in the United States, and if an applicable income tax treaty so provides, the gain is attributable to a permanent establishment or fixed base maintained by the Non-U.S. Holder in the United States; in these cases, the Non-U.S. Holder will be taxed on a net income basis at the regular tax rates and in the manner applicable to U.S. persons, and, if the Non-U.S. Holder is a corporation, an additional branch profits tax at a rate of 30%, or a lower rate as may be specified by an applicable income tax treaty, may also apply;
● the Non-U.S. Holder is a nonresident alien present in the United States for 183 days or more in the taxable year of the disposition and certain other requirements are met, in which case the Non-U.S. Holder will be subject to a 30% tax (or such lower rate as may be specified by an applicable income tax treaty between the United States and such holder’s country of residence) on the net gain derived from the disposition, which may be offset by certain U.S.-source capital losses of the Non-U.S. Holder, if any; or
● the common stock constitutes a U.S. real property interest because we are, or have been at any time during the five-year period preceding such disposition (or the Non-U.S. Holder’s holding period of the common stock, Pre-Funded Warrants or Common Warrants, if shorter), a “U.S. real property holding corporation,” unless the common stock is regularly traded on an established securities market, as defined by applicable Treasury Regulations, and the Non-U.S. Holder held no more than 5% of our outstanding common stock, directly or indirectly, during the shorter of the five-year period ending on the date of the disposition or the period that the Non-U.S. Holder held the common stock. Special rules may apply to the determination of the 5% threshold in the case of a holder of Pre-Funded Warrants or Common Warrants. Non-U.S. Holders are urged to consult their own tax advisors regarding the effect of holding Pre-Funded Warrants or Common Warrants on the calculation of such 5% threshold. Generally, a corporation is a “U.S. real property holding corporation” if the fair market value of its “U.S. real property interests” (as defined in the Code and applicable regulations) equals or exceeds 50% of the sum of the fair market value of its worldwide real property interests plus its other assets used or held for use in a trade or business. Although there can be no assurance, we believe that we are not currently, and we do not anticipate becoming, a “U.S. real property holding corporation” for U.S. federal income tax purposes. No assurance can be provided that the common stock will be regularly traded on an established securities market for purposes of the rules described above. Non-U.S. Holders are urged to consult their own tax advisors regarding the U.S. federal income tax considerations that could result if we are, or become, a “U.S. real property holding corporation.
See the sections titled “—Backup Withholding and Information Reporting” and “—Foreign Accounts” for additional information regarding withholding rules that may apply to proceeds of a disposition of the common stock, Pre-Funded Warrants or Common Warrants paid to foreign financial institutions or non-financial foreign entities.
Backup Withholding and Information Reporting
We must report annually to the IRS and to each Non-U.S. Holder the gross amount of the distributions (including constructive distributions) on the common stock, Pre-Funded Warrants or Common Warrants paid to such holder and the tax withheld, if any, with respect to such distributions. Non-U.S. Holders may have to comply with specific certification procedures to establish that the holder is not a U.S. person (as defined in the Code) in order to avoid backup withholding at the applicable rate, currently 24%, with respect to dividends (or constructive dividends) on the common stock, Pre-Funded Warrants or Common Warrants. Generally, a holder will comply with such procedures if it provides a properly executed IRS Form W-8BEN (or other applicable Form W-8) or otherwise meets documentary evidence requirements for establishing that it is a Non-U.S. Holder, or otherwise establishes an exemption. Dividends paid to Non-U.S. Holders subject to withholding of U.S. federal income tax, as described above under the heading “Distributions,” will generally be exempt from U.S. backup withholding.
Information reporting and backup withholding generally will apply to the proceeds of a disposition of the common stock, Pre-Funded Warrants or Common Warrants by a Non-U.S. Holder effected by or through the U.S. office of any broker, U.S. or foreign, unless the holder certifies its status as a Non-U.S. Holder and satisfies certain other requirements, or otherwise establishes an exemption. Generally, information reporting and backup withholding will not apply to a payment of disposition proceeds to a Non-U.S. Holder where the transaction is effected outside the United States through a non-U.S. office of a broker. However, for information reporting purposes, dispositions effected through a non-U.S. office of a broker with substantial U.S. ownership or operations generally will be treated in a manner similar to dispositions effected through a U.S. office of a broker. Non-U.S. Holders should consult their own tax advisors regarding the application of the information reporting and backup withholding rules to them.
Copies of information returns may be made available to the tax authorities of the country in which the Non-U.S. Holder resides or is incorporated under the provisions of a specific treaty or agreement.
Backup withholding is not an additional tax. Any amounts withheld under the backup withholding rules from a payment to a Non-U.S. Holder can be refunded or credited against the Non-U.S. Holder’s U.S. federal income tax liability, if any, provided that an appropriate claim is timely filed with the IRS.
Foreign Accounts
The Foreign Account Tax Compliance Act, or FATCA, generally imposes a 30% withholding tax on dividends (including constructive dividends) on the common stock, Pre-Funded Warrants and Common Warrants if paid to a non-U.S. entity unless (i) if the non-U.S. entity is a “foreign financial institution,” the non-U.S. entity undertakes certain due diligence, reporting, withholding, and certification obligations, (ii) if the non-U.S. entity is not a “foreign financial institution,” the non-U.S. entity identifies certain of its U.S. investors, if any, or (iii) the non-U.S. entity is otherwise exempt under FATCA.
Withholding under FATCA generally will apply to payments of dividends (including constructive dividends) on our common stock, Pre-Funded Warrants and Common Warrants. While withholding under FATCA would have also applied to payments of gross proceeds from a sale or other disposition of the common stock, Pre-Funded Warrants or Common Warrants, under proposed U.S. Treasury Regulations withholding on payments of gross proceeds is not required. Although such regulations are not final, applicable withholding agents may rely on the proposed regulations until final regulations are issued.
An intergovernmental agreement between the United States and an applicable foreign country may modify the requirements described in this section. Under certain circumstances, a holder may be eligible for refunds or credits of the tax. Holders should consult their own tax advisors regarding the possible implications of FATCA on their investment in the common stock, Pre-Funded Warrants or Common Warrants.
Federal Estate Tax
Common stock owned or treated as owned by an individual who is not a citizen or resident of the United States (as specially defined for U.S. federal estate tax purposes) at the time of death will be included in the individual’s gross estate for U.S. federal estate tax purposes and, therefore, may be subject to U.S. federal estate tax, unless an applicable estate tax or other treaty provides otherwise. The foregoing may also apply to Common Warrants and Pre-Funded Warrants. A Non-U.S. Holder should consult his, her, or its own tax advisor regarding the U.S. federal estate tax consequences of the ownership or disposition of shares of the common stock, Pre-Funded Warrants and Common Warrants.
The preceding discussion of material U.S. federal tax considerations is for informational purposes only. It is not tax advice. Prospective investors should consult their own tax advisors regarding the particular U.S. federal, state, local and non-U.S. tax consequences of purchasing, holding and disposing of the common stock, Pre-Funded Warrants or Common Warrants, including the consequences of any proposed changes in applicable laws.
UNDERWRITING
We are offering the securities described in this prospectus through the underwriters named below. Ladenburg Thalmann & Co. Inc. is acting as the representative (the “Representative”) of the underwriters in this offering. Subject to the terms and conditions of the underwriting agreement, the underwriters have agreed to purchase the number of our securities set forth opposite its name below.
|
Underwriters |
Number of Common Stock Units |
Number of PFW Units
|
||||||
|
Ladenburg Thalmann & Co. Inc. |
||||||||
|
Total |
||||||||
A copy of the underwriting agreement has been filed as an exhibit to the registration statement of which this prospectus is part.
We have been advised by the underwriters that they propose to offer the Common Stock Units and PFW Units directly to the public at the public offering prices set forth on the cover page of this prospectus. Any securities sold by the underwriters to securities dealers will be sold at the public offering price less a selling concession not in excess of $____ per Common Stock Unit and $____ per PFW Unit.
The underwriting agreement provides that the underwriters’ obligation to purchase the securities we are offering is subject to conditions contained in the underwriting agreement.
No action has been taken by us or the underwriters that would permit a public offering of the Common Stock Units and PFW Units in any jurisdiction outside the United States where action for that purpose is required. None of our securities included in this offering may be offered or sold, directly or indirectly, nor may this prospectus or any other offering material or advertisements in connection with the offer and sales of any of the securities offering hereby be distributed or published in any jurisdiction except under circumstances that will result in compliance with the applicable rules and regulations of that jurisdiction. Persons who receive this prospectus are advised to inform themselves about and to observe any restrictions relating to this offering of securities and the distribution of this prospectus. This prospectus is neither an offer to sell nor a solicitation of any offer to buy the securities in any jurisdiction where that would not be permitted or legal.
The underwriters have advised us that they do not intend to confirm sales to any account over which they exercise discretionary authority.
Underwriting Discount and Expenses
The following table summarizes the underwriting discount and commission to be paid to the underwriters by us.
|
Per Common Stock Unit(1) |
Per |
Total |
Total |
|||||||||||||
|
Public offering price |
$ | $ | $ | $ | ||||||||||||
|
Underwriting discounts and commissions to be paid to underwriters by us(3)(4) |
$ | $ | $ | $ | ||||||||||||
|
Proceeds, before expenses, to us |
$ | $ | $ | $ | ||||||||||||
________________
|
(1) |
The public offering price and underwriting discount in respect of the Common Stock Units corresponds to a public offering price per share of common stock and accompanying Common Warrant of $____ ($____ net of the underwriting discount). |
|
(2) |
The public offering price and underwriting discount in respect of the PFW Units corresponds to a public offering price per PFW Unit of $____ ($____ net of the underwriting discount). |
|
(3) |
We have also agreed to reimburse the accountable expenses of the representative, including a pre-closing expense allowance of up to a maximum of $30,000 and an additional closing expense allowance up to a maximum of $85,000. |
|
(4) |
We have granted the underwriters an option for a period of up to 45 days from the date of this prospectus to purchase up to 104,822 additional shares of our common stock and/or Series A warrants to purchase up to an additional 104,822 shares of our common stock, or any combination thereof, as determined by the underwriters, at the assumed public offering price, less underwriting discounts and commissions, in each case solely to cover over-allotments, if any. |
We estimate the total expenses payable by us for this offering to be approximately $1,020,000, which amount includes (i) the underwriting discount of $800,000, (ii) reimbursement of the accountable expenses of the underwriters, including the legal fees of the representative, in an amount not to exceed a total of $115,000 consisting of: $30,000 for pre-closing expenses plus $85,000 for closing expenses and (iii) other estimated company expenses of approximately $105,000, which includes legal, accounting, printing costs, and various fees associated with the registration and listing of our shares.
The securities we are offering are being offered by the underwriters subject to certain conditions specified in the underwriting agreement.
Over-allotment Option
We have granted the underwriters an option for a period of up to 45 days from the date of this prospectus to purchase up to 104,822 additional shares of our common stock and/or Series A warrants to purchase up to an additional 104,822 shares of our common stock, or any combination thereof, as determined by the underwriters, at the assumed public offering price, less underwriting discounts and commissions.
The underwriters may exercise the option solely to cover overallotments, if any, made in connection with this offering. If any additional shares of common stock and/or Series A warrants are purchased, the underwriters will offer these shares of common stock and/or Series A warrants on the same terms as those on which the other securities are being offered.
Representative Warrants
Upon completion of this offering, we have agreed to issue to the representative as compensation, warrants to purchase up to 41,928 shares of common stock (or 48,218 shares of common stock assuming the exercise of the over-allotment option in full), which equals 6.0% of the aggregate number of the Shares and the shares of common stock underlying the Pre-Funded Warrants sold in this offering, but excluding shares of Common Stock underlying the Series A Warrants (the “Representative Warrants”). The Representative Warrants will be exercisable at a per share exercise price equal to 155% of the public offering price per Common Stock Unit in this offering. The Representative Warrants are exercisable at any time and from time to time, in whole or in part, during the four and one half year period commencing 180 days following the commencement of sales of the securities issued in this offering.
The Representative Warrants have been deemed compensation by FINRA and are therefore subject to a 180-day lock-up pursuant to Rule 5110(e)(1)(A) of FINRA. The representative of the Underwriters (or permitted assignees under Rule 5110(e)(2)) will not sell, transfer, assign, pledge, or hypothecate these Representative Warrants or the shares of common stock underlying these warrants, nor will they engage in any hedging, short sale, derivative, put, or call transaction that would result in the effective economic disposition of the warrants or the underlying securities for a period of 180 days following the commencement of sales of the securities issued in this offering.. The exercise price and number of shares issuable upon exercise of the Representative Warrants may be adjusted in certain circumstances including in the event of a stock dividend or our recapitalization, reorganization, merger or consolidation. However, the exercise price of the Representative Warrants or the underlying shares of common stock will not be adjusted for issuances of shares of common stock at a price below the warrant exercise price.
Right of First Refusal
We have granted to Ladenburg Thalmann & Co. Inc. (“Ladenburg”) the right of first refusal for a period of nine (9) months following the closing of this offering the opportunity to participate as a sole bookrunner or exclusive placement agent or exclusive sales agent in respect of any future financing by us on terms and conditions mutually acceptable to us and Ladenburg.
Fee Obligation
Within nine (9) months after the date of termination or expiration of the engagement agreement that we entered into with Ladenburg dated May 21, 2024, the securities or securities convertible into or exchangeable for the securities that are sold by us to investors contacted by Ladenburg, then we shall pay to Ladenburg, at the time of each such sale or transaction, the fees set forth above with respect to any such sale.
Listing
Our shares of common stock are listed on the Nasdaq Capital Market under the symbol “AMIX.”
The last reported sale price of our common stock on October 31, 2024 was $14.31 per share. The actual public offering price per Common Stock Unit or PFW Unit, as the case may be, will be determined between us, the underwriters and the investors in the offering, and may be at a discount to the current market price of our common stock. Therefore, the assumed public offering price used throughout this prospectus may not be indicative of the final offering price. There is no established public trading market for the Common Warrants or Pre-Funded Warrants, and we do not expect such a market to develop. In addition, we do not intend to apply for a listing of the Common Warrants, Pre-Funded Warrants or the Representative Warrants on any national securities exchange or other nationally recognized trading system.
See “Prospectus Summary” for information about the listing of our common stock.
Lock-up Agreements
Our officers, directors and each of their respective affiliates and associated partners have agreed with the underwriters to be subject to a lock-up period of 90 days following the date of this prospectus. This means that, during the applicable lock-up period, such persons may not offer for sale, contract to sell, sell, distribute, grant any option, right or warrant to purchase, pledge, hypothecate or otherwise dispose of, directly or indirectly, any shares of our common stock or any securities convertible into, or exercisable or exchangeable for, shares of our common stock. Certain limited transfers are permitted during the lock-up period if the transferee agrees to these lock-up restrictions. We have also agreed, in the underwriting agreement, to similar lock-up restrictions on the issuance and sale of our securities for 90 days following the closing of this offering, although we will be permitted to issue stock options or stock awards to directors, officers and employees under our existing plans. The Representative may, in its sole discretion and without notice, waive the terms of any of these lock-up agreements.
Transfer Agent and Registrar
The transfer agent and registrar for our common stock is Equity Stock Transfer, LLC.
Stabilization, Short Positions and Penalty Bids
The underwriters may engage in syndicate covering transactions stabilizing transactions and penalty bids or purchases for the purpose of pegging, fixing or maintaining the price of our common stock:
|
• |
Syndicate covering transactions involve purchases of securities in the open market after the distribution has been completed in order to cover syndicate short positions. Such a naked short position would be closed out by buying securities in the open market. A naked short position is more likely to be created if the underwriters are concerned that there could be downward pressure on the price of the securities in the open market after pricing that could adversely affect investors who purchase in the offering. |
|
• |
Stabilizing transactions permit bids to purchase the underlying security so long as the stabilizing bids do not exceed a specific maximum. |
|
• |
Penalty bids permit the underwriters to reclaim a selling concession from a syndicate member when the securities originally sold by the syndicate member are purchased in a stabilizing or syndicate covering transaction to cover syndicate short positions. |
These syndicate covering transactions, stabilizing transactions, and penalty bids may have the effect of raising or maintaining the market prices of our securities or preventing or retarding a decline in the market prices of our securities. As a result, the price of our common stock may be higher than the price that might otherwise exist in the open market. Neither we nor the underwriters make any representation or prediction as to the effect that the transactions described above may have on the price of our common stock. These transactions may be effected on the Nasdaq Stock Market, in the over-the-counter market or on any other trading market and, if commenced, may be discontinued at any time.
In connection with this offering, the underwriters also may engage in passive market making transactions in our common stock in accordance with Regulation M during a period before the commencement of offers or sales of shares of our common stock in this offering and extending through the completion of the distribution. In general, a passive market maker must display its bid at a price not in excess of the highest independent bid for that security. However, if all independent bids are lowered below the passive market maker’s bid that bid must then be lowered when specific purchase limits are exceeded. Passive market making may stabilize the market price of the securities at a level above that which might otherwise prevail in the open market and, if commenced, may be discontinued at any time.
Neither we, nor the underwriters make any representation or prediction as to the direction or magnitude of any effect that the transactions described above may have on the prices of our securities. In addition, neither we nor the underwriters make any representation that the underwriters will engage in these transactions or that any transactions, once commenced will not be discontinued without notice.
Indemnification
We have agreed to indemnify the underwriters against certain liabilities, including certain liabilities arising under the Securities Act, or to contribute to payments that the underwriters may be required to make for these liabilities.
Other
From time to time, certain of the underwriters and/or their affiliates may in the future provide, various investment banking and other financial services for us for which they may receive customary fees. In the course of their businesses, the underwriters and their affiliates may actively trade our securities or loans for their own account or for the accounts of customers, and, accordingly, the underwriters and their affiliates may at any time hold long or short positions in such securities or loans. Except for services provided in connection with this offering, no underwriter has provided any investment banking or other financial services to us during the 180-day period preceding the date of this prospectus and we do not expect to retain any underwriter to perform any investment banking or other financial services for at least 90 days after the date of this prospectus.
Electronic Distribution
A prospectus in electronic format may be made available on the websites maintained by the underwriters, if any, participating in this offering and the underwriters may distribute prospectuses electronically. Other than the prospectus in electronic format, the information on these websites is not part of this prospectus or the registration statement of which this prospectus forms a part, has not been approved or endorsed by us or the underwriters, and should not be relied upon by investors.
Offer Restrictions Outside the United States
Other than in the United States, no action has been taken by us or the underwriters that would permit a public offering of the securities offered by this prospectus in any jurisdiction where action for that purpose is required. The securities offered by this prospectus may not be offered or sold, directly or indirectly, nor may this prospectus or any other offering material or advertisements in connection with the offer and sale of any such securities be distributed or published in any jurisdiction, except under circumstances that will result in compliance with the applicable rules and regulations of that jurisdiction. Persons into whose possession this prospectus comes are advised to inform themselves about and to observe any restrictions relating to the offering and the distribution of this prospectus. This prospectus does not constitute an offer to sell or a solicitation of an offer to buy any securities offered by this prospectus in any jurisdiction in which such an offer or a solicitation is unlawful.
Australia
This prospectus is not a disclosure document under Chapter 6D of the Australian Corporations Act, has not been lodged with the Australian Securities and Investments Commission and does not purport to include the information required of a disclosure document under Chapter 6D of the Australian Corporations Act. Accordingly, (i) the offer of the securities under this prospectus is only made to persons to whom it is lawful to offer the securities without disclosure under Chapter 6D of the Australian Corporations Act under one or more exemptions set out in section 708 of the Australian Corporations Act, (ii) this prospectus is made available in Australia only to those persons as set forth in clause (i) above, and (iii) the offeree must be sent a notice stating in substance that by accepting this offer, the offeree represents that the offeree is such a person as set forth in clause (i) above, and, unless permitted under the Australian Corporations Act, agrees not to sell or offer for sale within Australia any of the securities sold to the offeree within 12 months after its transfer to the offeree under this prospectus.
LEGAL MATTERS
The validity of the securities offered hereby will be passed upon for us by ArentFox Schiff LLP, Washington, DC. The underwriters are being represented by Blank Rome LLP, New York, New York in connection with this offering.
EXPERTS
The financial statements of Autonomix Medical, Inc. as of March 31, 2024 and 2023 and for each of the years in the two-year period ended March 31, 2024, have been audited by Forvis Mazars, LLP, independent registered public accounting firm, as set forth in their report thereon, included in this registration statement. Such financial statements have been included herein in reliance upon such report given on the authority of such firm as experts in accounting and auditing.
The report of Forvis Mazars, LLP contains an explanatory paragraph regarding substantial doubt about the Company’s ability to continue as a going concern.
WHERE YOU CAN FIND MORE INFORMATION
We have filed with the SEC a registration statement on Form S-1 under the Securities Act for the securities being offered by this prospectus. This prospectus, which is part of the registration statement, does not contain all of the information included in the registration statement and the exhibits. For further information about us and the securities offered by this prospectus, you should refer to the registration statement and its exhibits. References in this prospectus to any of our contracts or other documents are not necessarily complete, and you should refer to the exhibits attached to the registration statement for copies of the actual contract or document. SEC filings are also available to the public at the SEC’s website at www.sec.gov.
We are subject to the reporting and information requirements of the Exchange Act and, as a result, we file periodic and current reports, proxy statements and other information with the SEC. We make our periodic reports and other information filed with or furnished to the SEC, available, free of charge, through our website as soon as reasonably practicable after those reports and other information are filed with or furnished to the SEC. Additionally, these periodic reports, proxy statements and other information are available at the SEC’s website at www.sec.gov.
REVERSE SPLIT FINANCIAL DATA:
On October 23, 2024, the Company announced that it is filing a certificate of amendment to its articles of incorporation with the Secretary of State of the State of Delaware to effect a 1-for-20 reverse stock split of its common stock. The table below sets forth the impact of the Reverse Stock Split on the Company's net loss per common share - basic and diluted; weighted average common shares outstanding - basic and diluted; and shares issued and outstanding, for the years ended March 31, 2024 and 2023; and the three months ended June 30, 2024 and 2023:
|
Pre Split (1) |
Post Split |
|||||||||||||||
|
Year Ended March 31, |
Year Ended March 31, |
|||||||||||||||
|
2024 |
2023 |
2024 |
2023 |
|||||||||||||
|
Net loss (in thousands, except share and per share data) |
$ | (15,426 | ) | $ | (1,990 | ) | $ | (15,426 | ) | $ | (1,990 | ) | ||||
|
Net loss per common share - basic |
$ | (1.05 | ) | $ | (0.17 | ) | $ | (21.09 | ) | $ | (3.31 | ) | ||||
|
Net loss per common share - diluted |
$ | (1.05 | ) | $ | (0.17 | ) | $ | (21.09 | ) | $ | (3.31 | ) | ||||
|
Weighted average common shares outstanding - basic |
14,626,282 | 12,023,112 | 731,372 | 601,166 | ||||||||||||
|
Weighted average common shares outstanding - diluted |
14,626,282 | 12,023,112 | 731,372 | 601,166 | ||||||||||||
|
Pre Split (1) |
Post Split |
|||||||||||||||
|
3 Months Ended June 30, |
3 Months Ended June 30, |
|||||||||||||||
|
2024 |
2023 |
2024 |
2023 |
|||||||||||||
|
Net loss (in thousands, except share and per share data) |
$ | (2,699 | ) | $ | (865 | ) | $ | (2,699 | ) | $ | (865 | ) | ||||
|
Net loss per common share - basic |
$ | (0.14 | ) | $ | (0.07 | ) | $ | (2.85 | ) | $ | (1.34 | ) | ||||
|
Net loss per common share - diluted |
$ | (0.14 | ) | $ | (0.07 | ) | $ | (2.85 | ) | $ | (1.34 | ) | ||||
|
Weighted average common shares outstanding - basic |
18,902,248 | 12,884,604 | 945,382 | 644,241 | ||||||||||||
|
Weighted average common shares outstanding - diluted |
18,902,248 | 12,884,604 | 945,382 | 644,241 | ||||||||||||
(1) The pre-split amounts represent the amounts reported in the Company's Annual Report on Form 10-K or Quarterly Report on Form 10-Q for the respective periods.
Index to Financial Statements
|
Page |
|
|
Report of Independent Registered Public Accounting Firm |
89 |
|
Balance Sheets as of March 31, 2024 and 2023 |
90 |
|
Statements of Operations for the years ended March 31, 2024 and 2023 |
91 |
|
Statements of Changes in Stockholders' Equity for the years ended March 31, 2024 and 2023 |
92 |
|
Statements of Cash Flows for the years ended March 31, 2024 and 2023 |
93 |
|
Notes to Financial Statements |
94 |
|
Unaudited Condensed Balance Sheets as of June 30, 2024 and March 31, 2024 |
111 |
|
Unaudited Condensed Statements of Operations for the three months ended June 30, 2024 and 2023 |
112 |
|
Unaudited Condensed Statements of Changes in Stockholders' Equity for the three months ended June 30, 2024 and 2023 |
113 |
|
Unaudited Condensed Statements of Cash Flows for the three months ended June 30, 2024 and 2023 |
114 |
|
Notes to the Unaudited Condensed Financial Statements |
115 |
Report of Independent Registered Public Accounting Firm
To the Shareholders, Board of Directors, and Audit Committee
Autonomix Medical, Inc.
Opinion on the Financial Statements
We have audited the accompanying balance sheets of Autonomix Medical, Inc. (the “Company”) as of March 31, 2024 and 2023, the related statements of operations, changes in stockholders’ equity, and cash flows for each of the years in the two-year period ended March 31, 2024, and the related notes (collectively referred to as the “financial statements”). In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of the Company as of March 31, 2024 and 2023, and the results of its operations and its cash flows for each of the years in the two-year period ended March 31, 2024, in conformity with accounting principles generally accepted in the United States of America.
Going Concern
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 1 to the financial statements, the Company has an accumulated deficit since inception, has not generated revenue from operations, and does not expect to experience positive cash flows from operating activities in the near term. These conditions raise substantial doubt about the Company’s ability to continue as a going concern. Management’s plans in regard to this matter are also described in Note 1. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits.
We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (“PCAOB”) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits, we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures include examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements.
Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
/s/ FORVIS, LLP
We have served as the Company’s auditor since 2022.
Atlanta, Georgia
May 31, 2024
Balance Sheets
|
(in thousands, except par value and share data) |
As of |
|||||||
|
March 31, |
||||||||
|
2024 |
2023 |
|||||||
|
Assets |
||||||||
|
Current assets: |
||||||||
|
Cash |
$ | 8,608 | $ | 865 | ||||
|
Other current assets |
783 | — | ||||||
|
Total current assets |
9,391 | 865 | ||||||
|
Long-term assets: |
||||||||
|
Fixed assets, net |
16 | — | ||||||
|
Total long-term assets |
16 | — | ||||||
|
Total Assets |
$ | 9,407 | $ | 865 | ||||
|
Liabilities and Stockholders' Equity |
||||||||
|
Current liabilities: |
||||||||
|
Accounts payable |
$ | 492 | $ | 173 | ||||
|
Accrued expenses |
285 | 48 | ||||||
|
Total current liabilities |
777 | 221 | ||||||
|
Long-term liabilities: |
||||||||
|
Long term debt - convertible notes, net of unamortized debt discount |
1,002 | — | ||||||
|
Total long-term liabilities |
1,002 | — | ||||||
|
Total Liabilities |
$ | 1,779 | $ | 221 | ||||
|
Commitments and contingencies (Note 5) |
||||||||
|
Stockholders' equity: |
||||||||
|
Preferred stock, $0.001 par value, 10,000,000 shares authorized as of March 31, 2024, no shares issued and outstanding, and 7,100,000 shares authorized as of March 31, 2023, no shares issued and outstanding |
$ | — | $ | — | ||||
|
Common stock, $0.001 par value, 500,000,000 shares authorized as of March 31, 2024, 18,846,094 shares issued and outstanding, and 25,000,000 shares authorized as of March 31, 2023, 12,336,571 shares issued and outstanding |
19 | 12 | ||||||
|
Additional paid-in capital |
46,578 | 24,175 | ||||||
|
Accumulated deficit |
(38,969 | ) | (23,543 | ) | ||||
|
Total Stockholders' Equity |
7,628 | 644 | ||||||
|
Total Liabilities and Stockholders' Equity |
$ | 9,407 | $ | 865 | ||||
See accompanying notes to the financial statements
Statements of Operations
|
For the Years Ended |
||||||||
|
March 31, |
||||||||
|
(in thousands, except share and per share data) |
2024 |
2023 |
||||||
|
Operating expenses: |
||||||||
|
General and administrative |
$ | 5,249 | $ | 1,245 | ||||
|
Research and development |
2,225 | 745 | ||||||
|
Warrant expense - termination agreement |
4,556 | — | ||||||
|
Total operating expenses |
12,030 | 1,990 | ||||||
|
Loss from operations |
(12,030 | ) | (1,990 | ) | ||||
|
Other (expense) income: |
||||||||
|
Warrant liability - mark-to-market |
(3,444 | ) | — | |||||
|
Interest expense |
(79 | ) | — | |||||
|
Interest income |
127 | — | ||||||
|
Total other expense |
(3,396 | ) | — | |||||
|
Loss before income taxes |
(15,426 | ) | (1,990 | ) | ||||
|
Income taxes |
— | — | ||||||
|
Net loss |
$ | (15,426 | ) | $ | (1,990 | ) | ||
|
Loss per share - basic and diluted |
$ | (1.05 | ) | $ | (0.17 | ) | ||
|
Weighted average shares outstanding - basic and diluted |
14,626,282 | 12,023,112 | ||||||
See accompanying notes to the financial statements
Statements of Changes in Stockholders' Equity
|
Additional |
Total |
|||||||||||||||||||||||||||
|
Preferred Stock |
Common Stock |
Paid-in |
Accumulated |
Stockholders' |
||||||||||||||||||||||||
|
(in thousands) |
Shares |
Amount |
Shares |
Amount |
Capital |
Deficit |
Equity |
|||||||||||||||||||||
|
Balance March 31, 2022 |
— | $ | — | 11,999 | $ | 12 | $ | 23,500 | $ | (21,553 | ) | $ | 1,959 | |||||||||||||||
|
Net loss |
— | — | — | — | — | (1,990 | ) | (1,990 | ) | |||||||||||||||||||
|
Issuance of common stock |
— | — | 338 | — | 675 | — | 675 | |||||||||||||||||||||
|
Balance March 31, 2023 |
— | — | 12,337 | 12 | 24,175 | (23,543 | ) | 644 | ||||||||||||||||||||
|
Net loss |
— | — | — | — | — | (15,426 | ) | (15,426 | ) | |||||||||||||||||||
|
Stock-based compensation |
— | — | — | — | 618 | — | 618 | |||||||||||||||||||||
|
Issuance of common stock |
— | — | 1,420 | 2 | 2,838 | — | 2,840 | |||||||||||||||||||||
|
Issuance of common stock from IPO, net of costs |
— | — | 2,234 | 2 | 9,873 | — | 9,875 | |||||||||||||||||||||
|
Issuance of common stock for extinguishment of convertible debt |
— | — | 335 | 1 | 499 | — | 500 | |||||||||||||||||||||
|
Issuance of common stock - warrants exercised |
— | — | 2,485 | 2 | (2 | ) | — | — | ||||||||||||||||||||
|
Issuance of restricted common stock |
— | — | 35 | — | — | — | — | |||||||||||||||||||||
|
Warrants issued for debt issuance costs |
— | — | — | — | 577 | — | 577 | |||||||||||||||||||||
|
Fair value of warrants issued - termination agreement |
— | — | — | — | 8,000 | — | 8,000 | |||||||||||||||||||||
|
Balance March 31, 2024 |
— | $ | — | 18,846 | $ | 19 | $ | 46,578 | $ | (38,969 | ) | $ | 7,628 | |||||||||||||||
See accompanying notes to the financial statements
Statements of Cash Flows
|
For the Years Ended March 31, |
||||||||
|
(in thousands) |
2024 |
2023 |
||||||
|
Cash Flows from Operating Activities: |
||||||||
|
Net loss |
$ | (15,426 | ) | $ | (1,990 | ) | ||
|
Adjustments to reconcile net loss to net cash used in operating activities: |
||||||||
|
Stock-based compensation |
618 | — | ||||||
|
Depreciation and amortization expense |
81 | — | ||||||
|
Warrant expense - termination agreement |
4,556 | — | ||||||
|
Warrant liability - mark-to-market |
3,444 | — | ||||||
|
Changes in operating assets - (increase)/decrease: |
||||||||
|
Other current assets |
(478 | ) | 9 | |||||
|
Changes in operating liabilities - increase: |
||||||||
|
Accounts payable |
320 | 81 | ||||||
|
Accrued expenses |
237 | 46 | ||||||
|
Net cash used in operating activities |
(6,648 | ) | (1,854 | ) | ||||
|
Cash Flows from Investing Activities: |
||||||||
|
Purchase of property and equipment |
(19 | ) | — | |||||
|
Net cash used in investing activities |
(19 | ) | — | |||||
|
Cash Flows from Financing Activities (increase/decrease): |
||||||||
|
Issuance of common stock |
2,840 | 675 | ||||||
|
Issuance of convertible debt |
2,000 | — | ||||||
|
Issuance of common stock from IPO |
10,866 | — | ||||||
|
IPO issuance costs |
(1,296 | ) | — | |||||
|
Net cash provided by financing activities |
14,410 | 675 | ||||||
|
Net change in cash and cash equivalents |
7,743 | (1,179 | ) | |||||
|
Cash and cash equivalents, at beginning of period |
865 | 2,044 | ||||||
|
Cash and cash equivalents, at end of period |
$ | 8,608 | $ | 865 | ||||
|
Supplemental cash flow disclosures: |
||||||||
|
Non-cash financing activities: |
||||||||
|
Warrants issued for debt issuance costs |
$ | 577 | $ | — | ||||
|
Proceeds from cashless exercise of warrants |
$ | 2 | $ | — | ||||
|
Fair value of warrants issued for issuance costs as part of IPO |
$ | 225 | $ | — | ||||
|
Holdback of IPO proceeds |
$ | 305 | $ | — | ||||
|
Convertible notes converted into common stock |
$ | 670 | $ | — | ||||
|
Settlement/conversion to common shares for debt issuance costs |
$ | (170 | ) | $ | — | |||
See accompanying notes to the financial statements
Notes to the Financial Statements
Note 1 – Description of the Business, Basis of Presentation and Summary of Significant Accounting Policies
Description of the Business
Autonomix Medical, Inc (“we,” “our,” the “Company”) is a medical device company organized as a Delaware corporation on June 10, 2014. The Company is a pre-revenue, clinical stage life sciences company focused on advancing innovative technologies for sensing and treating disorders relating to the peripheral nervous system.
Liquidity and Going Concern
The Company's financial statements are prepared on a going concern basis, which contemplates the realization of assets and the satisfaction of liabilities and commitments in the normal course of business. The Company is an early-stage company that is subject to all the risks associated with early-stage and emerging growth companies and has incurred losses since inception. For the years ended March 31, 2024 and 2023, the Company has net losses of approximately $15.4 million and $2.0 million, respectively and had net cash flows used in operating activities of $6.6 million and $1.9 million, respectively. The Company had no revenues for the years ended March 31, 2024 and 2023, respectively, accumulated deficit of $39.0 million and working capital of approximately $8.6 million as of March 31, 2024. The Company does not expect to generate positive cash flows from operating activities in the near future. These conditions, and the Company's ability to comply with such conditions, raise substantial doubt about the Company's ability to continue as a going concern within one year after the date that the financial statements are issued. The accompanying financial statements have been prepared on a going concern basis and do not include any adjustments that might be necessary if the Company is unable to continue as a going concern.
On January 26, 2024, the Company completed its initial public offering (“IPO”) of common stock. During the IPO, the Company sold a total of 2,234,222 shares of common stock at a purchase price of $5.00 per share for gross proceeds of $11.2 million and net proceeds of $9.8 million. On May 13, 2024, the Company cancelled 1,050 shares represented in the IPO due to payment disputes. As part of the IPO closing, $0.3 million was retained by the Company’s marketing partner as a holdback to be paid 90 days after the IPO. This $0.3 million was recorded in other current assets on the Company's balance sheet. In connection with the closing of the IPO, a portion of the Company’s convertible notes were converted into 335,000 shares of the Company’s common stock. Upon the closing of the IPO, certain notes were to be automatically converted according to their terms into the Company’s common stock to the extent and provided that certain holders of these notes are not permitted to convert such notes to the extent that the holders or any of its affiliates would beneficially own in excess of 4.99% of the Company’s common stock after such conversion. Due to this 4.99% limitation, principal representing $1.3 million, or 665,000 shares, of these convertible notes remained outstanding. The Company's existing cash resources, unexercised warrants and the cash received from the IPO are not expected to provide sufficient funds to carry out the Company's operations and clinical trials through the next twelve months.
The Company paid a cash commission of 7.0% to the selling agent on sales of the shares of common stock in the IPO. In addition, the Company has issued the selling agent warrants to purchase up to a total number of shares of common stock equal to 2.675% of the total number of shares sold in the IPO at an exercise price equal to 125% of the public offering price of the shares sold in the IPO. The selling agent warrants will be exercisable at any time, and from time to time, in whole or in part, commencing from the date that is six months after the commencement date of sales in the IPO and expiring on the fifth anniversary of the commencement date of sales in the IPO. The selling agent warrants will have a cashless exercise provision and will provide for registration rights with respect to the registration of the shares underlying the warrants.
The Company estimates its current cash resources, including the approximately $9.8 million of net proceeds from the IPO is sufficient to fund its operations into but not beyond the second calendar quarter of 2025. The Company recognizes it will need to raise additional capital to continue to execute its business plan, including obtaining regulatory clearance for its products currently under development and commercializing and generating revenues from products under development. There is no assurance that additional financing will be available when needed or that management will be able to obtain financing on terms acceptable to the Company. A failure to raise sufficient capital, generate sufficient product revenues, control expenditures and regulatory matters, among other factors, will adversely impact the Company’s ability to meet its financial obligations as they become due and payable and to achieve its intended business objectives. If the Company is unable to raise sufficient additional funds, it will have to scale back its operations.
Basis of Presentation
Use of Estimates in Financial Statement Presentation
The preparation of these financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities at the date of the financial statements and the reported amounts of expenses during the reporting period. The Company's significant estimates and assumptions include the valuation of equity related instruments, and initial and recurring fair value measurements for the warrant liability. Although the Company believes that its estimates and assumptions are reasonable, they are based upon information available at the time the estimates and assumptions were made. Some of these judgments can be subjective and complex, and, consequently, actual results could differ from those estimates.
Cash and Cash Equivalents
The Company considers all highly liquid accounts with original maturities of three months or less at the date of acquisition to be cash equivalents. Periodically, the Company may carry cash balances at financial institutions in excess of the federally insured limit of $250,000. The Company has not experienced losses on these accounts and management believes, based upon the quality of the financial institutions, that the credit risk with regard to these deposits is not significant.
Offering Costs
Offering costs consist of professional costs incurred through the balance sheet date that are direct and incremental related to the Company’s anticipated IPO. These costs, together with the selling agent fees, were reclassified to additional paid-in capital upon completion of the Company’s IPO on January 26, 2024. Costs associated with salaries and other period costs were expensed as incurred.
During the year ended March 31, 2024, the Company paid $1.3 million of offering costs related to its IPO.
Property and Equipment
Convertible Notes
The Company evaluates embedded redemption, conversion and other features within its debt to determine whether any embedded features should be bifurcated from the host instrument and accounted for as a derivative at fair value, with changes in fair value recorded in the statement of operations.
The Company’s debt is carried on the balance sheet on a historical cost basis net of unamortized discounts and premiums because the Company has not elected the fair value option of accounting. Costs associated with acquiring debt, including detachable warrants issued in connection with the financing, are capitalized as a debt discount. The debt discount is presented in the balance sheet as a direct deduction from the carrying amount of the debt liability. The costs are amortized over the estimated contractual life of the related debt instrument using the effective interest method and are included in interest expense in the statement of operations.
If the Company incurs costs associated with its convertible notes, in advance of the receipt of proceeds, the Company will record a deferred asset. Upon receipt of proceeds the Company will reclassify the deferred asset as a direct deduction from the carrying amount, as described above. In addition, since the instruments included a substantive conversion feature as of time of issuance, the issuance of equity securities were accounted for as a contractual conversion with no gain or loss recognized related to the equity securities issued to settle the instrument.
Fair Value of Financial Instruments
Fair value is defined as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. Valuation techniques used to measure fair value maximize the use of observable inputs and minimize the use of unobservable inputs. The Company utilizes a three-level valuation hierarchy for disclosures of fair value measurements, defined as follows:
Level 1 – inputs to the valuation methodology are quoted prices (unadjusted) for identical assets or liabilities in active markets.
Level 2 – inputs to the valuation methodology include quoted prices for similar assets and liabilities in active markets, and inputs that are observable for the assets or liability, either directly or indirectly, for substantially the full term of the financial instruments.
Level 3 – inputs to the valuation methodology are unobservable and significant to the fair value and require significant judgment and estimation.
The carrying value of short-term instruments, including cash, accounts payable, accrued expenses and convertible notes included in long-term debt, approximate fair value due to the relatively short period to maturity for these instruments.
Related Parties
The Company follows ASC 850, Related Party Disclosures, for the identification of related parties and disclosure of related party transactions. See further discussion in the Notes below on this matter.
Income Taxes
The Company uses the asset and liability method of accounting for income taxes. Under this method, deferred tax assets and liabilities are determined based on the differences between the financial reporting and the tax basis of reported assets and liabilities and are measured using the enacted tax rates and laws that will be in effect when the differences are expected to reverse. The Company must then assess the likelihood that the resulting deferred tax assets will be realized. A valuation allowance is provided when it is more likely than not that some portion or all of a deferred tax asset will not be realized. As of March 31, 2024 and March 31, 2023 the Company determined a full valuation allowance was required to offset its deferred tax assets as a result of recurring operating losses.
The Company accounts for uncertain tax positions in accordance with the provisions of ASC 740-10 which prescribes a recognition threshold and measurement attribute for financial statement disclosure of tax positions taken, or expected to be taken, on its tax return. The Company evaluates and records any uncertain tax positions based on the amount that management deems is more likely than not to be sustained upon examination and ultimate settlement with the tax authorities in the tax jurisdictions in which it operates. As of March 31, 2024 and March 31, 2023 the Company had no uncertain tax positions.
Stock-based Compensation
Employee share-based compensation is measured at the grant date, based on the fair value of the award, and is recognized as an expense over the requisite service period. For awards with a performance condition, compensation expense is recognized over the requisite service period if it is probable that the performance condition will be satisfied. For awards to non-employees, the Company recognizes compensation expense in the same manner as if the Company had paid cash for the goods or services. The Company estimates the fair value of options and equity classified warrants granted using an options pricing model. Expense is recognized within general and administrative and research and development expenses and forfeitures are recognized as they are incurred.
Warrants
The Company accounts for warrants as either equity-classified or liability-classified instruments based on an assessment of the warrant’s specific terms and applicable authoritative guidance in FASB ASC 480, Distinguishing Liabilities from Equity (“ASC 480”) and ASC 815, Derivatives and Hedging (“ASC 815”). The assessment considers whether the warrants are freestanding financial instruments pursuant to ASC 480, meet the definition of a liability pursuant to ASC 480, and whether the warrants meet all of the requirements for equity classification under ASC 815, including whether the warrants are indexed to the Company’s own ordinary shares and whether the warrant holders could potentially require “net cash settlement” in a circumstance outside of the Company’s control, among other conditions for equity classification. This assessment, which requires the use of professional judgment, is conducted at the time of warrant issuance and as of each subsequent quarterly period end date while the warrants are outstanding.
For issued or modified warrants that meet all of the criteria for equity classification, the warrants are required to be recorded as a component of additional paid-in capital at the time of issuance. For issued or modified warrants that do not meet all the criteria for equity classification, the warrants are required to be recorded at their initial fair value on the date of issuance, and each balance sheet date thereafter. Changes in the estimated fair value of the warrants are recognized as a non-cash gain or loss on the statements of operations. The fair value of the warrants is estimated using a Black-Scholes pricing model or a Monte Carlo simulation.
The Company issued warrants to purchase shares of common stock (i) in connection with the Bridge Offering, (ii) as part of selling agent compensation in 2024, and (iii) in connection with the Exclusive License Termination Agreement (the “Termination Agreement”). Based on the guidance noted above, we determined that warrants issued in connection with the Termination Agreement should be accounted for as a liability and the remaining warrants issued meet the requirements for equity classification. Liability classified warrants are subject to remeasurement at each balance sheet date, while equity classified warrants are valued at inception only. As discussed in Note 2, the liability warrants subsequently met equity classification.
Loss Per Common Share
Basic loss per common share is computed by dividing net loss by the weighted-average number of common shares outstanding during the period. Diluted loss per common share is determined using the weighted-average number of common shares outstanding during the period, adjusted for the dilutive effect of common stock equivalents. In periods when losses are reported, the weighted-average number of common shares outstanding excludes common stock equivalents, because their inclusion would be anti-dilutive. Generally, the Company’s outstanding warrants are non-participating securities as they are not entitled to non-forfeitable rights to dividends or dividend equivalents during the vesting term and have no obligation to fund losses. The dilutive effect of convertible securities is calculated using the “if-converted method.” Under the if-converted method, securities are assumed to be converted at the beginning of the period, and the resulting common shares are included in the denominator of the diluted calculation for the entire period being presented. However, the warrants described in Note 2 are participating securities as they receive a right to dividends, but they are not obligated to fund losses. In periods of loss, since no income is allocated to these securities, the Company's use of the treasury stock method derives the same result.
For the twelve months ended March 31, 2024 and 2023, dilutive securities that were not included in the calculations of the loss per common share because they would be anti-dilutive included the following:
|
March 31, |
||||||||
|
2024 |
2023 |
|||||||
|
Equity based warrants to purchase common shares |
5,744,569 | 6,569,929 | ||||||
|
Convertible Notes - common shares (1) |
665,000 | — | ||||||
|
Convertible Notes - equity-based warrants to purchase common shares |
500,000 | — | ||||||
|
Stock options granted under Company's incentive plan |
2,003,600 | — | ||||||
|
Total potentially dilutive securities |
8,913,169 | 6,569,929 | ||||||
|
(1) |
Shares relating to the conversion of the convertible notes as of March 31, 2024 |
Research and Development Costs
Research and development costs are expensed as incurred.
Advertising
It is our policy to expense advertising costs as incurred. Advertising expenses are included within general and administrative expenses within the statement of operations. For the years ended March 31, 2024 and 2023, the Company recorded $1.7 million and $0.1 million, respectively.
Fair Value of Common Stock
Prior to establishing a public market for the Company’s common stock, the estimated fair value of the Company’s common stock was determined by the Company’s Board of Directors (the "Board") as of the date of each option grant, with input from management, considering the Company’s most recently available third-party valuations of common stock, recent sales of common stock to third parties, and the Company’s board of directors’ assessment of additional objective and subjective factors that it believed were relevant and which may have changed from the date of the most recent valuation through the date of the grant.
JOBS Act Accounting Election
The Company qualifies as an emerging growth company ("EGC"), as defined in the Jumpstart Our Business Startups Act of 2012 (the “JOBS Act”). The JOBS Act exempts emerging growth companies from being required to comply with new or revised financial accounting standards until private companies are required to comply with the new or revised financial accounting standards. The JOBS Act provides that a company can elect to opt out of the extended transition period and comply with the requirements that apply to non-emerging growth companies but any such election to opt out is irrevocable. The Company has elected not to opt out of such extended transition period which means that when a standard is issued or revised and it has different application dates for public or private companies, the Company, as an emerging growth company, can adopt the new or revised standard at the time private companies adopt the new or revised standard. This may make comparison of the Company’s financial statements with another public company which is neither an early-stage company nor an emerging growth company which has opted out of using the extended transition period difficult or impossible because of the potential differences in accounting standards used.
Segments
The Company currently operates in one reportable segment based on management’s view of its business for purposes of evaluating performance and making operating decisions. Based upon this business model, the Company’s Chief Executive Officer, whom the Company has determined to be its chief operating decision-maker, reviews financial information as one operating segment.
Recent Accounting Pronouncements
In December 2023, the FASB issued ASU 2023-09, “Income Taxes (Topic 740): Improvements to Income Tax Disclosures,” which requires disaggregated information about a reporting entity’s effective tax rate reconciliation as well as information on income taxes paid. The guidance is effective for the Company’s fiscal years beginning after December 15, 2024, with early adoption permitted. The Company does not expect the adoption of this standard to have any material impact on its financial statements.
In June 2016, the FASB issued ASU No. 2016-13, “Financial Instruments – Credit Losses (Topic 326): Measurement of Credit Losses on Financial Instruments,” which introduced a new model for recognizing credit losses on financial instruments based on an estimate of current expected credit losses (“CECL”). The new guidance applies to loans, accounts receivable, trade receivables, other financial assets measured at amortized cost, loan commitments and other off-balance sheet credit exposures. The new guidance also applies to debt securities and other financial assets measured at fair value through other comprehensive income. Estimated credit losses under CECL consider relevant information about past events, current conditions and reasonable and supporting forecasts that affect the collectability of financial assets. Given the non-revenue nature of the Company, the adoption of this standard did not result in an adoption adjustment or material impact.
The Company does not believe that any recently issued effective pronouncements, or pronouncements issued but not yet effective, if adopted, would have a material effect on the accompanying financial statements.
Reclassifications
Certain prior year amounts have been reclassified for consistency with the current year presentation. These reclassifications had no effect on the reported results of operations.
Correction of an Immaterial Error in the Prior Period Financial Statements
During the fourth quarter of 2024 (March 31, 2024), the Company determined that the prior year financial statements had an error caused by an immaterial classification error of certain research and development expense in accordance with ASC 730. As a result, certain prior year amounts have been revised for consistency with the current year presentation. The Company assessed the materiality of this change in presentation on prior period financial statements in accordance with SEC Staff Accounting Bulletin No. 99, “Materiality,” (ASC Topic 250, Accounting Changes and Error Corrections). Based on this assessment, the Company concluded that these classification error corrections in its Statements of Operations are not material to any previously presented financial statements based upon overall considerations of both quantitative and qualitative factors. The corrections had no impact on the fiscal year 2023 Balance Sheet, Statements of Cash Flows, or Statement of Changes in Stockholders’ Equity. Further, the immaterial corrections did not result in a change in operating losses, net loss, or basic or diluted earnings per share in the Income Statement. Accordingly, the Company corrected the previously reported immaterial errors for the year ended March 31, 2023 in this Annual Report on Form 10-K.
A summary of immaterial corrections reflecting the prior period impact to the Company’s Statement of Operations, for the year ended March 31, 2023 is shown below (in thousands):
|
As Revised |
||||||||||||
|
March 31, 2023 |
Correction |
March 31, 2023 |
||||||||||
|
General and administrative expense |
$ | 1,333 | $ | (88 | ) | $ | 1,245 | |||||
|
Research and development expense |
657 | 88 | 745 | |||||||||
|
Net Loss |
$ | 1,990 | $ | - | $ | 1,990 | ||||||
Note 2 – Warrant Liability and Fair Value of Financial Instruments
Financial assets and financial liabilities are classified in their entirety based on the lowest level of input that is significant to the fair value measurement. While the Company believes that its valuation methods are appropriate, the Company recognizes that the use of different methodologies or assumptions to determine the fair value could result in a different estimate of fair value at the reporting date. The primary assumptions that would significantly affect the fair values are the probability weighting of the different settlement outcomes used.
The Company did not have any assets or liabilities measured at fair value as of or during the 12-month period ending March 31, 2023. There were not any transfers into or out of Level 3 as of March 31, 2024 and March 31, 2023.
The following table summarizes the activity of the Level 3 fair value measurements (in thousands):
|
Warrant Liabilities |
||||
|
Balance as of March 31, 2023 |
$ | — | ||
|
Additions |
4,556 | |||
|
Change in fair value measurements - warrants mark-to-market |
3,444 | |||
|
Settlement and reclassification to equity |
(8,000 | ) | ||
|
Balance as of March 31, 2024 |
$ | — | ||
The Company recognized the initial warrant expense as a component of operating expenses on the statement of operations under warrant expense – termination agreement for $4.6 million and the changes in the fair value under warrant liability – mark-to-market for $3.4 million. There were no changes to the valuation approaches or techniques used for Level 3 measurements.
Warrant Liabilities
As more fully detailed in Note 6 – Related Party Transactions, on July 7, 2023, the Company entered into an Exclusive License Termination Agreement (the “Termination Agreement”) with a licensee in exchange for the issuance, upon the closing of the Company’s IPO within one year of the agreement’s execution, of a warrant (the "Warrant") to purchase shares of the Company for a variable number of shares.
The fair value of the warrant liability has been estimated using a discounted cash flow model under various scenarios and used the probability-weighted expected return method (“PWERM”) comparing the probabilities of different outcomes. The outcomes considered included (i) the closing of a qualified financing as part of the Company’s IPO at various points in time and (ii) the possibility of default whereby the licensee receives nothing. Key assumptions for the model were as follows for the initial measurement:
|
Discount rate at issuance (1) |
20.00% |
|
|
Probability (2) |
70% - 10% - 20% |
|
|
Payment (3) |
$0 - $8,000,000 |
|
|
Expected term (in years) |
0.48 - 0.98 |
| (1) |
The initial discount rate was chosen based on private equity rates of return as described in the AICPA Practice Aid on Valuation of Privately-Held-Company Equity securities issued as compensation. For the recurring fair value measurement, the Company updated the discount rate based upon yield curves estimated to be similar in credit quality to the Company; |
| (2) |
Scenario probability as of issuance was based on timing expectations of management that a qualified offering occurring as of December 31, 2023 was estimated at 70%, respectively; a qualified offering occurring as of June 30, 2024 was estimated at 10%; and no qualified offering occurring was estimated at 20%; |
| (3) |
The warrant has a $0.01 strike price, however, the strike price is low relative to the stock price, making the warrant value close to the value of a stock unit. The agreement has a fixed payment value of $8.0 million, see Note 6 – Related Party Transactions. |
On January 29, 2024, the Company issued 1.6 million warrant shares pursuant to the Termination Agreement.
Note 3 – Convertible Notes Payable
On September 9, 2023, the Company's Board authorized an offering up to $2.0 million in unsecured, non-interest bearing convertible promissory notes (the “Notes”) and accompanying warrants (the “Bridge Financing Warrants”) (collectively, the “Bridge Offering”) that will mature on December 31, 2025. The Notes provided that, on the closing date of the IPO, the outstanding principal would be automatically converted into common stock at the conversion price of $2.00. Each dollar in principal amount of Notes purchased were accompanied by a five-year Bridge Financing Warrant to purchase 0.25 shares of Common stock with an exercise price of $1.00 per share. The Company records the Bridge Financing Warrants as a discount to the Notes.
The Bridge Financing Warrants can be exercised from the date of Notes issuance through the five-year anniversary of the issuance of the Notes. The shares issuable pursuant to the Notes and Bridge Financing Warrants have a 180-day lock-up after the Company’s IPO. Thereafter, the foregoing lock-up agreement will cease to apply to 25% of the purchased shares each month for a period of four months. The Note holders are not permitted to convert their Notes when the holders or any of their affiliates would beneficially own in excess of 4.99% of the Company’s common stock after such conversion.
As of March 31, 2024, the Company received proceeds of $2.0 million of Notes executed from the Bridge Offering. Upon the closing of the IPO, certain notes were to be automatically converted according to their terms into the Company’s common stock to the extent and provided that certain holders of these notes are not permitted to convert such notes to the extent that the holders or any of its affiliates would beneficially own in excess of 4.99% of the Company’s common stock after such conversion. Due to this 4.99% limitation, principal representing $1.3 million, or 665,000 shares, of these notes remained outstanding. As discussed in Note 1, the remaining notes converted into common stock in accordance with their original terms on the IPO.
The table below summarizes the Company’s outstanding convertible notes payable as of March 31, 2024 (in thousands).
|
Principal Amount |
Amortized Debt Discount |
Net Carrying Amount |
||||||||||
|
Zero-coupon convertible notes payable due on December 31, 2025 |
$ | 1,330 | $ | 328 | $ | 1,002 | ||||||
Warrants
The Company issued the Notes with detachable warrants for the purchase of shares of the Company’s common stock. The Company utilized a Monte Carlo simulation model to determine the fair value of each Bridge Offering Warrant. During the year ended March 31, 2024, the Company issued warrants valued at $0.6 million. The key inputs to the Monte Carlo simulation used to determine the fair value of each warrant include, the Company’s stock price fair value which was determined through a back solve calculation such that the stock price results in the average total value of the Notes and the Bridge Offering Warrants being equal to the cash proceeds received, volatility based on a selection of publicly held peer companies of 101.88%, expected term of 5 years, risk free rate of 4.40%, discount rate of 20.00% and a discount for lack of marketability of 15.77%.
During the year ended March 31, 2024, the Company recorded less than $0.1 million in interest expense related to the amortization of the debt discount.
The following table presents a summary of activity for the warrants issued in connection with the Company’s Notes:
|
Warrants |
Weighted-Average Exercise Price Per Share |
Remaining Life (In Years) |
Aggregate Intrinsic Value* |
|||||||||||||
|
Outstanding and exercisable, March 31, 2023 |
— | $ | — | — | $ | — | ||||||||||
|
Granted |
500,000 | 1.00 | — | — | ||||||||||||
|
Exercised |
— | — | — | — | ||||||||||||
|
Forfeited/Cancelled |
— | — | — | — | ||||||||||||
|
Expired |
— | — | — | — | ||||||||||||
|
Outstanding, March 31, 2024 |
500,000 | $ | 1.00 | 4.48 | $ | 1,010,000 | ||||||||||
|
Exercisable, March 31, 2024 |
500,000 | $ | 1.00 | 4.48 | $ | 1,010,000 | ||||||||||
*Aggregate Intrinsic Value = Excess of market value over the exercise price of all in-the-money warrants.
Note 4 – Equity
On November 29, 2023, the Company’s Board of Directors and applicable shareholders approved to amend and restate the Company’s certificate of incorporation and increased the authorized shares to 500,000,000 shares of common stock, with a par value of $.001 per share, and 10,000,000 shares of preferred stock, with a par value of $.001 per share. The specific rights of the preferred stock shall be determined by the Board of Directors.
Preferred Stock
As of March 31, 2024, the Company had no shares of preferred stock outstanding.
Restricted Stock
On February 15, 2024, the Company issued 35,000 restricted shares of common stock to the Company's marketing consultant at the closing price of $3.80 of the Company's common stock. The total value of these shares is $133,000. These shares vest monthly over a 12-month period beginning on the issue date.
|
Year ended March 31, |
||||||||
|
2024 |
2023 |
|||||||
|
Recognized in general and administrative expense |
$ | 16,625 | $ | — | ||||
|
Total |
$ | 16,625 | $ | — | ||||
For the year ended March 31, 2024, there was $116,375 of unrecognized stock-based compensation expense related to unvested Restricted Stock, which is expected to be recognized over the period April 2024 through February 2025.
A summary of activity regarding Restricted Stock issued is as follows:
|
Grant Date |
||||||||
|
Number of Shares |
Fair Value Per Share |
|||||||
|
Outstanding, March 31, 2023 |
— | $ | — | |||||
|
Granted |
35,000 | $ | 3.80 | |||||
|
Vested |
(2,917 | ) | $ | 3.80 | ||||
|
Unvested, March 31, 2024 |
32,083 | $ | 3.80 | |||||
Common Stock
On April 6, 2023, the Board of Directors approved a private placement offering of up to 2,000,000 common shares at a price of $2.00 per share. During the year ended March 31, 2024, the Company sold 1,420,000 shares for cash proceeds of $2,840,000. The Company did not incur any costs that were direct and incremental to the private placement.
On September 9, 2023, the Board approved a Bridge Offering. See Note 3 Convertible Notes Payable for additional detail as these notes are convertible into common stock.
Stock Plan and Stock Options
In June 2023, the Company adopted, and the Company’s shareholders approved, the Autonomix Medical, Inc. 2023 Stock Plan (the “Plan”). The Plan is a stock-based compensation plan that provides for discretionary grants of stock options, stock awards and stock unit awards to key employees, non-employee directors, and consultants, subject to certain individual threshold limitations. The Plan provides for up to 4,000,000 shares to be issued. Shares that are surrendered because of forfeiture, expiration, termination, or cancellation are available for re-issuance. As of March 31, 2024, the Plan has 1,996,400 shares remaining available to be issued.
In August 2023, the Plan was amended to allow for an automatic increase of the available shares for issuance, whereby on the 1st of each fiscal year, beginning on April 1, 2024 and ending on (and including) April 1, 2033 in an amount equal to five percent (5%) of the total number of shares of Common Stock outstanding on the March 31st immediately preceding the applicable date. However, the Board may act prior to the automatic increase of a given year to provide that there will be no increase for such year, or that the increase for such year will be a lesser number of shares of Common Stock. The Board did not take any such action and on April 1, 2024, the increase took place.
The following table summarizes the stock option activity for the year ended March 31, 2024. There were no options outstanding during the year ended March 31, 2023.
|
Options |
Weighted-Average Exercise Price Per Share |
Weighted-Average Remaining Life (In Years) |
Aggregate Intrinsic Value* |
|||||||||||||
|
Outstanding and exercisable, March 31, 2023 |
— | $ | — | — | $ | — | ||||||||||
|
Granted |
2,003,600 | 2.33 | — | — | ||||||||||||
|
Exercised |
— | — | — | — | ||||||||||||
|
Forfeited/Cancelled |
— | — | — | — | ||||||||||||
|
Expired |
— | — | — | — | ||||||||||||
|
Outstanding, March 31, 2024 |
2,003,600 | $ | 2.33 | 9.35 | $ | 1,680,672 | ||||||||||
|
Exercisable, March 31, 2024* |
239,217 | $ | 2.00 | 8.96 | $ | 244,001 | ||||||||||
*Aggregate Intrinsic Value = Excess of market value over the exercise price of all in-the-money stock.
During the year ended March 31, 2024, the Company granted certain individuals options to purchase 2,003,600 shares of common stock with contractual terms ranging from three years to ten years, and vesting periods that included monthly over one year, quarterly over one year, monthly over four years, and annually over four years. The options had an aggregate grant date fair value of $3.7 million that was calculated using the Black-Scholes option pricing model. Variables used in the Black-Scholes option pricing model included the following: (1) fair value of common stock on the measurement date of $2.00 per share for options granted as of September 30, 2023, $5.00 per share for options granted subsequent to September 30, 2023 but prior to our IPO and $2.70 for options granted subsequent to our IPO; (2) discount rate ranging from 4.02% to 4.98% based on the daily yield curve rates for U.S. Treasury obligations, (3) expected life ranging from 1.77 years to 6.25 years based on the simplified method (vesting plus contractual term divided by two), and (4) expected volatility ranging from 95% to 119% based on the historical volatility of comparable companies' stock.
All options issued and outstanding are being amortized over their respective vesting periods. The unrecognized compensation expense at March 31, 2024 was $3.1 million. During the year ended March 31, 2024, the Company recorded stock-based compensation - option expense of $0.6 million, of which $0.5 million was recorded in general and administrative expenses and $0.1 million was recorded in research and development expenses in the statements of operations. There was no recorded stock-based compensation - option expense for the year ended March 31, 2023.
Equity-Based Stock Warrants
On March 26, 2024, the Company issued five-year warrants to the selling agent in the Company's IPO to purchase 59,765 shares of common stock at an exercise price of $6.25. Under the fair value method, the fair value of these warrants was estimated on the grant date using the Black-Scholes option pricing model. Variables used in the Black-Scholes warrant pricing model included the following: (1) fair value of common stock on the measurement date of $5.00 per share; (2) discount rate of 4.04% based on the daily yield curve rates for U.S. Treasury obligations; (3) expected life of 5 years and (4) expected volatility of 104% based on the historical volatility of comparable companies' stock. The costs associated with these shares were reclassified to additional paid-in capital upon completion of the Company’s IPO on January 26, 2024.
The Company will periodically grant warrants to investors in connection with equity financing or to third-party service providers in exchange for services rendered. The following table summarizes the stock warrant activity for the year ended March 31, 2024:
|
Warrants |
Weighted-Average Exercise Price Per Share |
Weighted-Average Remaining Life (In Years) |
Aggregate Intrinsic Value* |
|||||||||||||
|
Outstanding and exercisable, March 31, 2023 |
6,569,929 | $ | 0.02 | 5.99 | $ | 12,982,587 | ||||||||||
|
Granted |
1,679,765 | 0.25 | — | — | ||||||||||||
|
Exercised** |
(2,485,301 | ) | 0.03 | — | — | |||||||||||
|
Forfeited/Cancelled |
(19,824 | ) | 1.50 | — | — | |||||||||||
|
Expired |
— | — | — | — | ||||||||||||
|
Outstanding, March 31, 2024 |
5,744,569 | $ | 0.08 | 4.80 | $ | 17,072,147 | ||||||||||
|
Exercisable, March 31, 2024 |
5,736,236 | $ | 0.08 | 4.80 | $ | 17,063,647 | ||||||||||
*Aggregate Intrinsic Value = Excess of market value over the exercise price of all in-the-money stock.
**All exercised shares utilized the “cashless exercise” option.
The unrecognized compensation expense at March 31, 2024 was less than $0.1 million. During the year ended March 31, 2024, the Company recorded stock-based compensation - warrant expense of less than $0.1 million, respectively. There was no recorded stock-based compensation - warrant expense for the year ended March 31, 2023.
Under the fair value method, the fair value of each warrant was estimated on the grant date using the Black-Scholes option pricing model. Variables used in the Black-Scholes warrant pricing model included the following:
|
Range |
||||||||
|
2023 |
2024 |
|||||||
|
Fair value of common stock on the measurement date (per share) |
— |
$2.00 - to $5.00 |
||||||
|
Discount rate based on the daily yield curve rates for U.S. Treasury obligations |
— | 4.04% to 4.54% | ||||||
|
Expected life |
— |
3 to 5 years |
||||||
|
Expected volatility based on the historical volatility of comparable companies' stock |
— | 104% to 119% | ||||||
Note 5 – Commitments and Contingencies
Legal Proceedings
From time to time, we may be involved in claims that arise during the ordinary course of business. Although the results of litigation and claims cannot be predicted with certainty, we do not currently have any pending litigation to which we are a party or to which our property is subject that we believe to be material. Regardless of the outcome, litigation can be costly and time consuming, and it can divert management’s attention from important business matters and initiatives, negatively impacting our overall operations.
Employment Agreements
The Company has agreements with key employees to provide certain benefits, including salary and other wage-related benefits, in the event of termination. In addition, the Company has adopted a severance policy for certain key members of executive management to provide certain benefits, including salary and other wage-related benefits, in the event of termination without cause. In total, these benefits would amount to $0.8 million using the rate of compensation in effect at March 31, 2024.
Note 6 – Related Party Transactions
The Company utilizes a consulting firm that is owned by the Company’s former Chief Financial Officer to provide accounting and financial reporting services and pays certain expenses on behalf of the Company. During the year ended March 31, 2024 and 2023, the Company incurred fees of less than $0.1 million, respectively, for these services, excluding officer compensation. As of March 31, 2024 and March 31, 2023, the Company owed the consulting firm less than $0.1 million, respectively, for services and expenses.
As of March 31, 2024, members of the Company’s management/Board and an immediate family member of the Company’s management (related party), collectively purchased $0.5 million ($0.4 million and $0.1 million, respectively) of the Bridge Offering.
On December 21, 2021, the Company entered into a perpetual, worldwide, exclusive license agreement (the “License” or “License Agreement”) with a company controlled by a significant stockholder of the Company (the “Licensee”). The License allows the Licensee to use certain intellectual property and technology related to the diagnosis and treatment of cardiovascular conditions held by the Company. Upon 90 days following the completion of an IPO or special purpose acquisition company transaction, the Licensee may enter into sublicenses of the licensed intellectual property and technology.
On July 7, 2023, the Company and the Licensee entered into an Exclusive License Termination Agreement (the “Termination Agreement”) in exchange for the issuance, upon the closing of the Company’s IPO within one year of the agreement’s execution, of a warrant to purchase shares of the Company for a variable number of shares. Upon the Company's closing of its IPO on January 29, 2024, 1.6 million warrant shares were issued at $5.00 per share for a fixed value of $8.0 million. The warrants are exercisable at a price of $0.001 per share and may be exercised any time after the issuance date, subject to a beneficial ownership limitation, and expire five years from the original issuance. The warrants contain dividend rights commensurate with the holders of common stock. The warrants do not include any other stockholder rights or privileges prior to exercise.
Note 7 – Income Taxes
The Company files U.S. federal and various U.S. state income tax returns. Due to the Company’s losses, there was no income tax expense for the years ended March 31, 2024 and 2023 (in thousands):
| March 31, | March 31, | |||||||||||||||
| 2024 | 2023 | |||||||||||||||
|
Amount |
% |
Amount |
% |
|||||||||||||
|
Tax benefit at the U.S. federal statutory rate |
$ | (3,239 | ) | 21.00 | % | $ | (418 | ) | 21.00 | % | ||||||
|
Tax rate change |
— | — | $ | — | — | % | ||||||||||
|
Permanent differences |
1,697 | (11.01 | )% | $ | — | — | % | |||||||||
|
Return to provision |
(69 | ) | 0.45 | % | $ | — | — | % | ||||||||
|
Change in state rate |
(190 | ) | 1.23 | % | $ | — | — | % | ||||||||
|
State tax (net of federal benefit) |
(192 | ) | 1.24 | % | $ | — | — | % | ||||||||
|
Valuation allowance |
1,993 | (12.91 | )% | $ | 418 | (21.00 | )% | |||||||||
|
Effective income tax rate |
$ | — | — | % | $ | — | — | % | ||||||||
The effective income tax rate varied from the statutory rate in 2024 primarily due to permanent differences and the increase in the valuation allowance. The effective income tax rate varied from the statutory rate in 2023 as a result of the increase in the valuation allowance.
Deferred tax assets and liabilities consist of the following (in thousands):
|
March 31, |
March 31, |
|||||||
|
2024 |
2023 |
|||||||
|
Assets related to: |
||||||||
|
Capitalized R&D costs |
$ | 602 | $ | 124 | ||||
|
Net operating losses |
2,643 | 1,342 | ||||||
|
Accrual to cash |
72 | — | ||||||
|
Stock-based compensation |
142 | — | ||||||
|
Total deferred tax assets |
3,459 | 1,466 | ||||||
|
Valuation allowance for deferred tax assets |
(3,459 | ) | (1,466 | ) | ||||
|
Net deferred tax |
— | — | ||||||
|
Net deferred tax assets |
$ | — | $ | — | ||||
At March 31, 2024, the Company had U.S. federal net operating loss ("NOL") carry forwards of $11.2 million. Approximately $3.4 million of the U.S. federal NOLs will start expiring in 2034. Additionally, the Company generated a U.S. federal NOL carry forward of approximately $7.7 million post-2017 to 2024. Under the new Tax Act, post-2017 federal NOL carry forwards do not expire, but can only offset 80% of taxable income in the year the loss carry forward is used. The Company also had state NOL carry forwards of approximately $11.1 million which begin to expire in 2034.
Sections 382 and 383 of the Internal Revenue Code limit the annual use of NOL carry forwards and tax credit carry forwards, respectively, following an ownership change. NOL carry forwards may be subject to annual limitations under Internal Revenue Code Section 382 (Section 382) (or comparable provisions of state law) if certain changes in ownership were to occur. The Company is pre-revenue and has been generating net operating losses. Therefore, no NOLs are being utilized nor subject to any utilization limitations. Determination of ownership change or limitation hasn’t been calculated; however, the Company will perform the NOL limitation analysis under Section 382 before any NOLs are expected to be utilized.
The Company has recorded a full valuation allowance against its net total deferred tax assets as of March 31, 2024 and 2023 because management determined that it is not more-likely-than not that those assets will be realized. In assessing the realization of deferred tax assets, management considers whether it is more likely than not that some portion or all of deferred assets will not be realized. The ultimate realization of the deferred tax assets is dependent upon the generation of future taxable income during the periods in which those temporary differences become deductible. During the year ended March 31, 2024, the valuation allowance increased by $1.9 million mainly due to additional net operating losses and capitalized R&D Costs.
The Company is subject to income taxes in the U.S. federal jurisdiction, and various state jurisdictions. Tax regulations within each jurisdiction are subject to the interpretation of the related tax laws and regulations and require significant judgment to apply. As of March 31, 2024, all of the tax years remained open to examination by the federal and state taxing authorities, for three or four years from the tax year in which net operating losses or tax credits are utilized completely.
As of March 31, 2024, the Company has no reserve for uncertain tax positions.
Note 8 – Subsequent Events
On April 1, 2024, pursuant to the provisions of the Company's 2023 Stock Plan, the shares of Common Stock underlying the Plan increased by five percent (5%) of the total number of shares of Common Stock outstanding on the March 31st immediately preceding the applicable date. This resulted in increasing the available shares of Common Stock under the Plan by 942,305.
On April 5, 2024, the Company granted 75,000 stock options to a new employee. These stock options vest in four equal annual installments.
Autonomix Medical, Inc.
Condensed Balance Sheets
(Unaudited)
|
(in thousands, except share and per share data) |
As of |
|||||||
|
June 30, |
March 31, |
|||||||
|
2024 |
2024 |
|||||||
|
Assets |
||||||||
|
Current assets: |
||||||||
|
Cash and cash equivalents |
$ | 6,751 | $ | 8,608 | ||||
|
Other current assets |
325 | 783 | ||||||
|
Total current assets |
7,076 | 9,391 | ||||||
|
Long term assets: |
||||||||
|
Fixed assets, net |
19 | 16 | ||||||
|
Total long term assets |
19 | 16 | ||||||
|
Total Assets |
$ | 7,095 | $ | 9,407 | ||||
|
Liabilities and Stockholders' Equity |
||||||||
|
Current liabilities: |
||||||||
|
Accounts payable |
$ | 287 | $ | 492 | ||||
|
Accrued expenses |
476 | 285 | ||||||
|
Total current liabilities |
763 | 777 | ||||||
|
Long term liabilities: |
||||||||
|
Long term debt - convertible notes, net of unamortized debt discount |
1,043 | 1,002 | ||||||
|
Total long term liabilities |
1,043 | 1,002 | ||||||
|
Total Liabilities |
$ | 1,806 | $ | 1,779 | ||||
|
Commitments and contingencies (Note 5) |
||||||||
|
Stockholders' equity: |
||||||||
|
Preferred stock, $0.001 par value, 10,000,000 shares authorized as of June 30, 2024 and March 31, 2024, respectively, no shares issued and outstanding as of June 30, 2024 and March 31, 2024, respectively |
$ | - | $ | - | ||||
|
Common stock, $0.001 par value, 500,000,000 shares authorized as of June 30, 2024 and March 31, 2024, respectively, 19,242,081 and 18,846,094 shares issued and outstanding as of June 30, 2024 and March 31, 2024, respectively |
19 | 19 | ||||||
|
Additional paid-in capital |
46,938 | 46,578 | ||||||
|
Accumulated deficit |
(41,668 | ) | (38,969 | ) | ||||
|
Total Stockholders' Equity |
5,289 | 7,628 | ||||||
|
Total Liabilities and Stockholders' Equity |
$ | 7,095 | $ | 9,407 | ||||
See accompanying notes to the unaudited condensed financial statements.
Autonomix Medical, Inc.
Condensed Statements of Operations
(Unaudited)
|
Three Months Ended |
||||||||
|
June 30, |
||||||||
|
(in thousands, except share and per share data) |
2024 |
2023 |
||||||
|
Operating expenses: |
||||||||
|
General and administrative |
$ | 1,799 | $ | 503 | ||||
|
Research and development |
954 | 368 | ||||||
|
Total operating expenses |
2,753 | 871 | ||||||
|
Loss from operations |
(2,753 | ) | (871 | ) | ||||
|
Other (expense) income: |
||||||||
|
Interest expense |
(41 | ) | - | |||||
|
Interest income |
95 | 6 | ||||||
|
Total other income |
54 | 6 | ||||||
|
Loss before income taxes |
(2,699 | ) | (865 | ) | ||||
|
Income taxes |
- | - | ||||||
|
Net loss |
$ | (2,699 | ) | $ | (865 | ) | ||
|
Loss per share - basic and diluted |
$ | (0.14 | ) | $ | (0.07 | ) | ||
|
Weighted average shares outstanding - basic and diluted |
18,902,248 | 12,884,604 | ||||||
See accompanying notes to the unaudited condensed financial statements.
Autonomix Medical, Inc.
Condensed Statements of Changes in Stockholders' Equity
(Unaudited)
|
Additional |
Total |
|||||||||||||||||||||||||||
|
Preferred Stock |
Common Stock |
Paid-in |
Accumulated |
Stockholders' |
||||||||||||||||||||||||
|
(in thousands) |
Shares |
Amount |
Shares |
Amount |
Capital |
Deficit |
Equity |
|||||||||||||||||||||
|
Balance March 31, 2023 |
- | - | 12,337 | 12 | 24,175 | (23,543 | ) | 644 | ||||||||||||||||||||
|
Net loss |
- | - | - | - | - | (865 | ) | (865 | ) | |||||||||||||||||||
|
Issuance of common stock |
- | - | 1,420 | 2 | 2,838 | - | 2,840 | |||||||||||||||||||||
|
Balance June 30, 2023 |
- | - | 13,757 | 14 | 27,013 | (24,408 | ) | 2,619 | ||||||||||||||||||||
|
Balance March 31, 2024 |
- | - | 18,846 | 19 | 46,578 | (38,969 | ) | 7,628 | ||||||||||||||||||||
| - | - | |||||||||||||||||||||||||||
|
Net loss |
- | - | - | - | - | (2,699 | ) | (2,699 | ) | |||||||||||||||||||
|
Stock-based compensation |
- | - | - | - | 360 | - | 360 | |||||||||||||||||||||
|
Refund of common stock from IPO |
- | - | (1 | ) | - | - | - | - | ||||||||||||||||||||
|
Issuance of common stock - warrants exercised |
- | - | 397 | - | - | - | - | |||||||||||||||||||||
|
Balance June 30, 2024 |
- | - | 19,242 | 19 | 46,938 | (41,668 | ) | 5,289 | ||||||||||||||||||||
See accompanying notes to the unaudited condensed financial statements.
Autonomix Medical, Inc.
Condensed Statements of Cash Flows
(Unaudited)
|
Three Months Ended June 30, |
||||||||
|
(in thousands) |
2024 |
2023 |
||||||
|
Cash Flows from Operating Activities: |
||||||||
|
Net loss |
$ | (2,699 | ) | $ | (865 | ) | ||
|
Adjustments to reconcile net loss to net cash used in operating activities: |
||||||||
|
Stock-based compensation |
360 | - | ||||||
|
Depreciation and amortization expense |
43 | - | ||||||
|
Changes in operating assets - decrease/(increase): |
||||||||
|
Other current assets |
458 | (25 | ) | |||||
|
Changes in operating liabilities - (decrease)/increase: |
||||||||
|
Accounts payable |
(205 | ) | 209 | |||||
|
Accrued expenses |
191 | 20 | ||||||
|
Net cash used in operating activities |
(1,852 | ) | (661 | ) | ||||
|
Cash Flows from Investing Activities: |
||||||||
|
Purchase of property and equipment |
(5 | ) | - | |||||
|
Net cash used in investing activities |
(5 | ) | - | |||||
|
Cash Flows from Financing Activities: |
||||||||
|
Issuance of common stock |
- | 2,840 | ||||||
|
Payment of offering costs |
- | (105 | ) | |||||
|
Net cash provided by financing activities |
- | 2,735 | ||||||
|
Net change in cash and cash equivalents |
(1,857 | ) | 2,074 | |||||
|
Cash and cash equivalents, at beginning of period |
8,608 | 865 | ||||||
|
Cash and cash equivalents, at end of period |
$ | 6,751 | $ | 2,939 | ||||
|
Supplemental cash flow disclosures: |
||||||||
|
Non-cash financing activities: |
||||||||
|
Cashless exercise of warrants |
$ | 4 | $ | - | ||||
See accompanying notes to the unaudited condensed financial statements.
Autonomix Medical, Inc.
Notes to the Unaudited Condensed Financial Statements
Note 1 – Description of the Business, Basis of Presentation and Summary of Significant Accounting Policies
Description of the Business
Autonomix Medical, Inc. (“we,” “our,” the “Company”) is a medical device company organized as a Delaware corporation on June 10, 2014. The Company is a pre-revenue, clinical stage life sciences company focused on advancing innovative technologies for sensing and treating disorders relating to the peripheral nervous system.
Liquidity and Going Concern
The Company's financial statements are prepared on a going concern basis, which contemplates the realization of assets and the satisfaction of liabilities and commitments in the normal course of business. The Company is an early-stage company that is subject to all the risks associated with early-stage and emerging growth companies and has incurred losses since inception.
For the three months ended June 30, 2024 and 2023, the Company incurred net losses of $2.7 million and $0.9 million, respectively, and had net cash flows used in operating activities of $1.9 million and $0.7 million, respectively. The Company had no revenues for the three months ended June 30, 2024 and 2023, respectively, accumulated deficit of $41.7 million, working capital of $6.3 million and cash of $6.8 million. The Company does not expect to generate positive cash flows from operating activities in the near future.
The Company estimates its current cash resources is sufficient to fund its operations into but not beyond the second calendar quarter of 2025. The Company recognizes it will need to raise additional capital to continue to execute its business plan, including obtaining regulatory clearance for its products currently under development and commercializing and generating revenues from products under development. There is no assurance that additional financing will be available when needed or that management will be able to obtain financing on terms acceptable to the Company. A failure to raise sufficient capital, generate sufficient product revenues, control expenditures and regulatory matters, among other factors, will adversely impact the Company’s ability to meet its financial obligations as they become due and payable and to achieve its intended business objectives. If the Company is unable to raise sufficient additional funds, it will have to scale back its operations.
These factors raise substantial doubt about the Company's ability to continue as a going concern within one year after the date the financial statements are issued. The accompanying condensed financial statements have been prepared on a going concern basis and do not include any adjustments that might be necessary if the Company is unable to continue as a going concern.
Basis of Presentation
The accompanying condensed interim financial statements are unaudited. These unaudited condensed interim financial statements have been prepared in accordance with the rules and regulations of the U.S. Securities and Exchange Commission (“SEC”) for interim financial information. Accordingly, they do not include all the information and notes required by generally accepted accounting principles in the United States of America (“GAAP”) for complete financial statements. The Company’s fiscal year end is March 31st. These unaudited condensed interim financial statements should be read in conjunction with the audited financial statements and accompanying notes for the year ended March 31, 2024 as found in the Annual Report in our Form 10-K filed with the SEC on May 31, 2024. In the opinion of management, the unaudited condensed interim financial statements reflect all the adjustments (consisting of normal recurring adjustments) necessary to state fairly the Company’s financial position, results of operations and cash flows for the quarterly and year-to-date periods, as applicable. The interim results of operations are not necessarily indicative of the results that may occur for the full fiscal year. The March 31, 2024 audited condensed balance sheet included herein was derived from the audited financial statements, but does not include all disclosures, including notes, required by GAAP for complete financial statements.
Use of Estimates in Financial Statement Presentation
The preparation of these unaudited condensed interim financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities at the date of the financial statements and the reported amounts of expenses during the reporting period. The Company's significant estimates and assumptions include work performed but not yet billed by contract manufacturers, engineers and research organizations and the valuation of equity related instruments. Although the Company believes that its estimates and assumptions are reasonable, they are based upon information available at the time the estimates and assumptions were made. Some of these judgments can be subjective and complex, and, consequently, actual results could differ from those estimates.
Cash and Cash Equivalents
The Company considers all highly liquid accounts with original maturities of three months or less at the date of acquisition to be cash equivalents. Periodically, the Company may carry cash balances at financial institutions in excess of the federally insured limit of $250,000. The Company has not experienced losses on these accounts and management believes, based upon the quality of the financial institutions, that the credit risk with regard to these deposits is not significant.
Offering Costs
Offering costs consist of professional costs incurred through the balance sheet date that are direct and incremental related to the Company’s initial public offering ("IPO"). These costs, together with the selling agent fees, were reclassified to additional paid-in capital upon completion of the Company’s IPO on January 26, 2024. Costs associated with salaries and other period costs were expensed as incurred.
During the three months ended June 30, 2024 and 2023, the Company paid zero and $0.1 million, respectively, of offering costs related to its IPO.
Property and Equipment
Property and equipment (comprised of computer and IT equipment) are stated at historical cost and depreciated on a straight-line basis over their estimated useful lives, generally three years. Upon disposition of the assets, the costs and related accumulated depreciation are removed from the accounts and any resulting gain or loss is included in the results of operations.
Convertible Notes
The Company evaluates embedded redemption, conversion and other features within its debt to determine whether any embedded features should be bifurcated from the host instrument and accounted for as a derivative at fair value, with changes in fair value recorded in the condensed statements of operations.
The Company’s debt is carried on the condensed balance sheets on a historical cost basis net of unamortized discounts and premiums because the Company has not elected the fair value option of accounting. Costs associated with acquiring debt, including detachable warrants issued in connection with the financing, are capitalized as a debt discount. The debt discount is presented in the condensed balance sheets as a direct deduction from the carrying amount of the debt liability. The costs are amortized over the estimated contractual life of the related debt instrument using the effective interest method and are included in interest expense in the condensed statements of operations.
If the Company incurs costs associated with its convertible notes, in advance of the receipt of proceeds, the Company will record a deferred asset. Upon receipt of proceeds the Company will reclassify the deferred asset as a direct deduction from the carrying amount, as described above.
In addition, since the instruments included a substantive conversion feature as of time of issuance, the issuance of equity securities to settle the outstanding notes with the conversion were accounted for as a contractual conversion with no gain or loss recognized related to the equity securities issued to settle the instrument.
Fair Value of Financial Instruments
Fair value is defined as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. Valuation techniques used to measure fair value maximize the use of observable inputs and minimize the use of unobservable inputs. The Company utilizes a three-level valuation hierarchy for disclosures of fair value measurements, defined as follows:
Level 1 – inputs to the valuation methodology are quoted prices (unadjusted) for identical assets or liabilities in active market
Level 2 – inputs to the valuation methodology include quoted prices for similar assets and liabilities in active markets, and inputs that are observable for the assets or liability, either directly or indirectly, for substantially the full term of the financial instrument
Level 3 – inputs to the valuation methodology are unobservable and significant to the fair value and require significant judgment and estimation.
Financial assets and financial liabilities are classified in their entirety based on the lowest level of input that is significant to the fair value measurement. While the Company believes that its valuation methods are appropriate, the Company recognizes that the use of different methodologies or assumptions to determine the fair value could result in a different estimate of fair value at the reporting date. The primary assumptions that would significantly affect the fair values are the probability weighting of the different settlement outcomes used.
The Company did not have any assets or liabilities measured at fair value as of or during the three-month period ending June 30, 2024 and March 31, 2024. There were not any transfers into or out of Level 3 as of June 30, 2024 and March 31, 2024.
As of June 30, 2024, the Company determined that the estimated fair value of debt was approximately $1.0 million. The fair value of debt was estimated using market rates the Company believes would be available for similar types of financial instruments and represents a Level 2 measurement.
The carrying value of short-term instruments, including cash, accounts payable and accrued expenses, approximate fair value due to the relatively short period to maturity for these instruments.
Related Parties
The Company follows Accounting Standards Codification ("ASC") 850, Related Party Disclosures, for the identification of related parties and disclosure of related party transactions. See further discussion in Note 5 below on this matter.
Income Taxes
The Company uses the asset and liability method of accounting for income taxes. Under this method, deferred tax assets and liabilities are determined based on the differences between the financial reporting and the tax basis of reported assets and liabilities and are measured using the enacted tax rates and laws that will be in effect when the differences are expected to reverse. The Company must then assess the likelihood that the resulting deferred tax assets will be realized. A valuation allowance is provided when it is more likely than not that some portion or all of a deferred tax asset will not be realized. As of June 30, 2024 and March 31, 2024 the Company determined a full valuation allowance was required to offset its deferred tax assets as a result of recurring operating losses.
The Company accounts for uncertain tax positions in accordance with the provisions of ASC 740-10 which prescribes a recognition threshold and measurement attribute for financial statement disclosure of tax positions taken, or expected to be taken, on its tax return. The Company evaluates and records any uncertain tax positions based on the amount that management deems is more likely than not to be sustained upon examination and ultimate settlement with the tax authorities in the tax jurisdictions in which it operates. As of June 30, 2024 and March 31, 2024 the Company had no uncertain tax positions.
The Company does not expect to pay any significant federal, state, or foreign income taxes in our fiscal year 2025 (ending March 31, 2025) as a result of the losses recorded during the three months ended June 30, 2024 and the additional losses expected for the remainder of our fiscal year 2025 and cumulative net operating loss carryforwards. Accounting standards require the consideration of a valuation allowance for deferred tax assets if it is “more likely than not” that some component or all of the benefits of deferred tax assets will not be realized.
The Company recorded no income tax provision for the three months ended June 30, 2024 and 2023, respectively. The effective tax rate for the three months ended June 30, 2024 and 2023 is zero. The Company estimates its annual effective tax rate at the end of each quarterly period. Jurisdictions with a projected loss for the year where no tax benefit can be recognized due to the valuation allowance could result in a higher or lower effective tax rate during a particular quarter depending on the mix and timing of actual earnings versus annual projections.
Stock-based Compensation
Employee and non-employee share-based compensation is measured at the grant date, based on the fair value of the award, and is recognized as an expense over the requisite service period. For awards with a performance condition, compensation expense is recognized over the requisite service period if it is probable that the performance condition will be satisfied. For awards to non-employees, the Company recognizes compensation expense in the same manner as if the Company had paid cash for the goods or services. The Company estimates the fair value of options and equity classified warrants granted using an options pricing model. Expense is recognized within general and administrative expenses and forfeitures are recognized as they are incurred.
Warrants
The Company accounts for warrants as either equity-classified or liability-classified instruments based on an assessment of the warrant’s specific terms and applicable authoritative guidance in FASB ASC 480, Distinguishing Liabilities from Equity (“ASC 480”) and ASC 815, Derivatives and Hedging (“ASC 815”). The assessment considers whether the warrants are freestanding financial instruments pursuant to ASC 480, meet the definition of a liability pursuant to ASC 480, and whether the warrants meet all of the requirements for equity classification under ASC 815, including whether the warrants are indexed to the Company’s own ordinary shares and whether the warrant holders could potentially require “net cash settlement” in a circumstance outside of the Company’s control, among other conditions for equity classification. This assessment, which requires the use of professional judgment, is conducted at the time of warrant issuance and as of each subsequent quarterly period end date while the warrants are outstanding.
For issued or modified warrants that meet all of the criteria for equity classification, the warrants are required to be recorded as a component of additional paid-in capital at the time of issuance. For issued or modified warrants that do not meet all the criteria for equity classification, the warrants are required to be recorded at their initial fair value on the date of issuance, and each balance sheet date thereafter. Changes in the estimated fair value of the warrants are recognized as a non-cash gain or loss on the statements of operations. The fair value of the warrants is estimated using a Black-Scholes pricing model or a Monte Carlo simulation.
Loss Per Common Share
Basic loss per common share is computed by dividing net loss by the weighted-average number of common shares outstanding during the period. Diluted loss per common share is determined using the weighted-average number of common shares outstanding during the period, adjusted for the dilutive effect of common stock equivalents. In periods when losses are reported, the weighted-average number of common shares outstanding excludes common stock equivalents, because their inclusion would be anti-dilutive. Generally, the Company’s outstanding warrants are non-participating securities as they are not entitled to non-forfeitable rights to dividends or dividend equivalents during the vesting term and have no obligation to fund losses.
However, the warrants described in Note 5 are participating securities as they receive a right to dividends, but they are not obligated to fund losses. In periods of loss, since no income is allocated to these securities, the Company's use of the treasury stock method derives the same result. The dilutive effect of convertible securities is calculated using the “if-converted method.” Under the if-converted method, securities are assumed to be converted at the beginning of the period, and the resulting common shares are included in the denominator of the diluted calculation for the entire period being presented.
For the three months ended June 30, 2024 and 2023, dilutive securities that were not included in the calculations of the loss per common share because they would be anti-dilutive included the following:
|
June 30, |
||||||||
|
2024 |
2023 |
|||||||
|
Equity based warrants to purchase common shares |
5,344,569 | 6,569,929 | ||||||
|
Convertible Notes - common shares (1) |
665,000 | - | ||||||
|
Convertible Notes - equity-based warrants to purchase common shares |
500,000 | - | ||||||
|
Stock options granted under Company's incentive plan |
4,329,579 | 933,600 | ||||||
|
Total potentially dilutive securities |
10,839,148 | 7,503,529 | ||||||
Research and Development Costs
Research and development costs are expensed as incurred.
Advertising
It is our policy to expense advertising costs as incurred. Advertising expenses are included within general and administrative expenses within the statement of operations. For the three months ended June 30, 2024 and 2023, the Company recorded less than $0.1 million and $0.1 million, respectively.
Fair Value of Common Stock
Prior to establishing a public market for the Company’s common stock, the estimated fair value of the Company’s common stock was determined by the Company’s board of directors as of the date of each option grant, with input from management, considering the Company’s most recently available third-party valuations of common stock, recent sales of common stock to third parties, and the Company’s board of directors’ assessment of additional objective and subjective factors that it believed were relevant and which may have changed from the date of the most recent valuation through the date of the grant.
JOBS Act Accounting Election
The Company qualifies as an emerging growth company (“EGC”), as defined in the Jumpstart Our Business Startups Act of 2012 (the “JOBS Act”). The JOBS Act exempts emerging growth companies from being required to comply with new or revised financial accounting standards until private companies are required to comply with the new or revised financial accounting standards. The JOBS Act provides that a company can elect to opt out of the extended transition period and comply with the requirements that apply to non-emerging growth companies but any such election to opt out is irrevocable. The Company has elected not to opt out of such extended transition period which means that when a standard is issued or revised and it has different application dates for public or private companies, the Company, as an emerging growth company, can adopt the new or revised standard at the time private companies adopt the new or revised standard. This may make comparison of the Company’s financial statements with another public company which is neither an early-stage company nor an emerging growth company which has opted out of using the extended transition period difficult or impossible because of the potential differences in accounting standards used.
Segments
The Company currently operates in one reportable segment based on management’s view of its business for purposes of evaluating performance and making operating decisions. Based upon this business model, the Company’s Chief Executive Officer, whom the Company has determined to be its chief operating decision-maker, reviews financial information as one operating segment.
Recent Accounting Pronouncements
In December 2023, the FASB issued ASU 2023-09, “Income Taxes (Topic 740): Improvements to Income Tax Disclosures,” which requires disaggregated information about a reporting entity’s effective tax rate reconciliation as well as information on income taxes paid. The guidance is effective for the Company’s fiscal years beginning after December 15, 2024, with early adoption permitted. The Company does not expect the adoption of this standard to have any material impact on its financial statements.
The Company does not believe that any recently issued effective pronouncements, or pronouncements issued but not yet effective, if adopted, would have a material effect on the accompanying financial statements.
Correction of an Immaterial Error in the Prior Period Financial Statements
During the fourth quarter of fiscal 2024 (March 31, 2024), the Company determined that the prior year financial statements had an error caused by an immaterial classification error of certain research and development expense in accordance with ASC 730 Research and Development Costs. As a result, certain prior year amounts have been revised for consistency with the current year presentation. The Company assessed the materiality of this change in presentation on prior period financial statements in accordance with SEC Staff Accounting Bulletin No. 99, “Materiality,” (ASC Topic 250, Accounting Changes and Error Corrections). Based on this assessment, the Company concluded that these classification error corrections in its Statements of Operations are not material to any previously presented financial statements based upon overall considerations of both quantitative and qualitative factors. The corrections had no impact on the fiscal year 2023 Balance Sheet, Statements of Cash Flows, or Statement of Changes in Stockholders’ Equity. Further, the immaterial corrections did not result in a change in operating losses, net loss, or basic or diluted earnings per share in the Income Statement.
A summary of immaterial corrections reflecting the prior period impact to the Company’s Statement of Operations, for the quarter ended June 30, 2023 is shown below (in thousands):
|
As Revised |
||||||||||||
|
June 30, 2023 |
Correction |
June 30, 2023 |
||||||||||
|
General and administrative expense |
$ | 523 | $ | (20 | ) | $ | 503 | |||||
|
Research and development expense |
348 | 20 | 368 | |||||||||
|
Net Loss |
$ | 871 | $ | - | $ | 871 | ||||||
Note 2 – Convertible Notes Payable
On September 9, 2023, the Company's Board of Directors (the “Board”) authorized an offering up to $2.0 million in unsecured, non-interest bearing convertible promissory notes (the “Notes”) and accompanying warrants (the “Bridge Financing Warrants”) (collectively, the “Bridge Offering”) that will mature on December 31, 2025. The Notes provided that, on the closing date of the IPO, the outstanding principal would be automatically converted into common stock at the conversion price of $2.00. Each dollar in principal amount of Notes purchased were accompanied by a five-year Bridge Financing Warrant to purchase 0.25 shares of Common stock with an exercise price of $1.00 per share. The Company records the Bridge Financing Warrants as a discount to the Notes.
The Bridge Financing Warrants can be exercised from the date of Notes issuance through the five-year anniversary of the issuance of the Notes. The shares issuable pursuant to the Notes and Bridge Financing Warrants have a 180-day lock-up after the Company’s IPO. Thereafter, the foregoing lock-up agreement will cease to apply to 25% of the purchased shares each month for a period of four months. The Note holders are not permitted to convert their Notes when the holders or any of their affiliates would beneficially own in excess of 4.99% of the Company’s common stock after such conversion.
As of June 30, 2024, the Company received proceeds of $2.0 million of Notes executed from the Bridge Offering, which would convert into 1.0 million shares of common stock. The Company’s effective interest rate for the Notes is 15.3% due to the amortization of the discount stemming from the issuance of the Bridge Financing Warrants. On January 26, 2024, we consummated our initial public offering (“IPO”). In connection with the closing of the IPO, a portion of our convertible notes were converted into 335,000 shares of our common stock. Upon the closing of the IPO, certain notes were to be automatically converted according to their terms into our common stock to the extent and provided that certain holders of these notes are not permitted to convert such notes to the extent that the holders or any of its affiliates would beneficially own in excess of 4.99% of our common stock after such conversion. Due to this 4.99% limitation, principal representing $1.3 million, or 665,000 shares, of these notes remains outstanding.
The table below summarizes the Company’s outstanding convertible notes payable as of June 30, 2024 (in thousands).
|
Principal Amount |
Amortized Debt Discount |
Net Carrying Amount |
||||||||||
|
Zero-coupon convertible notes payable due on December 31, 2025 |
$ | 1,330 | $ | 287 | $ | 1,043 | ||||||
Warrants
The Company issued the Notes with detachable warrants for the purchase of shares of the Company’s common stock. The Company utilized a Monte Carlo simulation model to determine the fair value of each Bridge Offering Warrant. The key inputs to the Monte Carlo simulation used to determine the fair value of each warrant include, the Company’s stock price fair value which was determined through a back solve calculation such that the stock price results in the average total value of the Notes and the Bridge Offering Warrants being equal to the cash proceeds received, volatility based on a selection of publicly held peer companies of 101.88%, expected term of 5 years, risk free rate of 4.40%, discount rate of 20.00% and a discount for lack of marketability of 15.77%.
During the three months ended June 30, 2024, the Company recorded less than $0.1 million in interest expense related to the amortization of the debt discount.
The following table presents a summary of activity for the warrants issued in connection with the Company’s Notes:
|
Weighted-Average |
||||||||||||||||
|
Exercise Price |
Remaining Life |
Aggregate |
||||||||||||||
|
Warrants |
Per Share |
(In Years) |
Intrinsic Value* |
|||||||||||||
|
Outstanding and exercisable, March 31, 2024 |
500,000 | $ | 1.00 | 4.48 | $ | 1,010,000 | ||||||||||
|
Granted |
- | - | - | - | ||||||||||||
|
Exercised |
- | - | - | - | ||||||||||||
|
Forfeited/Cancelled |
- | - | - | - | ||||||||||||
|
Expired |
- | - | - | - | ||||||||||||
|
Outstanding, June 30, 2024 |
500,000 | $ | 1.00 | 4.23 | $ | - | ||||||||||
|
Exercisable, June 30, 2024 |
500,000 | $ | 1.00 | 4.23 | $ | - | ||||||||||
*Aggregate Intrinsic Value = Excess of market value over the exercise price of all in-the-money warrants. No outstanding and exercisable warrants issued in connection with the Company's Notes were in-the-money as of June 30, 2024.
Note 3 – Equity
On January 26, 2024, we consummated our IPO. In the IPO, we sold a total of 2,234,222 shares of common stock at a purchase price of $5.00 per share for gross proceeds of $11.2 million and net proceeds of $9.8 million. In connection with the closing of the IPO, a portion of our convertible notes were converted into 335,000 shares of our common stock. Total shares of common stock outstanding at the closing of the IPO amounted to 18,687,061 shares, prior to the May 13, 2024 cancellation of 1,050 shares represented in the IPO for payment disputes. Upon the closing of the IPO, certain notes were to be automatically converted according to their terms into our common stock to the extent and provided that certain holders of these notes are not permitted to convert such notes to the extent that the holders or any of its affiliates would beneficially own in excess of 4.99% of our common stock after such conversion. Due to this 4.99% limitation, principal representing $1.3 million, or 665,000 shares, of these notes remains outstanding.
On November 29, 2023, the Company’s Board of Directors and applicable shareholders approved to amend and restate the Company’s certificate of incorporation and increased the authorized shares to 500,000,000 shares of common stock, with a par value of $.001 per share, and 10,000,000 shares of preferred stock, with a par value of $.001 per share. The specific rights of the preferred stock shall be determined by the Board of Directors.
Restricted Stock
On February 15, 2024, the Company issued 35,000 restricted shares of common stock to the Company's marketing consultant at the closing price of $3.80 of the Company's common stock. The total value of these shares is $133,000. These shares vest monthly over a 12-month period beginning on the issue date.
|
Quarter ended June 30, |
||||||||
|
2024 |
2023 |
|||||||
|
Recognized in general and administrative expense |
$ | 33,250 | $ | — | ||||
|
Total |
$ | 33,250 | $ | — | ||||
For the quarter ended June 30, 2024, there was $83,125 of unrecognized stock-based compensation expense related to unvested Restricted Stock, which is expected to be recognized over the period July 2024 through February 2025.
A summary of activity regarding Restricted Stock issued is as follows:
|
Grant Date |
||||||||
|
Number of Shares |
Fair Value Per Share |
|||||||
|
Unvested, March 31, 2024 |
32,083 | $ | 3.80 | |||||
|
Granted |
— | $ | — | |||||
|
Vested |
(8,750 | ) | $ | 3.80 | ||||
|
Unvested, June 30, 2024 |
23,333 | $ | 3.80 | |||||
Common Stock
On April 6, 2023, the Board of Directors approved a private placement offering of up to 2,000,000 common shares at a price of $2.00 per share. During the three months ended June 30, 2023, the Company sold 1,420,000 shares for cash proceeds of $2,840,000. The Company did not incur any costs that were direct and incremental to the private placement.
On September 9, 2023, the Board approved a Bridge Offering. See Note 3 Convertible Notes Payable for additional detail as these notes are convertible into common stock.
Stock Plan and Stock Options
In June 2023, the Company adopted, and the Company’s shareholders approved, the Autonomix Medical, Inc. 2023 Stock Plan (the “Plan”). The Plan is a stock-based compensation plan that provides for discretionary grants of stock options, stock awards and stock unit awards to key employees, non-employee directors, and consultants, subject to certain individual threshold limitations. The Plan provides for up to 4,000,000 shares to be issued. Shares that are surrendered because of forfeiture, expiration, termination, or cancellation are available for re-issuance.
In August 2023, the Plan was amended to allow for an automatic increase of the available shares for issuance, whereby on the 1st of each fiscal year, beginning on April 1, 2024 and ending on (and including) April 1, 2033 in an amount equal to five percent (5%) of the total number of shares of Common Stock outstanding on the March 31st immediately preceding the applicable date. However, the Board may act prior to the automatic increase of a given year to provide that there will be no increase for such year, or that the increase for such year will be a lesser number of shares of Common Stock. On April 1, 2024, the Plan was increased by 942,305 shares.
The following table summarizes the stock option activity for the three months ended June 30, 2024:
|
Weighted-Average |
Weighted-Average |
|||||||||||||||
|
Exercise Price |
Remaining Life |
Aggregate |
||||||||||||||
|
Options |
Per Share |
(In Years) |
Intrinsic Value* |
|||||||||||||
|
Outstanding and exercisable, March 31, 2024 |
2,003,600 | $ | 2.33 | 9.35 | $ | 1,680,672 | ||||||||||
|
Granted |
2,325,979 | 1.38 | - | - | ||||||||||||
|
Exercised |
- | - | - | - | ||||||||||||
|
Forfeited/Cancelled |
- | - | - | - | ||||||||||||
|
Expired |
- | - | - | - | ||||||||||||
|
Outstanding, June 30, 2024 |
4,329,579 | $ | 1.82 | 9.57 | $ | - | ||||||||||
|
Exercisable, June 30, 2024* |
321,317 | $ | 2.00 | 8.69 | $ | - | ||||||||||
*Aggregate Intrinsic Value = Excess of market value over the exercise price of all in-the-money stock. No outstanding or exercisable options were in-the-money as of June 30, 2024.
During the three months ended June 30, 2024, the Company granted certain individuals options to purchase 2,325,979 shares of common stock with an average exercise price of $1.38 per share and a contractual term that vests annually over four years on the anniversary date. The options had an aggregate grant date fair value of $2.6 million that was calculated using the Black-Scholes option pricing model. Variables used in the Black-Scholes option pricing model included the following: (1) fair value of common stock on the measurement date; (2) discount rate ranging from 4.25% to 4.39% based on the daily yield curve rates for U.S. Treasury obligations, (3) expected life ranging of 6.25 years based on the simplified method (vesting plus contractual term divided by two) and (4) expected volatility ranging from 110% to 130% based on the historical volatility of comparable companies' stock.
All options issued and outstanding are being amortized over their respective vesting periods. The unrecognized compensation expense at June 30, 2024 was $5.4 million. During the three months ended June 30, 2024, the Company recorded stock-based compensation - option expense of $0.3 million in general and administrative expense and less than $0.1 million in research and development expense. There was no recorded stock-based compensation - option expense for the three months ended June 30, 2023.
Equity-Based Stock Warrants
The Company will periodically grant warrants to investors in connection with equity financing or to third-party service providers in exchange for services rendered. The following table summarizes the stock warrant activity for the three months ended June 30, 2024:
|
Weighted-Average |
Weighted-Average |
|||||||||||||||
|
Exercise Price |
Remaining Life |
Aggregate |
||||||||||||||
|
Warrants |
Per Share |
(In Years) |
Intrinsic Value* |
|||||||||||||
|
Outstanding, March 31, 2024 |
5,744,569 | $ | 0.08 | 4.80 | $ | 17,072,147 | ||||||||||
|
Granted |
- | - | - | - | ||||||||||||
|
Exercised** |
(397,037 | ) | 0.01 | - | - | |||||||||||
|
Forfeited/Cancelled |
(2,963 | ) | 0.01 | - | - | |||||||||||
|
Expired |
- | - | - | - | ||||||||||||
|
Outstanding, June 30, 2024 |
5,344,569 | $ | 0.09 | 4.54 | $ | 5,009,401 | ||||||||||
|
Exercisable, June 30, 2024 |
5,341,236 | $ | 0.09 | 4.54 | $ | 5,009,401 | ||||||||||
|
* |
Aggregate Intrinsic Value = Excess of market value over the exercise price of all in-the-money warrants. 5,257,929 outstanding and exercisable warrants were in-the-money as of June 30, 2024. |
|
** |
All exercised warrants utilized the “cashless exercise” option. |
The unrecognized compensation expense at June 30, 2024 was less than $0.1 million. During the three months ended June 30, 2024, the Company recorded stock-based compensation - warrant expense of less than $0.1 million. There was no recorded stock-based compensation - warrant expense for the three months ended June 30, 2023.
Note 4 – Commitments and Contingencies
Legal Proceedings
From time to time, we may be involved in claims that arise during the ordinary course of business. Although the results of litigation and claims cannot be predicted with certainty, we do not currently have any pending litigation to which we are a party or to which our property is subject that we believe to be material. Regardless of the outcome, litigation can be costly and time consuming, and it can divert management’s attention from important business matters and initiatives, negatively impacting our overall operations.
Employment Agreements
We have agreements with key employees to provide certain benefits, including salary and other wage-related benefits, in the event of termination. In addition, the Company has adopted a severance policy for certain key members of executive management to provide certain benefits, including salary and other wage-related benefits, in the event of termination. In total, these benefits would amount to a range of $1.1 million to $1.6 million using the rate of compensation in effect at June 30, 2024.
Brad Hauser - Chief Executive Officer
On June 17, 2024, we entered into an employment agreement with Brad Hauser pursuant to which Mr. Hauser agreed to serve as our chief executive officer and president for an initial three-year period, which may be extended on a year-to-year basis. Mr. Hauser’s agreement provides for an initial annual base salary of $450,000 (subject to an annual review and increase at the discretion of our Compensation Committee) and a target annual bonus of 60% of his base salary. Pursuant to the agreement, Mr. Hauser was granted a ten-year option (the “Inducement Options”) to purchase 900,000 shares of common stock at an exercise price equal to the closing price of our common stock on the date of the employment agreement. The option vests in four equal annual installments (or 225,000 shares each installment) on each of the succeeding four anniversary dates of the execution of the employment agreement, provided Mr. Hauser is employed by us on each vesting date. In the event of a “change of control” or the termination of the agreement by us without “cause” or by Mr. Hauser for “good reason,” all of the unvested options shall immediately vest. The Inducement Options were granted outside of our 2023 Stock Plan as an inducement material to Mr. Hauser’s entering into employment with us in accordance with Nasdaq Stock Market Listing Rule 5635(c)(4). Commencing with the year ending March 31, 2025, Mr. Hauser will be eligible to receive annual option grants as determined by the Compensation Committee of the Board of Directors, based on criteria established by the Compensation Committee. The number of shares underlying the target annual option grant will be equal to $1,000,000 divided by the Black-Scholes value per share of our common stock on the date of grant.
If Mr. Hauser’s employment is terminated at our election without “cause,” or by Mr. Hauser for “good reason,” Mr. Hauser shall be entitled to receive severance payments equal to twelve months of Mr. Hauser’s base salary and 100% of the target bonus for the year in which such termination occurs; provided that such amounts shall be increased by 50% if Mr. Hauser’s agreement is terminated without “cause” or by Mr. Hauser for “good reason” within three months prior to or twelve months after a “change of control.” In the event that any payments or benefits provided to Mr. Hauser would trigger the excise tax under Section 4999 of the Internal Revenue Code or any similar provision, the Company agreed to provide Mr. Hauser with a gross-up payment to ensure that, after payment of all taxes (including the excise tax, federal, state, and local income taxes, and employment taxes) imposed on the gross-up payment, Mr. Hauser receives a net amount equal to the payments or benefits Mr. Hauser would have received if the excise tax didn't apply
Lori Bisson - Vice Chair (former Chief Executive Officer)
On June 17, 2024, we entered into an employment agreement with Lori Bisson pursuant to which Ms. Bisson agreed to serve as our Executive Vice Chair and Strategic Adviser to the Chief Executive Officer (“Vice Chair”) for a two-year period. Ms. Bisson’s agreement provides for an initial annual base salary of $150,000 (subject to an annual review and increase at the discretion of our Compensation Committee) and a target annual bonus of 50% of her base salary. Pursuant to the agreement, Ms. Bisson continued to vest in the option grants issued to Ms. Bisson in her role as chief executive officer and president in accordance with the vesting schedule set out in her initial employment agreement. In the event of a “change of control” or the termination of the agreement by us without “cause” or by Ms. Bisson for “good reason,” all of the unvested options shall immediately vest. Ms. Bisson is entitled to receive any compensation, including incentive compensation, for the fiscal year ended March 31, 2024 that has not been paid as of the date of the agreement. Commencing with the year ending March 31, 2025, Ms. Bisson will be eligible to receive annual option grants as determined by the Compensation Committee of the Board of Directors, based on criteria established by the Compensation Committee. Ms. Bisson agreed to waive any severance payments due to her in connection with the termination of the prior employment agreement that we entered into with her on June 30, 2023.
Note 5 – Related Party Transactions
The Company utilizes a consulting firm that is owned by the Company’s former Chief Financial Officer to provide accounting and financial reporting services and pays certain expenses on behalf of the Company. During the three months ended June 30, 2024 and 2023, the Company incurred fees of $0 and less than $0.1 million, respectively, for these services, excluding officer compensation. As of June 30, 2024 and March 31, 2024, the Company owed the consulting firm $0 and less than $0.1 million, respectively, for services and expenses.
As of June 30, 2024, members of the Company’s management/Board and an immediate family member of the Company’s management (related party), collectively purchased $0.5 million ($0.4 million and $0.1 million, respectively) of the Bridge Offering.
On December 21, 2021, the Company entered into a perpetual, worldwide, exclusive license agreement (the “License” or “License Agreement”) with a company controlled by a significant stockholder of the Company (the “Licensee”). The License allows the Licensee to use certain intellectual property and technology related to the diagnosis and treatment of cardiovascular conditions held by the Company. Upon 90 days following the completion of an initial public offering or special purpose acquisition company transaction, the Licensee may enter into sublicenses of the licensed intellectual property and technology.
On July 7, 2023, the Company and the Licensee entered into an Exclusive License Termination Agreement (the “Termination Agreement”) in exchange for the issuance, upon the closing of the Company’s initial public offering within one year of the agreement’s execution, of a warrant to purchase shares of the Company for a variable number of shares. The variable number of shares issued was based upon a fixed value of $8.0 million divided by the price per share in the offering. The warrants are exercisable at a price of $0.001 per share and may be exercised any time after the issuance date, subject to a beneficial ownership limitation, and expires five years from the original issuance. The warrants provide voting rights, dividend rights, and other rights of a shareholder prior to exercise. The shares underlying the warrant will be subject to a lockup agreement for a period of six months after the closing of the offering with respect to 12.5% of the shares issued and twelve months after the closing of the offering for the remainder of the shares.
On January 29, 2024, we issued a warrant to purchase 1,600,000 shares (the “Warrant”) pursuant to the Termination Agreement with Impulse Medical, Inc. ("Impulse"). The warrants are exercisable at a price of $0.001 per share and may be exercised any time after the issuance date, subject to a beneficial ownership limitation, and expires five years from the original issuance. The warrants provide voting rights, dividend rights, and other rights of a shareholder prior to exercise. The shares underlying the Warrant are subject to a lockup agreement for a period of six months after the closing of the IPO with respect to 12.5% of the shares issued and twelve months after the closing of the IPO for the remainder of the shares. In connection with the Termination Agreement, the Company agreed to register the resale of the shares of common stock underlying the Warrant upon a notice of 20 business days by the Warrant holder.
Note 6 – Subsequent Events
On July 10, 2024, we entered into a license agreement (the “Agreement”) with RF Innovations, Inc. (“RFI”), a privately held medical technology company, to license products utilizing RFI’s intellectual property related to its Apex 6 Radiofrequency Generator (the “Licensed Products”). The Apex 6 Generator is a United States Food and Drug Administration (“FDA”) cleared ablation technology designed to lesion neural tissue for pain management in the peripheral nervous system. Pursuant to the Agreement, RFI granted us a perpetual non-exclusive worldwide royalty free fully paid license related to the Licensed Products, provided that the license did not include the right to sell certain products to customers for the treatment of spine pain. In connection with the Agreement, we issued RFI 250,000 unregistered shares of our common stock as consideration for the license. The Agreement provides RFI the right to terminate the license if we breach any representation, warranty or covenant contained in the Agreement, subject to any relevant cure periods, or if we are subject to a bankruptcy or insolvency event.
In July 2024, 3,594,000 warrants were exercised on a cashless basis resulting in a net share amount of 3,544,852 at an exercise price of $0.01.
Up to 698,812 Common Stock Units
Each Common Stock Unit Consisting of One Share of Common Stock and One Series A Warrant to Purchase One Share of Common Stock
Up to 698,812 Shares of Common Stock Issuable Upon Exercise of Series A Warrants
Up to 698,812 PFW Units
Each PFW Unit Consisting of One Pre-Funded Warrant to Purchase One Share of Common Stock and One Series A Warrant to Purchase One Share of Common Stock
Up to 698,812 Shares of Common Stock Issuable Upon Exercise of Pre-Funded Warrants
Up to 698,812 Shares of Common Stock Underlying the Series A Warrants
Representative Warrants to Purchase up to 41,928 Shares of Common Stock
Up to 41,928 Shares of Common Stock Issuable Upon Exercise of Representative Warrants

Ladenburg Thalmann
PROSPECTUS
________________, 2024
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
Item 13. Other Expenses of Issuance and Distribution.
The following table sets forth the estimated costs and expenses to be incurred in connection with the issuance and distribution of the securities of Autonomix Medical, Inc. (the “Registrant”) which are registered under this Registration Statement on Form S-1 (this “Registration Statement”), other than underwriting discounts and commissions. All amounts are estimates except the Securities and Exchange Commission registration fee and the Financial Industry Regulatory Authority, Inc. filing fee.
The following expenses will be borne solely by the Registrant.
|
Amount to be |
||
|
SEC Registration fee |
$ |
3,949.00 |
|
Financial Industry Regulatory Authority, Inc. filing fee |
4,369.00 |
|
|
Legal fees and expenses |
150,000.00 |
|
|
Accounting fees and expenses |
56,000.00 |
|
|
Transfer Agent’s fees |
1,000.00 |
|
|
Miscellaneous fees and expenses |
4,682.00 |
|
|
Total |
$ |
220,000.00 |
Item 14. Indemnification of Directors and Officers.
Pursuant to Section 145 of the Delaware General Corporation Law (the “DGCL”), a corporation shall have the power to indemnify any person who was or is a party or is threatened to be made a party to any threatened, pending or completed action, suit or proceeding, whether civil, criminal, administrative or investigative (other than a derivative action by or in the right of such corporation) by reason of the fact that such person is or was a director, officer, employee or agent of such corporation, or serving at the request of such corporation in such capacity for another corporation, partnership, joint venture, trust or other enterprise, against expenses (including attorneys’ fees), judgments, fines and amounts paid in settlement actually and reasonably incurred in connection with such action, suit or proceeding, if such person acted in good faith and in a manner he or she reasonably believed to be in or not opposed to the best interests of such corporation, and, with respect to any criminal action or proceeding, had no reasonable cause to believe his or her conduct was unlawful.
The DGCL also permits indemnification by a corporation under similar circumstances for expenses (including attorneys’ fees) actually and reasonably incurred by such persons in connection with the defense or settlement of a derivative action or suit, except that no indemnification shall be made in respect of any claim, issue or matter as to which such person shall have been adjudged to be liable to such corporation unless the Delaware Court of Chancery or the court in which such action or suit was brought shall determine upon application that such person is fairly and reasonably entitled to indemnity for such expenses which such court shall deem proper.
To the extent a present or former director or officer is successful in the defense of such an action, suit or proceeding referenced above, or in defense of any claim, issue or matter therein, a corporation is required by the DGCL to indemnify such person for actual and reasonable expenses incurred in connection therewith. Expenses (including attorneys’ fees) incurred by such persons in defending any action, suit or proceeding may be paid in advance of the final disposition of such action, suit or proceeding upon in the case of a current officer or director, receipt of an undertaking by or on behalf of such person to repay such amount if it is ultimately determined that such person is not entitled to be so indemnified.
The DGCL provides that the indemnification described above shall not be deemed exclusive of other indemnification that may be granted by a corporation pursuant to its bylaws, disinterested directors’ vote, stockholders’ vote and agreement or otherwise.
Section 102(b)(7) of the DGCL enables a corporation, in its certificate of incorporation or an amendment thereto, to eliminate or limit the personal liability of a director to the corporation or its stockholders for monetary damages for violations of the directors’ fiduciary duty, except (i) for any breach of the director’s duty of loyalty to the corporation or its stockholders, (ii) for acts or omissions not in good faith or which involve intentional misconduct or a knowing violation of law, (iii) pursuant to Section 174 of the DGCL (providing for liability of directors for unlawful payment of dividends or unlawful stock purchases or redemptions) or (iv) for any transaction from which a director derived an improper personal benefit. The Registrant’s certificate of incorporation provides for such limitations on liability for its directors.
The DGCL also provides corporations with the power to purchase and maintain insurance on behalf of any person who is or was a director, officer, employee or agent of such corporation, or is or was serving at the request of such corporation in a similar capacity for another corporation, partnership, joint venture, trust or other enterprise, against any liability asserted against him or her in any such capacity or arising out of his or her status as such, whether or not the corporation would have the power to indemnify him or her against such liability as described above.
The Registrant’s amended and restated certificate of incorporation requires the Registrant to indemnify and hold harmless, to the fullest extent permitted by applicable law as it presently exists or may hereafter be amended, any person (a “covered person”) who was or is made or is threatened to be made a party or is otherwise involved in any threatened, pending or completed action, suit or proceeding, whether civil, criminal, administrative or investigative (a “proceeding”) by reason of the fact that he or she is or was a director, officer or member of a committee of the Registrant, or, while a director or officer of the Registrant, is or was serving at the request of the Registrant as a director or officer, employee or agent of another corporation, partnership, joint venture, trust or other enterprise or non-profit entity, including service with respect to employee benefit plans, against all liability and loss suffered and expenses (including attorneys’ fees), judgment, fines and amounts paid in settlement actually and reasonably incurred by such person in connection with a proceeding.
In addition, under the Registrant’s amended and restated certificate of incorporation, in certain circumstances, the Registrant shall pay the expenses (including attorneys’ fees) incurred by a covered person in defending a proceeding in advance of the final disposition of such proceeding; provided, however, that the Registrant shall not be required to advance any expenses to a person against whom the Registrant directly brings an action, suit or proceeding alleging that such person (1) committed an act or omission not in good faith or (2) committed an act of intentional misconduct or a knowing violation of law. Additionally, an advancement of expenses incurred by a covered person shall be made only upon delivery to the Registrant of an undertaking, by or on behalf of such covered person, to repay all amounts so advanced if it shall ultimately be determined by final judicial decision from which there is no further right to appeal or otherwise in accordance with Delaware law that such covered person is not entitled to be indemnified for such expenses.
In addition, the Registrant has entered into indemnification agreements with its directors and executive officers that provide for additional indemnification protections, which form of agreement has been filed as an exhibit to this registration statement.
Item 15. Recent Sales of Unregistered Securities.
Except as set forth below, in the three years preceding the filing of this Registration Statement, the Registrant has not issued any securities that were not registered under the Securities Act:
In December 2021, the Company entered into a Simple Agreement for Future Equity (“SAFE”) and received total proceeds of $400,000. The amount invested was convertible into equity instruments upon the closing of a subsequent equity financing. The SAFE investor also received a warrant to purchase 323,000 shares of common stock with a 7-year exercise period and an exercise price $0.20 per share.
On March 21, 2022, the Company entered into the Exchange Agreement with the Company’s convertible note holders, Series A preferred stock shareholders, option holders and common stockholders at the time, to convert all outstanding investments into the Company’s common stock for a fixed number of shares. Upon execution of the Exchange Agreement, there were 246,814 shares of common stock and 3,197 warrants to purchase common stock at an exercise price of $0.21 per share issued to existing equity and debt holders. A total of 52,883 shares of common stock were forfeited upon the execution of the Exchange Agreement.
In January and February of 2022, the Company issued a total of 292,000 shares of common stock to the Board of Directors, Officers and Scientific Advisor, as compensation.
In March 2022, the Company completed a common stock offering, in which the Company sold 50,000 shares of common stock for gross proceeds of $2,000,000 and issued 2,300 warrants to purchase common stock at an exercise price of $40.00 per share. In addition, 1,150 shares of common stock were issued as broker compensation.
In March 2023, the Company completed a common stock offering in which the Company sold 16,875 shares of common stock for net proceeds of $675,000.
From April to June 2023, the Company completed a common stock offering, in which the Company sold 71,001 shares of common stock for gross proceeds of $2,840,000.
During the year ended March 31, 2024 and prior to the Company’s initial public offering, the Company issued the following unregistered securities: i) $2.0 million in unsecured, non-interest bearing convertible promissory notes (the Notes) and accompanying warrants (the Bridge Financing Warrants). The Notes provided that, on the closing date of the IPO, the outstanding principal would be automatically converted into common stock at the conversion price of $40.00. Each dollar in principal amount of Notes purchased were accompanied by a five-year Bridge Financing Warrant to purchase approximately 0.0125 shares of common stock with an exercise price of $20.00 per share; and ii) 1,750 shares of restricted stock for a consultant for purposes of providing business advisory services.
On July 7, 2023, the Company entered into a Termination Agreement with the Licensee in exchange for the issuance, upon the closing of the Company’s IPO within one year of the agreement’s execution, of a warrant to purchase shares of the Company for a variable number of shares based on a value of $8.0 million. Upon the closing of the Company’s IPO on January 29, 2024, the Company issued the licensee a warrant to purchase 80,000 shares, which were issued at an assumed valuation of $100.00 per share for a fixed value of $8.0 million. The warrants are exercisable at a price of $0.02 per share and may be exercised any time after the issuance date, subject to a beneficial ownership limitation, and expire five years from the original issuance.
On September 1, 2023, the Company issued a warrant to purchase 1,000 shares of common stock to a consultant providing investor relation advisory services.
On June 17, 2024, the Company entered into an employment agreement with Brad Hauser. Pursuant to the agreement, Mr. Hauser was granted a ten-year option (the “Inducement Options”) to purchase 45,000 shares of common stock at an exercise price equal to the closing price of the Company’s common stock on the date of the employment agreement. The Inducement Options were granted outside of the Company’s 2023 Stock Plan as an inducement material to Mr. Hauser’s entering into employment with the Company in accordance with Nasdaq Stock Market Listing Rule 5635(c)(4).
On July 10, 2024, as consideration for a license agreement, the Company agreed to issue 12,500 shares of its common stock.
All of the securities above were issued in reliance on the exemption provided by Section 4(a)(2) of the Securities Act for the offer and sale of securities not involving a public offering, and/or Regulation D promulgated under the Securities Act.
Item 16. Exhibits and Financial Statement Schedules.
(a) Exhibits:
|
Exhibit |
Description of Document |
|
|
1.1* |
||
|
3.1 |
||
|
3.2 |
||
|
4.1 |
||
|
4.2 |
||
|
4.3* |
||
| 4.4* | Form of Series A Warrant | |
| 4.5* | Form of Representative Warrant | |
| 4.6* | Warrant Agency Agreement | |
|
5.1* |
||
|
10.1** |
||
|
10.2** |
||
|
10.3** |
||
|
10.4** |
||
|
10.5 |
||
|
10.6 |
||
|
10.7 |
||
|
10.8+ |
||
|
10.9 |
||
|
10.10 |
||
|
10.11** |
||
|
10.12** |
||
|
10.13 |
||
|
23.1* |
||
|
23.2* |
||
|
24.1* |
||
|
107* |
||
|
* |
Filed herewith. |
|
** |
Management contract or compensatory plan, contract or arrangement. |
|
+ |
Pursuant to Item 601(b)(10)(iv) of Regulation S-K promulgated by the SEC, certain portions of this exhibit have been redacted. The Company hereby agrees to furnish supplementally to the SEC, upon its request, an unredacted copy of this exhibit. |
(b) Consolidated Financial Statement Schedules: All schedules are omitted because the required information is inapplicable or the information is presented in the consolidated financial statements and the related notes.
Item 17. Undertakings
(a) The undersigned Registrant hereby undertakes that:
(1) To file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement:
(i) To include any prospectus required by Section 10(a)(3) of the Securities Act;
(ii) To reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in the registration statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of prospectus filed with the Commission pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than 20 percent change in the maximum aggregate offering price set forth in the “Calculation of Registration Fee” table in the effective registration statement;
(iii) To include any material information with respect to the plan of distribution not previously disclosed in the registration statement or any material change to such information in the registration statement;
provided, however, that paragraphs (i), (ii) and (iii) above do not apply if the information required to be included in a post-effective amendment by those paragraphs is contained in reports filed with or furnished to the Commission by the registrant pursuant to Section 13 or Section 15(d) of the Exchange Act that are incorporated by reference in this Registration Statement or is contained in a form of prospectus filed pursuant to Rule 424(b) that is part of the Registration Statement.
(2) That, for the purpose of determining any liability under the Securities Act, each such post-effective amendment shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
(3) To remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination of the offering.
(4) That, for the purpose of determining liability under the Securities Act to any purchaser in the initial distribution of the securities, the undersigned registrant undertakes that in a primary offering of securities of the undersigned registrant pursuant to this registration statement, regardless of the underwriting method used to sell the securities to the purchaser, if the securities are offered or sold to such purchaser by means of any of the following communications, the undersigned registrant will be a seller to the purchaser and will be considered to offer or sell such securities to such purchaser:
(i) Any preliminary prospectus or prospectus of the undersigned registrant relating to the offering required to be filed pursuant to Rule 424 (§230.424 of this chapter);
(ii) Any free writing prospectus relating to the offering prepared by or on behalf of the undersigned registrant or used or referred to by the undersigned registrant;
(iii) The portion of any other free writing prospectus relating to the offering containing material information about the undersigned registrant or its securities provided by or on behalf of the undersigned registrant; and
(iv) Any other communication that is an offer in the offering made by the undersigned registrant to the purchaser.
(b) The undersigned Registrant hereby undertakes that, for purposes of determining any liability under the Securities Act, each filing of the registrant’s annual report pursuant to section 13(a) or section 15(d) of the Exchange Act (and, where applicable, each filing of an employee benefit plan’s annual report pursuant to section 15(d) of the Exchange Act) that is incorporated by reference in the registration statement shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
(c) Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers and controlling persons of the registrant pursuant to the foregoing provisions, or otherwise, the Registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer, or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Act and will be governed by the final adjudication of such issue.
(d) The undersigned Registrant hereby undertakes that:
(1) For purposes of determining any liability under the Securities Act of 1933, the information omitted from the form of prospectus filed as part of this registration statement in reliance upon Rule 430A and contained in a form of prospectus filed by the registrant pursuant to Rule 424(b)(1) or (4) or 497(h) under the Securities Act shall be deemed to be part of this registration statement as of the time it was declared effective.
(2) For the purpose of determining any liability under the Securities Act of 1933, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
SIGNATURES
Pursuant to the requirements of the Securities Act, the registrant has duly caused this Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the city of Houston, Texas, on November 1, 2024.
|
|
AUTONOMIX MEDICAL, INC. (Registrant) |
|
|
|
|
|
|
|
|
|
By: |
/s/ Brad Hauser |
|
|
|
|
Brad Hauser |
|
|
|
|
Chief Executive Officer and President |
|
KNOW ALL MEN AND WOMEN BY THESE PRESENTS, that each person whose signature appears below constitutes and appoints either Brad Hauser or Trent Smith, her or his true and lawful attorneys-in-fact and agents, with full power of substitution and resubstitution for her or him and in her or his name, place and stead, in any and all capacities, to sign any and all amendments (including post-effective amendments) to this Registration Statement, and any subsequent registration statements pursuant to Rule 462 of the Securities Act of 1933 and to file the same, with all exhibits thereto, and other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorneys-in-fact and agents, and each of them, full power and authority to do and perform each and every act and thing requisite and necessary to be done in and about the premises, as fully to all intents and purposes as he or she might or could do in person, hereby ratifying and confirming all that each of said attorney-in-fact or her or his substitute or substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Act of 1933, as amended, this registration statement has been signed below by the following persons in the capacities and on the dates indicated:
|
Date: November 1, 2024 |
By: |
/s/ BRAD HAUSER |
|
Brad Hauser |
||
|
Date: November 1, 2024 |
/s/ TRENT SMITH |
|
|
Trent Smith Chief Financial Officer (Principal Financial and Accounting Officer) |
||
|
Date: November 1, 2024 |
/s/ WALTER KLEMP |
|
|
Walter Klemp Executive Chairman of the Board of Directors |
||
|
Date: November 1, 2024 |
/s/ LORI BISSON |
|
|
Lori Bisson |
||
|
Date: November 1, 2024 |
/s/ JONATHAN FOSTER |
|
|
Jonathan Foster Director |
||
|
Date: November 1, 2024 |
/s/ DAVID ROBINS |
|
|
David Robins |
||
|
Date: November 1, 2024 |
/s/ CHRISTOPHER CAPELLI, MD |
|
|
Christopher Capelli |
Exhibit 1.1
[●] SHARES OF COMMON STOCK
PRE-FUNDED WARRANTS TO PURCHASE [●] SHARES OF COMMON STOCK
SERIES A COMMON WARRANTS TO PURCHASE [●] SHARES OF COMMON STOCK
OF
AUTONOMIX MEDICAL, INC.
UNDERWRITING AGREEMENT
[●], 2024
Ladenburg Thalmann & Co. Inc.
As the Representative of the Several Underwriters,
if any, Named in Schedule I hereto
640 Fifth Avenue, 4th Floor
New York, NY 10019
Ladies and Gentlemen:
The undersigned, Autonomix Medical, Inc., a Delaware corporation (the “Company”), hereby confirms its agreement (this “Agreement”) with the several underwriters, if any (such underwriters, including the Representative (as defined below), the “Underwriters” and each an “Underwriter”) named in Schedule I hereto for which Ladenburg Thalmann & Co. Inc. is acting as representative to the several Underwriters (the “Representative” and if there are no Underwriters other than the Representative, references to multiple Underwriters shall be disregarded and the term Representative as used herein shall have the same meaning as Underwriter) on the terms and conditions set forth herein.
It is understood that the Public Securities are to be initially offered to the public by the several Underwriters at the public offering price set forth in the Prospectus. The Representative may from time to time thereafter change the public offering price and other selling terms.
It is further understood that you will act as the Representative for the other Underwriters, if any, in the offering and sale of the Closing Securities and, if any, the Option Securities (as each such term is defined below) in accordance with this Agreement.
ARTICLE I.
DEFINITIONS
1.1 Definitions. In addition to the terms defined elsewhere in this Agreement, for all purposes of this Agreement, the following terms have the meanings set forth in this Section 1.1:
“Action” shall have the meaning ascribed to such term in Section 3.1(k).
“Affiliate” means with respect to any Person, any other Person that, directly or indirectly through one or more intermediaries, controls or is controlled by or is under common control with such Person as such terms are used in and construed under Rule 405 under the Securities Act.
“Board of Directors” means the board of directors of the Company.
“Blank Rome” means Blank Rome LLP, counsel to the Underwriters, with offices located at 1271 Avenue of the Americas, New York, NY 10020.
“Business Day” means any day other than Saturday, Sunday or other day on which commercial banks in The City of New York are authorized or required by law to remain closed; provided, however, for clarification, commercial banks shall not be deemed to be authorized or required by law to remain closed due to “stay at home”, “shelter-in-place”, “non-essential employee” or any other similar orders or restrictions or the closure of any physical branch locations at the direction of any governmental authority so long as the electronic funds transfer systems (including for wire transfers) of commercial banks in The City of New York generally are open for use by customers on such day.
“Closing” means the closing of the purchase and sale of the Closing Securities pursuant to Section 2.1.
“Closing Date” means the hour and the date on the Trading Day on which all conditions precedent to (i) the Underwriters’ obligations to pay the Closing Purchase Price and (ii) the Company’s obligations to deliver the Closing Securities, in each case, have been satisfied or waived, but in no event later than 10:00 a.m. (New York City time) on the second (2nd) Trading Day following the date hereof or at such earlier time as shall be agreed upon by the Representative and the Company.
“Closing Common Warrants” shall have the meaning ascribed to such term in Section 2.1(a)(iii).
“Closing Pre-Funded Warrants” shall have the meaning ascribed to such term in Section 2.1(a)(ii).
“Closing Purchase Price” shall have the meaning ascribed to such term in Section 2.1(b), which aggregate purchase price shall be net of the underwriting discounts and commissions.
“Closing Securities” shall have the meaning ascribed to such term in Section 2.1(a)(iii).
“Closing Shares” shall have the meaning ascribed to such term in Section 2.1(a)(i).
“Commission” means the United States Securities and Exchange Commission.
“Common Stock” means the common stock of the Company, par value $0.001 per share, and any other class of securities into which such securities may hereafter be reclassified or changed.
“Common Stock Units” means each unit consisting of: (i) one share of the Company’s Common Stock; and (ii) a Common Warrant to purchase one share of Common Stock at an exercise price of $[●].
“Common Stock Unit Purchase Price” equals $[●], subject to adjustment for reverse and forward stock splits, stock dividends, stock combinations and other similar transactions of the Common Stock that occur after the date of this Agreement and prior to the Closing Date, provided that the purchase price per Pre-Funded Warrant Unit shall be the Common Stock Unit Purchase Price minus $0.001.
“Common Stock Equivalents” means any securities of the Company which would entitle the holder thereof to acquire at any time Common Stock, including, without limitation, any debt, preferred stock, right, option, warrant or other instrument that is at any time convertible into or exercisable or exchangeable for, or otherwise entitles the holder thereof to receive, Common Stock.
“Common Warrant Purchase Price” shall have the meaning ascribed to such term in Section 2.1(b), which aggregate purchase price shall be net of the underwriting discounts and commissions.
“Common Warrants” refers to the Series A Common Stock purchase warrants, which shall have an exercise price of $[●] per share (subject to adjustment as provided in the Common Warrants), delivered to the Underwriters in accordance with Section 2.1(a)(iii) in the form of Exhibit C attached hereto.
“Common Warrant Shares” means the shares of Common Stock issuable upon exercise of the Common Warrants.
“Company Auditor” means Forvis Mazars, LLP, with offices located at 191 Peachtree Road, NE Suite 2700, Atlanta, Georgia 30303.
“Company Counsel” means ArentFox Schiff LLP, with offices located at 1717 K Street NW, Washington, DC 20006.
“Exchange Act” means the Securities Exchange Act of 1934, as amended, and the rules and regulations promulgated thereunder.
“Execution Date” shall mean the date on which the parties execute and enter into this Agreement.
“Exempt Issuance” means the issuance of: (a) shares of Common Stock or options to employees, officers or directors of the Company pursuant to any stock or option plan duly adopted for such purpose by the non-employee members of the Board of Directors or the members of a committee of non-employee directors established for such purpose; (b) securities upon the exercise or exchange of or conversion of any Securities issued hereunder and/or other securities exercisable or exchangeable for or convertible into shares of Common Stock issued and outstanding on the date of this Agreement, provided that such securities have not been amended since the date of this Agreement to increase the number of such securities or to decrease the exercise price, exchange price or conversion price of such securities (other than in connection with (i) stock splits or combinations and (ii) such decreases in exercise, exchange or conversion price in accordance with the terms of such securities) or to extend the term of such securities; (c) any shares of Common Stock or Related Securities issued or issuable pursuant to any stock option, stock bonus, or other stock plan or arrangement described in the Registration Statement, a Preliminary Prospectus or the Prospectus that are filed on one or more registration statements on Form S-8, (d) shares of Common Stock issued in connection with the acquisition or license by the Company of the securities, business, property or other assets of another person or business entity or pursuant to any employee benefit plan assumed by the Company in connection with any such merger or acquisition, provided that such securities are issued as “restricted securities” (as defined in Rule 144 under the Securities Act) and carry no registration rights that require or permit the filing of any registration statement in connection therewith; or (e) shares of Common Stock or Related Securities issued in connection with any merger, joint venture, commercial relationship or other strategic or collaborative transactions, provided that such securities are issued as “restricted securities” (as defined in Rule 144 under the Securities Act) and carry no registration rights that require or permit the filing of any registration statement in connection therewith; provided, that in the case of immediately preceding clauses (d) and (e), (x) the aggregate number of shares issued or underlying such Related Securities issued in connection with all such acquisitions and other transactions does not exceed 5% of the aggregate number of shares of Common Stock outstanding immediately following the consummation of the offering of the Securities pursuant to this Agreement and (y) the recipients of the shares of Common Stock or Related Securities agree in writing to be bound by the same terms described in the Lock-Up Agreements. For purposes of the foregoing, “Related Securities” shall mean any options or warrants or other rights to acquire shares of Common Stock or any securities exchangeable or exercisable for or convertible into shares of Common Stock, or to acquire other securities or rights ultimately exchangeable or exercisable for, or convertible into, shares of Common Stock.
“FCPA” means the Foreign Corrupt Practices Act of 1977, as amended.
“FDA” means the United States Food and Drug Administration.
“FINRA” means the Financial Industry Regulatory Authority.
“GAAP” shall have the meaning ascribed to such term in Section 3.1(i).
“Indebtedness” means (a) any liabilities for borrowed money or amounts owed in excess of $50,000 (other than trade accounts payable incurred in the ordinary course of business), (b) all guaranties, endorsements and other contingent obligations in respect of indebtedness of others, whether or not the same are or should be reflected in the Company’s consolidated balance sheet (or the notes thereto), except guaranties by endorsement of negotiable instruments for deposit or collection or similar transactions in the ordinary course of business; and (c) the present value of any lease payments in excess of $50,000 due under leases required to be capitalized in accordance with GAAP.
“Intellectual Property Counsel” means Ryan, Mason & Lewis, LLP, with offices at 48 South Service Road, Suite 100, Melville, NY 11747.
“Liens” means a lien, charge, pledge, security interest, encumbrance, right of first refusal, preemptive right or other restriction.
“Lock-Up Agreements” means the lock-up agreements, in the form of Exhibit A attached hereto, delivered on the date hereof by each of the Company’s officers and directors.
“Material Adverse Effect” means (i) a material adverse effect on the legality, validity or enforceability of any Transaction Document, (ii) a material adverse effect on the results of operations, assets, business, prospects or condition (financial or otherwise) of the Company or (iii) a material adverse effect on the Company’s ability to perform in any material respect on a timely basis its obligations under any Transaction Document.
“Offering” shall have the meaning ascribed to such term in Section 2.1(c).
“Option Closing Date” shall have the meaning ascribed to such term in Section 2.2(c).
“Option Closing Purchase Price” shall have the meaning ascribed to such term in Section 2.2(b), which aggregate purchase price shall be net of the underwriting discounts and commissions.
“Option Securities” shall have the meaning ascribed to such term in Section 2.2(a).
“Option Shares” shall have the meaning ascribed to such term in Section 2.2(a).
“Over-Allotment Option” shall have the meaning ascribed to such term in Section 2.2.
“Person” means an individual or corporation, partnership, trust, incorporated or unincorporated association, joint venture, limited liability company, joint stock company, government (or an agency or subdivision thereof) or other entity of any kind.
“Pre-Funded Warrant Purchase Price” shall have the meaning ascribed to such term in Section 2.1(b), which aggregate purchase price shall be net of the underwriting discounts and commissions.
“Pre-Funded Warrants” refer to the pre-funded warrants, which shall have an exercise price of $0.001 per share (subject to adjustment as provided in the Pre-Funded Warrants), delivered to the Underwriters in accordance with Section 2.1(a)(ii) in the form of Exhibit B attached hereto.
“Pre-Funded Warrant Units” means, collectively, (i) a Pre-Funded Warrant to purchase one share of Common Stock, at an exercise price of $0.001 until such time as the Pre-Funded Warrant is exercised in full, subject to adjustment as provided in the Pre-Funded Warrant; and (ii) a Common Warrant to purchase one share of Common Stock at an exercise price of $[●].
“Pre-Funded Warrant Shares” means the shares of Common Stock issuable upon exercise of the Pre‑Funded Warrants.
“Preliminary Prospectus” means, if any, any preliminary prospectus relating to the Securities included in the Registration Statement or filed with the Commission pursuant to Rule 424(b).
“Prospectus” means the final prospectus filed for the Registration Statement.
“Prospectus Supplement” means, if any, any supplement to the Prospectus complying with Rule 424(b) of the Securities Act that is filed with the Commission.
“Public Securities” means, collectively, the Closing Securities and, if any, the Option Securities.
“Registration Statement” means, collectively, the various parts of the registration statement prepared by the Company on Form S-1 (File No. 333-[____]) with respect to the Securities, each as amended as of the date hereof, including the Prospectus and Prospectus Supplement, if any, the Preliminary Prospectus, if any, and all exhibits filed with or incorporated by reference into such registration statement, and includes any Rule 462(b) Registration Statement.
“Representative Warrant” shall have the meaning ascribed to such term in Section 2.3(viii).
“Representative Warrant Shares” means the shares of Common Stock issuable upon exercise of the Representative Warrant.
“Required Approvals” shall have the meaning ascribed to such term in Section 3.1(e).
“Rule 424” means Rule 424 promulgated by the Commission pursuant to the Securities Act, as such Rule may be amended or interpreted from time to time, or any similar rule or regulation hereafter adopted by the Commission having substantially the same purpose and effect as such Rule.
“Rule 462(b) Registration Statement” means any registration statement prepared by the Company registering additional Securities, which shall have been filed with the Commission on or prior to the date hereof and became automatically effective pursuant to Rule 462(b) promulgated by the Commission pursuant to the Securities Act.
“SEC Reports” shall have the meaning ascribed to such term in Section 3.1(i).
“Securities” means the Closing Securities, the Option Securities, and the Warrant Shares.
“Securities Act” means the Securities Act of 1933, as amended, and the rules and regulations promulgated thereunder.
“Share Purchase Price” shall have the meaning ascribed to such term in Section 2.1(b).
“Shares” means, collectively, the shares of Common Stock delivered to the Underwriters in accordance with Section 2.1(a)(i) and Section 2.2(a).
“Trading Day” means a day on which the principal Trading Market is open for trading.
“Trading Market” means any of the following markets or exchanges on which the Common Stock is listed or quoted for trading on the date in question: the NYSE American, the Nasdaq Capital Market, the Nasdaq Global Market, the Nasdaq Global Select Market, or the New York Stock Exchange (or any successors to any of the foregoing).
“Transaction Documents” means this Agreement, the Pre-Funded Warrants, the Common Warrants, the Representative Warrant, the Warrant Agency Agreement, the Lock-Up Agreements and any other documents or agreements executed in connection with the transactions contemplated hereunder.
“Transfer Agent” means Equity Stock Transfer, LLC and any successor transfer agent of the Company.
“Variable Rate Transaction” shall have the meaning ascribed to such term in Section 4.20(b).
“Warrant Agency Agreement” means the warrant agency agreement dated on or about the date hereof, between the Company and the Transfer Agent pursuant to which the Transfer Agent will act as warrant agent for the Common Warrants, the Pre-Funded Warrants and the Representative Warrants, in the form of Exhibit G attached hereto.
“Warrants” means each of the Common Warrants, the Pre-Funded Warrants and the Representative Warrants.
“Warrant Shares” means each of the Common Warrant Shares, the Pre-Funded Warrant Shares and the Representative Warrant Shares.
ARTICLE II.
PURCHASE AND SALE
2.1 Closing.
(a) Upon the terms and subject to the conditions set forth herein, the Company agrees to sell in the aggregate Common Stock Units and Pre-Funded Warrant Units consisting of: [●] shares of Common Stock, [●] Pre-Funded Warrants to purchase an aggregate of [●] shares of Common Stock and [●] Common Warrants to purchase an aggregate of [●] shares of Common Stock, and each Underwriter agrees to purchase, severally and not jointly, at the Closing, the following securities of the Company:
(i) the number of shares of Common Stock (the “Closing Shares”) set forth opposite the name of such Underwriter on Schedule I hereof;
(ii) the number of Pre-Funded Warrants (the “Closing Pre-Funded Warrants”) set forth opposite the name of such Underwriter on Schedule I hereof; and
(iii) Common Warrants to purchase up to the number of Common Warrant Shares equal to [●]% of the sum of the number of Closing Shares and Closing Pre-Funded Warrants set forth opposite the name of such Underwriter on Schedule I hereof (the “Closing Common Warrants”). The Closing Common Warrants, the Closing Pre‑Funded Warrants, and the Closing Shares are collectively referred to herein as the “Closing Securities.”
(b) The aggregate purchase price for the Closing Securities purchased by such Underwriter shall equal the amount set forth opposite the name of such Underwriter on Schedule I hereto (the “Closing Purchase Price”). The combined purchase price for one Share and one Common Warrant shall be $[●] (the “Share Purchase Price”), which shall be allocated as $[●] per Share and $[●] per Comon Warrant. The combined purchase price for one Pre-Funded Warrant and one Common Warrant shall be $[•] (the “Pre-Funded Warrant Purchase Price”), which shall be allocated as $[●] per Pre-Funded Warrant and $[●] per Common Warrant.
(c) On the Closing Date, each Underwriter shall deliver or cause to be delivered to the Company, via wire transfer, immediately available funds equal to such Underwriter’s Closing Purchase Price and the Company shall deliver to, or as directed by, such Underwriter its respective Closing Securities, and the Company shall deliver the other items required pursuant to Section 2.3 deliverable at the Closing. Upon satisfaction of the covenants and conditions set forth in Sections 2.3 and 2.4, the Closing shall occur at the offices of Blank Rome or such other location as the Company and Representative shall mutually agree. The Public Securities are to be offered initially to the public at the offering price set forth on the cover page of the Prospectus (the “Offering”).
(d) The Company acknowledges and agrees that, with respect to any Notice(s) of Exercise (as defined in each of the Pre-Funded Warrants and the Common Warrants, as applicable) delivered by a Holder (as defined in each of the Pre-Funded Warrants and the Common Warrants, as applicable) on or prior to 12:00 p.m. (New York City time) on the Closing Date, which Notice(s) of Exercise may be delivered at any time after the time of execution of this Agreement, the Company shall deliver the Pre-Funded Warrant Shares or the Common Warrant Shares, as applicable, subject to such notice(s) to the Holder by 4:00 p.m. (New York City time) on the Closing Date, and the Closing Date shall be the Warrant Share Delivery Date (as defined in each of the Pre-Funded Warrants and the Common Warrants, as applicable). The Company acknowledges and agrees that the Holders are third-party beneficiaries of this covenant of the Company.
2.2 Over-Allotment Option.
(a) For the purposes of covering any over-allotments in connection with the distribution and sale of the Closing Securities, the Representative is hereby granted an option (the “Over-Allotment Option”) to purchase, in the aggregate, up to [●] shares of Common Stock (the “Option Shares”), and/or Pre-Funded Warrants to purchase up to [●] shares of Common Stock (the “Option Pre-Funded Warrants”), and Common Warrants to purchase up to the number of Common Warrant Shares equal to [•]% of the sum of the number of Option Shares and Option Pre-Funded Warrants (the “Option Common Warrants,” and, together with the Option Pre-Funded Warrants, the “Option Warrants”), which may be purchased in any combination of Option Shares and/or Option Warrants at the Share Purchase Price and/or the Pre-Funded Warrant Purchase Price, as applicable. The Option Warrants and Option Shares together are referred to herein as “Option Securities.”
(b) In connection with an exercise of the Over-Allotment Option, (a) the purchase price to be paid for the Option Shares is equal to the product of the Share Purchase Price multiplied by the number of Option Shares to be purchased, and (b) the purchase price to be paid for the Option Pre-Funded Warrants is equal to the product of the Pre-Funded Warrant Purchase Price multiplied by the number of Option Pre-Funded Warrants to be purchased (the aggregate purchase price to be paid on an Option Closing Date, the “Option Closing Purchase Price”).
(c) The Over-Allotment Option granted pursuant to this Section 2.2 may be exercised by the Representative as to all (at any time) or any part (from time to time) of the Option Securities within 45 days after the Execution Date. An Underwriter will not be under any obligation to purchase any Option Securities prior to the exercise of the Over-Allotment Option by the Representative. The Over-Allotment Option granted hereby may be exercised by the giving of oral notice to the Company from the Representative, which must be confirmed in writing by overnight mail or facsimile or other electronic transmission setting forth the number of Option Shares and/or Option Pre-Funded Warrants to be purchased and the date and time for delivery of and payment for the Option Securities (each, an “Option Closing Date”), which will not be later than two (2) full Business Days after the date of the notice or such other time as shall be agreed upon by the Company and the Representative, at the offices of Blank Rome or at such other place (including remotely by facsimile or other electronic transmission) as shall be agreed upon by the Company and the Representative. Each Option Closing Date will be as set forth in the notice. Upon exercise of the Over-Allotment Option, the Company will become obligated to convey to the Underwriters, and, subject to the terms and conditions set forth herein, the Underwriters will become obligated to purchase, the number of Option Shares and/or Option Pre-Funded Warrants specified in such notice. The Representative may cancel the Over-Allotment Option at any time prior to the expiration of the Over-Allotment Option by written notice to the Company. On each Option Closing Date, if any, each Underwriter shall deliver or cause to be delivered to the Company, via wire transfer, immediately available funds equal to such Underwriter’s Option Closing Purchase Price and the Company shall deliver to, or as directed by, such Underwriter its respective Option Shares and the Company shall deliver the other items required pursuant to Section 2.3 deliverable at the Option Closing. Upon satisfaction of the covenants and conditions set forth in Sections 2.3 and 2.4, the Option Closing shall occur at the offices of Blank Rome or such other location as the Company and Representative shall mutually agree.
2.3 Deliveries. The Company shall deliver or cause to be delivered to each Underwriter (or DTC pursuant to the Warrant Agency Agreement (as applicable)) the following:
(i) At the Closing Date, the Closing Shares and, as to each Option Closing Date, if any, the applicable Option Shares, which shares shall be delivered via The Depository Trust Company (“DTC”) system for the accounts of the several Underwriters;
(ii) At the Closing Date, the Closing Common Warrants and, as to each Option Closing Date, if any, the applicable Option Common Warrants via DTC in accordance with the Warrant Agency Agreement;
(iii) At the Closing Date, the Closing Pre-Funded Warrants and, as to each Option Closing Date, if any, the applicable Option Pre-Funded Warrants via DTC in accordance with the Warrant Agency Agreement;
(iv) At the Closing Date, a legal opinion and a negative assurance letter of Company Counsel addressed to the Underwriters, in each case in form and substance reasonably satisfactory to the Representative and as to each Option Closing Date, if any, a bring-down opinion from Company Counsel in form and substance reasonably satisfactory to the Representative, including, without limitation, a negative assurance letter, in form and substance reasonably satisfactory to the Representative;
(v) At the Closing Date, a legal opinion of the Company’s Intellectual Property Counsel addressed to the Underwriters, including, without limitation, a negative assurance letter, in form and substance reasonably satisfactory to the Representative and as to each Option Closing Date, if any, a bring-down opinion from the Company’s Intellectual Property Counsel in form and substance reasonably satisfactory to the Representative, including, without limitation, a negative assurance letter, in form and substance reasonably satisfactory to the Representative;
(vi) Contemporaneously herewith, a cold comfort letter, addressed to the Underwriters and in form and substance satisfactory in all respects to the Representative from the Company Auditor, dated as of the date of this Agreement and a bring-down letter dated as of the Closing Date and each Option Closing Date, if any;
(vii) On the Closing Date and on each Option Closing Date, if any, the duly executed and delivered Officers’ Certificate, substantially in the form required by Exhibit D attached hereto;
(viii) On the Closing Date and on each Option Closing Date, if any, the duly executed and delivered Secretary’s Certificate, substantially in the form required by Exhibit E attached hereto;
(ix) On the Closing Date, and each Option Closing Date, if any, to the Representative, or its designees, a warrant (the “Representative Warrant”) in the form attached hereto as Exhibit F, to purchase up to a number of shares of Common Stock equal to 6.0% of the shares of Common Stock and Pre-Funded Warrants issued on such Closing Date and Option Closing Date, as applicable, for the account of the Representative (or its designees), which Representative Warrant shall have an exercise price of $[●]1, subject to adjustment therein, and registered in the name of the Representative;
(x) Contemporaneously herewith, the duly executed and delivered Lock‑Up Agreements;
(xi) Contemporaneously herewith and as of the Closing Date and each Option Closing Date, a Certificate of the Chief Financial Officer of the Company, in form and substance satisfactory in all respects to the Representative; and
(xii) On or prior to the Closing Date or the Option Closing Date, if any, the Company shall have furnished to the Representative such further certificates and documents as the Representative may reasonably request.
2.4 Closing Conditions. The respective obligations of each Underwriter hereunder in connection with the Closing and each Option Closing Date are subject to the following conditions being met:
(i) the accuracy in all material respects when made and on the date in question (other than representations and warranties of the Company already qualified by materiality, which shall be true and correct in all respects) of the representations and warranties of the Company contained herein (unless as of a specific date therein);
(ii) all obligations, covenants and agreements of the Company required to be performed at or prior to the date in question shall have been performed;
(iii) the delivery by the Company of the items set forth in Section 2.3 of this Agreement;
(iv) the Registration Statement shall be effective on the date of this Agreement and at each of the Closing Date and each Option Closing Date, if any, no stop order suspending the effectiveness of the Registration Statement shall have been issued and no proceedings for that purpose shall have been instituted or shall be pending or contemplated by the Commission and any request on the part of the Commission for additional information shall have been complied with to the reasonable satisfaction of the Representative;
(v) by the Execution Date, if required by FINRA, the Underwriters shall have received clearance from FINRA as to the amount of compensation allowable or payable to the Underwriters as described in the Registration Statement;
(vi) the Closing Shares, the Warrant Shares, and the Option Shares have been approved for listing on the Trading Market;
1 155% of the price per Closing Share.
(vii) prior to and on each of the Closing Date and each Option Closing Date, if any: (i) there shall have been no material adverse change in the condition or business activities, financial or otherwise, of the Company from the latest dates as of which such condition is set forth in the Registration Statement and Prospectus; (ii) no action, suit or proceeding, at law or in equity, shall have been pending or threatened against the Company or any Affiliate of the Company before or by any court or federal or state commission, board or other administrative agency wherein an unfavorable decision, ruling or finding may materially adversely affect the business, operations, prospects or financial condition or income of the Company, except as set forth in the Registration Statement and Prospectus; (iii) no stop order shall have been issued under the Securities Act with respect to the Registration Statement and no proceedings therefor shall have been initiated or threatened by the Commission; and (iv) the Registration Statement and the Prospectus and any amendments or supplements thereto shall contain all material statements which are required to be stated therein in accordance with the Securities Act and the rules and regulations thereunder and shall conform in all material respects to the requirements of the Securities Act and the rules and regulations thereunder, and neither the Registration Statement nor the Prospectus nor any amendment or supplement thereto shall contain any untrue statement of a material fact or omit to state any material fact required to be stated therein or necessary to make the statements therein, in light of the circumstances under which they were made, not misleading; and
(viii) No action shall have been taken and no statute, rule, regulation or order shall have been enacted, adopted or issued by any federal, state or foreign governmental or regulatory authority that would, as of the Closing Date or the Option Closing Date, if any, prevent the issuance or sale of the Securities; and no injunction or order of any federal, state or foreign court shall have been issued that would, as of the Closing Date or the Option Closing Date, if any, prevent the issuance or sale of the Securities.
ARTICLE III.
REPRESENTATIONS AND WARRANTIES
3.1 Representations and Warranties of the Company. The Company represents and warrants to the Underwriters as of the Execution Date, as of the Closing Date and as of each Option Closing Date, if any, as follows:
(a) Subsidiaries. Each subsidiary of the Company (each a “Subsidiary” and collectively, the “Subsidiaries”) that is a significant subsidiary, as defined in Rule 1-02(w) of Regulation S-X of the Exchange Act (each a “Significant Subsidiary” and collectively, the “Significant Subsidiaries”), has been duly incorporated or organized and is validly existing as a corporation, limited liability company or limited partnership, as the case may be, in good standing under the law of the jurisdiction of its incorporation or organization, has corporate power and authority to own, lease and operate its properties and conduct its business as described in the Prospectus and is duly qualified as a foreign corporation, limited liability company or limited partnership, as the case may be, to transact business and is in good standing in each jurisdiction in which such qualification is required, whether by reason of the ownership or leasing of property or the conduct of business, except where the failure to so qualify would not have a Material Adverse Effect. All of the issued and outstanding capital stock of, or other ownership interests in, each such Significant Subsidiary has been duly authorized and validly issued, is fully paid and non-assessable and, except for directors’ qualifying shares, is owned by the Company, directly or through subsidiaries, free and clear of any security interest, mortgage, pledge, lien, encumbrance, claim or equity. At the date of filing with the Commission, the Company did not have any Significant Subsidiary not listed on Exhibit 21.1 to the Company’s most recent Annual Report on Form 10-K which was required to be so listed.
(b) Organization and Qualification. The Company is an entity duly incorporated or otherwise organized, validly existing and in good standing under the laws of the jurisdiction of its incorporation or organization, with the requisite power and authority to own and use its properties and assets and to carry on its business as currently conducted. The Company is not in violation nor default of any of the provisions of its respective certificate or articles of incorporation, bylaws or other organizational or charter documents. The Company is duly qualified to conduct business and is in good standing as a foreign corporation or other entity in each jurisdiction in which the nature of the business conducted or property owned by it makes such qualification necessary, except where the failure to be so qualified or in good standing, as the case may be, could not have or reasonably be expected to result in a Material Adverse Effect and no proceeding has been instituted in any such jurisdiction revoking, limiting or curtailing or seeking to revoke, limit or curtail such power and authority or qualification.
(c) Authorization; Enforcement. The Company has the requisite corporate power and authority to enter into and to consummate the transactions contemplated by this Agreement and each of the other Transaction Documents to which it is a party and otherwise to carry out its obligations hereunder and thereunder. The execution and delivery of this Agreement and each of the other Transaction Documents by the Company and the consummation by it of the transactions contemplated hereby and thereby have been duly authorized by all necessary corporate action on the part of the Company and no further action is required by the Company, the Board of Directors or the Company’s stockholders in connection herewith or therewith other than in connection with the Required Approvals. This Agreement and each other Transaction Document to which the Company is a party has been (or upon delivery will have been) duly executed by the Company and, when delivered in accordance with the terms hereof and thereof, will constitute the valid and binding obligation of the Company enforceable against the Company in accordance with its terms, except (i) as limited by general equitable principles and applicable bankruptcy, insolvency, reorganization, moratorium and other laws of general application affecting enforcement of creditors’ rights generally, (ii) as limited by laws relating to the availability of specific performance, injunctive relief or other equitable remedies and (iii) insofar as indemnification and contribution provisions may be limited by applicable law.
(d) No Conflicts. Except as set forth in the Registration Statement or the Prospectus, the execution, delivery and performance by the Company of this Agreement and the other Transaction Documents to which it is a party, the issuance and sale of the Securities, Representative Warrants and Representative Warrant Shares and the consummation by it of the transactions contemplated hereby and thereby do not and will not (i) conflict with or violate any provision of the Company’s certificate or articles of incorporation, bylaws or other organizational or charter documents, or (ii) conflict with, or constitute a default (or an event that with notice or lapse of time or both would become a default) under, result in the creation of any Lien upon any of the properties or assets of the Company, or give to others any rights of termination, amendment, anti-dilution or similar adjustments, acceleration or cancellation (with or without notice, lapse of time or both) of, any agreement, credit facility, debt or other instrument (evidencing a Company debt or otherwise) or other understanding to which the Company is a party or by which any property or asset of the Company is bound or affected, or (iii) subject to the Required Approvals, conflict with or result in a violation of any law, rule, regulation, order, judgment, injunction, decree or other restriction of any court or governmental authority to which the Company is subject (including federal and state securities laws and regulations), or by which any property or asset of the Company is bound or affected; except in the case of each of clauses (ii) and (iii), such as could not have or reasonably be expected to result in a Material Adverse Effect.
(e) Filings, Consents and Approvals. The Company is not required to obtain any consent, waiver, authorization or order of, give any notice to, or make any filing or registration with, any court or other federal, state, local or other governmental authority or other Person in connection with the execution, delivery and performance by the Company of the Transaction Documents, other than such as have been obtained or made by the Company and are in full force and effect under the Securities Act and such as may be required under applicable state securities or blue sky laws, FINRA or the Trading Market (collectively, the “Required Approvals”).
(f) Registration Statement. The Company has filed with the Commission the Registration Statement for the registration of the Securities under the Securities Act, which Registration Statement has been prepared by the Company in all material respects in conformity with the requirements of the Securities Act and the rules and regulations of the Commission under the Securities Act. The Registration Statement was declared effective by the Commission on the Execution Date. No stop order suspending the effectiveness of the Registration Statement, or the use of the Prospectus has been issued, and no proceeding for any such purpose is pending or has been initiated or, to the Company’s knowledge, is threatened by the Commission. For purposes of this Agreement, “free writing prospectus” has the meaning set forth in Rule 405 under the Securities Act. The Company will not, without the prior consent of the Representative, prepare, use or refer to, any free writing prospectus.
(g) Issuance of Securities. The Securities are duly authorized and, when issued and paid for in accordance with the applicable Transaction Documents, will be duly and validly issued, fully paid and nonassessable, free and clear of all Liens imposed by the Company. The Warrant Shares, when issued in accordance with the terms of the applicable Warrants, will be validly issued, fully paid and nonassessable, free and clear of all Liens imposed by the Company. The Company has reserved, from its duly authorized capital stock, the maximum number of shares of Common Stock issuable pursuant to this Agreement and the Warrants. The holders of the Securities, Representative Warrants and Representative Warrant Shares will not be subject to personal liability by reason of being such holders. The Securities, Representative Warrants and Representative Warrant Shares are not and will not be subject to the preemptive rights of any holders of any security of the Company or similar contractual rights granted by the Company. All corporate action required to be taken for the authorization, issuance and sale of the Securities has been duly and validly taken. The Securities conform in all material respects to all statements with respect thereto contained in the Registration Statement. The Representative Warrant and Representative Warrant Shares conform in all material respects to all statements with respect thereto contained in the Registration Statement.
(h) Capitalization. The capitalization of the Company is as set forth in the Prospectus and Registration Statement. The Company has not issued any capital stock since September 30, 2024, other than pursuant to the exercise of employee stock options or vesting of restricted stock units under the Company’s equity incentive plans, the issuance of shares of Common Stock to employees pursuant to the Company’s employee stock purchase plans, pursuant to the conversion and/or exercise of Common Stock Equivalents outstanding as of such date, or in connection with rounding up of fractional shares following a reverse stock split. No Person has any right of first refusal, preemptive right, right of participation, or any similar right to participate in the transactions contemplated by the Transaction Documents, except for such rights as have been waived. Except as a result of the purchase and sale of the Securities and as set forth in the Registration Statement and the Prospectus, there are no outstanding options, warrants, scrip rights to subscribe to, calls or commitments of any character whatsoever relating to, or securities, rights or obligations convertible into or exercisable or exchangeable for, or giving any Person any right to subscribe for or acquire, any shares of Common Stock, or contracts, commitments, understandings or arrangements by which the Company is or may become bound to issue additional shares of Common Stock or Common Stock Equivalents. Except as set forth in the Registration Statement and Prospectus, the issuance and sale of the Securities will not obligate the Company to issue shares of Common Stock or other securities to any Person (other than the Underwriters). Except as set forth in the Registration Statement or the Prospectus, there are no outstanding securities or instruments of the Company with any provision that adjusts the exercise, conversion, exchange or reset price of such security or instrument upon an issuance of securities by the Company. All of the outstanding shares of capital stock of the Company are duly authorized, validly issued, fully paid and nonassessable, have been issued in compliance with all federal and state securities laws, and none of such outstanding shares was issued in violation of any preemptive rights or similar rights to subscribe for or purchase securities. The authorized shares of the Company conform in all material respects to all statements relating thereto contained in the Registration Statement and the Prospectus. The offers and sales of the Company’s securities were at all relevant times either registered under the Securities Act and the applicable state securities or Blue Sky laws or, based in part on the representations and warranties of the purchasers, exempt from such registration requirements. No further approval or authorization of any stockholder, the Board of Directors or others is required for the issuance and sale of the Securities. Except as set forth in the Registration Statement, there are no stockholders agreements, voting agreements or other similar agreements with respect to the Company’s capital stock to which the Company is a party or, to the knowledge of the Company, between or among any of the Company’s stockholders.
(i) SEC Reports; Financial Statements. The Company has filed all reports, schedules, forms, statements and other documents required to be filed by the Company under the Exchange Act, including pursuant to Section 13(a) or 15(d) thereof, for the two years preceding the date hereof (or such shorter period as the Company was required by law or regulation to file such material) (the foregoing materials, including the exhibits thereto and documents incorporated by reference therein, being collectively referred to herein as the “SEC Reports”) on a timely basis or has received a valid extension of such time of filing and has filed any such SEC Reports prior to the expiration of any such extension, except as could not have or reasonably be expected to result in a Material Adverse Effect. As of their respective dates, the SEC Reports complied in all material respects with the requirements of the Exchange Act, as applicable, and none of the SEC Reports, when filed, contained any untrue statement of a material fact or omitted to state a material fact required to be stated therein or necessary in order to make the statements therein, in light of the circumstances under which they were made, not misleading. The financial statements of the Company included in the Registration Statement comply in all material respects with applicable accounting requirements and the rules and regulations of the Commission with respect thereto as in effect at the time of filing. Such financial statements have been prepared in accordance with United States generally accepted accounting principles applied on a consistent basis during the periods involved (“GAAP”), except as may be otherwise specified in such financial statements or the notes thereto and except that unaudited financial statements may not contain all footnotes required by GAAP, and fairly present in all material respects the financial position of the Company as of and for the dates thereof and the results of operations and cash flows for the periods then ended, subject, in the case of unaudited statements, to normal, immaterial, year-end audit adjustments. The agreements and documents described in the Registration Statement, the Prospectus and any Prospectus Supplement conform to the descriptions thereof contained therein, and there are no agreements or other documents required by the Securities Act and the rules and regulations thereunder to be described in the Registration Statement, the Prospectus or any Prospectus Supplement, or to be filed with the Commission as exhibits to the Registration Statement, that have not been so described or filed. Each agreement or other instrument (however characterized or described) to which the Company is a party or by which it is or may be bound or affected and (i) that is referred to in the Registration Statement or the Prospectus or (ii) is material to the Company’s business, has been duly authorized and validly executed by the Company, is in full force and effect in all material respects and is enforceable against the Company and, to the Company’s knowledge, the other parties thereto, in accordance with its terms, except (x) as such enforceability may be limited by bankruptcy, insolvency, reorganization or similar laws affecting creditors’ rights generally, (y) as enforceability of any indemnification or contribution provision may be limited under the federal and state securities laws, and (z) that the remedy of specific performance and injunctive and other forms of equitable relief may be subject to the equitable defenses and to the discretion of the court before which any proceeding therefore may be brought. None of such agreements or instruments has been assigned by the Company, and neither the Company nor, to the best of the Company’s knowledge, any other party is in default thereunder and, to the best of the Company’s knowledge, no event has occurred that, with the lapse of time or the giving of notice, or both, would constitute a default thereunder. To the best of the Company’s knowledge, performance by the Company of the material provisions of such agreements or instruments will not result in a violation of any existing applicable law, rule, regulation, judgment, order or decree of any governmental agency or court, domestic or foreign, having jurisdiction over the Company or any of its assets or businesses, including, without limitation, those relating to environmental laws and regulations.
(j) Material Changes; Undisclosed Events, Liabilities or Developments. Since the date of the latest audited financial statements included within the Registration Statement, except as specifically disclosed in a subsequent SEC Report filed prior to the date hereof, (i) there has been no event, occurrence or development that has had or that could reasonably be expected to result in a Material Adverse Effect, (ii) the Company has not incurred any liabilities (contingent or otherwise) other than (A) trade payables and accrued expenses incurred in the ordinary course of business consistent with past practice and (B) liabilities not required to be reflected in the Company’s financial statements pursuant to GAAP or disclosed in filings made with the Commission, (iii) the Company has not altered its method of accounting, (iv) the Company has not declared or made any dividend or distribution of cash or other property to its stockholders or purchased, redeemed or made any agreements to purchase or redeem any shares of its capital stock, (v) the Company has not issued any equity securities to any officer, director or Affiliate, except pursuant to existing Company equity incentive plans and (vi) no officer or director of the Company has resigned from any position with the Company. The Company does not have pending before the Commission any request for confidential treatment of information. Except for the issuance of the Securities contemplated by this Agreement, no event, liability, fact, circumstance, occurrence or development has occurred or exists or is reasonably expected to occur or exist with respect to the Company or its business, prospects, properties, operations, assets or financial condition that would be required to be disclosed by the Company under applicable securities laws at the time this representation is made or deemed made that has not been publicly disclosed at least one (1) Trading Day prior to the date that this representation is made. Unless otherwise disclosed in an SEC Report filed prior to the date hereof and except as disclosed to the Representative, the Company has not: (i) issued any securities (except pursuant to any employee benefit plan maintained by the Company) or incurred any liability or obligation, direct or contingent, for borrowed money; or (ii) declared or paid any dividend or made any other distribution on or in respect of its capital stock.
(k) Litigation. Except as disclosed in the Company’s SEC Reports, the Registration Statement or Prospectus, there is no action, suit, inquiry, notice of violation, proceeding or investigation pending or, to the knowledge of the Company, threatened against or affecting the Company or any of its properties before or by any court, arbitrator, governmental or administrative agency or regulatory authority (federal, state, county, local or foreign) (collectively, an “Action”) which (i) adversely affects or challenges the legality, validity or enforceability of any of the Transaction Documents or the Securities or (ii) could, if there were an unfavorable decision, have or reasonably be expected to result in a Material Adverse Effect. Except as disclosed in the Company’s SEC Reports, the Registration Statement or the Prospectus, (x) neither the Company, nor any director or officer thereof, is or has been the subject of any Action involving a claim of violation of or liability under federal or state securities laws or a claim of breach of fiduciary duty and (y) there has not been, and to the knowledge of the Company, there is not pending or contemplated, any investigation by the Commission involving the Company or any current or former director or officer of the Company. The Commission has not issued any stop order or other order suspending the effectiveness of any registration statement filed by the Company under the Exchange Act or the Securities Act.
(l) Labor Relations. No labor dispute exists or, to the knowledge of the Company, is imminent with respect to any of the employees of the Company, which could reasonably be expected to result in a Material Adverse Effect. None of the Company’s employees is a member of a union that relates to such employee’s relationship with the Company, and the Company is not a party to a collective bargaining agreement, and the Company believes that its relationships with its employees are good. To the knowledge of the Company, no executive officer of the Company is, or is now expected to be, in violation of any material term of any employment contract, confidentiality, disclosure or proprietary information agreement or non-competition agreement, or any other contract or agreement or any restrictive covenant in favor of any third party, and the continued employment of each such executive officer does not subject the Company to any liability with respect to any of the foregoing matters. The Company is in compliance with all U.S. federal, state, local and foreign laws and regulations relating to employment and employment practices, terms and conditions of employment and wages and hours, except where the failure to be in compliance could not, individually or in the aggregate, reasonably be expected to have a Material Adverse Effect.
(m) Compliance. The Company (i) is not in default under and is not in violation of (and no event has occurred that has not been waived that, with notice or lapse of time or both, would result in a default by the Company under), nor has the Company received notice of a claim that it is in default under or that it is in violation of, any indenture, loan or credit agreement or any other agreement or instrument to which it is a party or by which it or any of its properties is bound (whether or not such default or violation has been waived), (ii) is not in violation of any judgment, decree or order of any court, arbitrator or other governmental authority and (iii) is not and has not been in violation of any statute, rule, ordinance or regulation of any governmental authority, including without limitation all foreign, federal, state and local laws relating to taxes, environmental protection, occupational health and safety, product quality and safety and employment and labor matters, except in each case as could not have or reasonably be expected to result in a Material Adverse Effect.
(n) Regulatory Permits. The Company possesses all required licenses, certificates, approvals, clearances, authorizations, registrations and permits (the “Permits”) issued by, and has made all required declarations and filings with, the appropriate federal, state, local or foreign governmental or regulatory authorities that are necessary to conduct its business as described in the Registration Statement and Prospectus, except where the failure to possess such permits could not reasonably be expected to result in a Material Adverse Effect (each, a “Material Permit”), and except as disclosed in the Registration Statement or the Prospectus, the Company has not received any notice of proceedings relating to the revocation, suspension, invalidation or termination of any Material Permit. The disclosures in the Registration Statement concerning the effects of Federal, State, local and all foreign regulation on the Company’s business as currently contemplated are correct in all material respects.
(o) Title to Assets. The Company has good and marketable title in fee simple to, or has valid and marketable rights to lease or otherwise use, all real property and all personal property that is material to the business of the Company, in each case free and clear of all Liens, except for (i) Liens as do not materially affect the value of such property and do not materially interfere with the use made and proposed to be made of such property by the Company and (ii) Liens for the payment of federal, state or other taxes, for which adequate reserves have been made in accordance with GAAP, and the payment of which is neither delinquent nor subject to penalties. Any real property and facilities held under lease by the Company are held by it under valid, subsisting and enforceable leases with which the Company is in compliance in all material respects.
(p) Intellectual Property. The Company has, or has rights to use, patents, patent applications, trademarks, and/or trademark applications and may also have, or have the rights to use, tradenames, trade secrets, and/or inventions for use in connection with its business as described in the Registration Statement and Prospectus and which the failure to do so could have a Material Adverse Effect (collectively, the “Intellectual Property Rights”). None of, and the Company has not received a notice (written or otherwise) that any of, the Intellectual Property Rights has unintentionally expired, terminated or been abandoned. The Company has not received any written notice of infringement, offer to license, notice of misappropriation, or notice of conflict with any patent rights of any third person. To the knowledge of the Company, all such Intellectual Property Rights which have been issued are enforceable and there is no existing infringement by another Person of any of the Intellectual Property Rights. The Company has taken reasonable security measures to protect the secrecy, confidentiality and value of all of their intellectual properties, except where failure to do so could not, individually or in the aggregate, reasonably be expected to have a Material Adverse Effect.
(q) Insurance. The Company is insured by insurers of recognized financial responsibility against such losses and risks and in such amounts as are prudent and customary in the businesses in which the Company is engaged, including, but not limited to, directors and officers insurance coverage. The Company does not have any reason to believe that it will not be able to renew its existing insurance coverage as and when such coverage expires or to obtain similar coverage from similar insurers as may be necessary to continue its business without a significant increase in cost.
(r) Transactions With Affiliates and Employees. Except as set forth in the Registration Statement and Prospectus, none of the officers or directors of the Company and, to the knowledge of the Company, none of the employees of the Company is presently a party to any transaction with the Company (other than for services as employees, officers and directors), including any contract, agreement or other arrangement providing for the furnishing of services to or by, providing for rental of real or personal property to or from, providing for the borrowing of money from or lending of money to or otherwise requiring payments to or from, any officer, director or such employee or, to the knowledge of the Company, any entity in which any officer, director, or any such employee has a substantial interest or is an officer, director, trustee, stockholder, member or partner, in each case in excess of $120,000 other than for (i) payment of cash compensation, including salary or consulting fees, for services rendered, (ii) reimbursement for expenses incurred on behalf of the Company and (iii) other employee benefits, including equity incentive agreements under any equity incentive plan of the Company.
(s) Sarbanes-Oxley; Internal Accounting Controls. The Company is in compliance in all material respects with any and all applicable requirements of the Sarbanes-Oxley Act of 2002 that are effective as of the date hereof, and any and all applicable rules and regulations promulgated by the Commission thereunder that are effective as of the date hereof and as of the Closing Date. Except as set forth in the Registration Statement and Prospectus, the Company maintains a system of internal accounting controls sufficient to provide reasonable assurance that: (i) transactions are executed in accordance with management’s general or specific authorizations, (ii) transactions are recorded as necessary to permit preparation of financial statements in conformity with GAAP and to maintain asset accountability, (iii) access to assets is permitted only in accordance with management’s general or specific authorization, and (iv) the recorded accountability for assets is compared with the existing assets at reasonable intervals and appropriate action is taken with respect to any differences. Except as set forth in the Registration Statement and Prospectus, the Company has established disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) for the Company and designed such disclosure controls and procedures to ensure that information required to be disclosed by the Company in the reports it files or submits under the Exchange Act is recorded, processed, summarized and reported, within the time periods specified in the Commission’s rules and forms. The Company’s certifying officers have evaluated the effectiveness of the disclosure controls and procedures of the Company as of the end of the period covered by the most recently filed periodic report under the Exchange Act (such date, the “Evaluation Date”). The Company presented in its most recently filed periodic report under the Exchange Act the conclusions of the certifying officers about the effectiveness of the disclosure controls and procedures based on their evaluations as of the Evaluation Date. Since the Evaluation Date, there have been no changes in the internal control over financial reporting (as such term is defined in the Exchange Act) of the Company that have materially affected, or are reasonably likely to materially affect, the internal control over financial reporting of the Company.
(t) Certain Fees. Except as set forth in the Prospectus, no brokerage or finder’s fees or commissions are or will be payable by the Company or Affiliate of the Company to any broker, financial advisor or consultant, finder, placement agent, investment banker, bank or other Person with respect to the transactions contemplated by the Transaction Documents. To the Company’s knowledge, there are no other arrangements, agreements or understandings of the Company or, to the Company’s knowledge, any of its stockholders that may affect the Underwriters’ compensation, as determined by FINRA. The Company has not made any direct or indirect payments (in cash, securities or otherwise) to: (i) any person, as a finder’s fee, consulting fee or otherwise, in consideration of such person raising capital for the Company or introducing to the Company persons who raised or provided capital to the Company; (ii) any FINRA member; or (iii) any person or entity that has any direct or indirect affiliation or association with any FINRA member, within the twelve months prior to the Execution Date. None of the net proceeds of the Offering will be paid by the Company to any participating FINRA member or its affiliates, except as specifically authorized herein.
(u) Investment Company. The Company is not, and is not an Affiliate of, and immediately after receipt of payment for the Securities will not be or be an Affiliate of, an “investment company” within the meaning of the Investment Company Act of 1940, as amended.
(v) Registration Rights. Except as set forth in the Registration Statement and Prospectus, no Person has any right to cause the Company to effect the registration under the Securities Act of any securities of the Company.
(w) Listing and Maintenance Requirements. The Common Stock is registered pursuant to Section 12(b) of the Exchange Act, and the Company has taken no action designed to, or which to its knowledge is likely to have the effect of, terminating the registration of the Common Stock under the Exchange Act nor has the Company received any notification that the Commission is contemplating terminating such registration. Except as set forth in the Registration Statement or the Prospectus, the Company has not, in the 12 months preceding the date hereof, received notice from any Trading Market on which the Common Stock is or has been listed or quoted to the effect that the Company is not in compliance with the listing or maintenance requirements of such Trading Market. Except as set forth in the Registration Statement or the Prospectus, the Company is, and has no reason to believe that it will not in the foreseeable future continue to be, in compliance with all such listing and maintenance requirements. The Common Stock is currently eligible for electronic transfer through DTC or another established clearing corporation and the Company is current in payment of the fees of DTC (or such other established clearing corporation) in connection with such electronic transfer.
(x) Application of Takeover Protections. The Company and the Board of Directors have taken all necessary action, if any, in order to render inapplicable any control share acquisition, business combination, poison pill (including any distribution under a rights agreement) or other similar anti-takeover provision under the Company’s certificate of incorporation (or similar charter documents) or the laws of its state of incorporation that is or could become applicable as a result of the Underwriters and the Company fulfilling their obligations or exercising their rights under the Transaction Documents.
(y) Disclosure; 10b-5. The Registration Statement (and any further documents to be filed with the Commission) contains all exhibits and schedules as required by the Securities Act. Each of the Registration Statement and any post-effective amendment thereto, if any, at the time it became effective, including any information deemed to be a part thereof at the time of effectiveness pursuant to Rule 430A under the Securities Act, complied in all material respects with the Securities Act and the Exchange Act and the applicable rules and regulations under the Securities Act and did not and, as amended or supplemented, if applicable, will not, contain any untrue statement of a material fact or omit to state a material fact required to be stated therein or necessary to make the statements therein not misleading. Each of the Preliminary Prospectus and the Prospectus, as of its respective date, complies in all material respects with the Securities Act and the Exchange Act and the applicable rules and regulations. The Prospectus, as amended or supplemented, did not and will not contain as of the date thereof any untrue statement of a material fact or omit to state a material fact necessary in order to make the statements therein, in light of the circumstances under which they were made, not misleading. No post-effective amendment to the Registration Statement reflecting any facts or events arising after the date thereof which represent, individually or in the aggregate, a fundamental change in the information set forth therein is required to be filed with the Commission. There are no documents required to be filed with the Commission in connection with the transaction contemplated hereby that (x) have not been filed as required pursuant to the Securities Act or (y) will not be filed within the requisite time period. There are no contracts or other documents required to be described in the Prospectus, or to be filed as exhibits or schedules to the Registration Statement, which have not been described or filed as required.
(z) No Integrated Offering. Neither the Company, nor any of its Affiliates, nor any Person acting on its or their behalf has, directly or indirectly, made any offers or sales of any security or solicited any offers to buy any security, under circumstances that would cause this offering of the Securities to be integrated with prior offerings by the Company for purposes of any applicable shareholder approval provisions of any Trading Market on which any of the securities of the Company are listed or designated.
(aa) Solvency. Based on the consolidated financial condition of the Company as of the Closing Date, after giving effect to the receipt by the Company of the proceeds from the sale of the Securities hereunder, (i) the fair saleable value of the Company’s assets exceeds the amount that will be required to be paid on or in respect of the Company’s existing debts and other liabilities (including known contingent liabilities) as they mature, (ii) the Company’s assets do not constitute unreasonably small capital to carry on its business as now conducted and as proposed to be conducted including its capital needs taking into account the particular capital requirements of the business conducted by the Company, consolidated and projected capital requirements and capital availability thereof, and (iii) the current cash flow of the Company, together with the proceeds the Company would receive, were it to liquidate all of its assets, after taking into account all anticipated uses of the cash, would be sufficient to pay all amounts on or in respect of its liabilities when such amounts are required to be paid. The Company does not intend to incur debts beyond its ability to pay such debts as they mature (taking into account the timing and amounts of cash to be payable on or in respect of its debt). The Company has no knowledge of any facts or circumstances which lead it to believe that it will file for reorganization or liquidation under the bankruptcy or reorganization laws of any jurisdiction within one year from the Closing Date. The Registration Statement and Prospectus set forth as of the date hereof all outstanding secured and unsecured Indebtedness of the Company or for which the Company has commitments.
(bb) Tax Status. Except for matters that would not, individually or in the aggregate, have or reasonably be expected to result in a Material Adverse Effect, the Company (i) has filed all United States federal, state and local, and foreign income and franchise tax returns, reports and declarations required to be filed through the date hereof (taking into account any valid extensions), (ii) has paid all taxes and other governmental assessments and charges that are material in amount, shown to be due on such returns, reports and declarations and (iii) has set aside on its books provision reasonably adequate for the payment of all material taxes for periods subsequent to the periods to which such returns, reports or declarations apply. There are no unpaid taxes in any material amount claimed to be due by the taxing authority of any jurisdiction. The provisions for taxes payable, if any, shown on the financial statements filed with or as part of the Registration Statement are sufficient for all accrued and unpaid taxes, whether or not disputed, and for all periods to and including the dates of such consolidated financial statements. The term “taxes” mean all federal, state, local, foreign, and other net income, gross income, gross receipts, sales, use, ad valorem, transfer, franchise, profits, license, lease, service, service use, withholding, payroll, employment, excise, severance, stamp, occupation, premium, property, windfall profits, customs, duties or other taxes, fees, assessments, or charges of a similar kind of nature, together with any interest and any penalties, additions to tax, or additional amounts with respect thereto. The term “returns” means all returns, declarations, reports, statements, and other documents required to be filed in respect to taxes.
(cc) Foreign Corrupt Practices. Neither the Company, nor to the knowledge of the Company, any agent or other person acting on behalf of the Company, has (i) directly or indirectly, used any funds for unlawful contributions, gifts, entertainment or other unlawful expenses related to foreign or domestic political activity, (ii) made any unlawful payment to foreign or domestic government officials or employees or to any foreign or domestic political parties or campaigns from corporate funds, (iii) failed to disclose fully any contribution made by the Company (or made by any person acting on its behalf of which the Company is aware) which is in violation of law, or (iv) violated in any material respect any provision of FCPA. The Company has taken reasonable steps to ensure that its accounting controls and procedures are sufficient to cause the Company to comply in all material respects with the FCPA.
(dd) Accountants. To the knowledge and belief of the Company, the Company Auditor is an independent registered public accounting firm as required by the Exchange Act. The Company Auditor has not, during the periods covered by the financial statements included in the Prospectus, provided to the Company any non-audit services, as such term is used in Section 10A(g) of the Exchange Act.
(ee) Regulatory Matters. As to each product subject to the jurisdiction of the U.S. Food and Drug Administration (“FDA”) under the Federal Food, Drug and Cosmetic Act, as amended, and the regulations thereunder (“FDCA”) that is manufactured, packaged, labeled, tested, distributed, sold, and/or marketed by the Company or any of its Subsidiaries (each such product, a “Pharmaceutical Product”), such Pharmaceutical Product is being manufactured, packaged, labeled, tested, distributed, sold and/or marketed by the Company in compliance with all applicable requirements under FDCA and similar laws, rules and regulations relating to registration, investigational use, premarket clearance, licensure, or application approval, good manufacturing practices, good laboratory practices, good clinical practices, product listing, quotas, labeling, advertising, record keeping and filing of reports, except where the failure to be in compliance would not have a Material Adverse Effect. There is no pending, completed or, to the Company’s knowledge, threatened, action (including any lawsuit, arbitration, or legal or administrative or regulatory proceeding, charge, complaint, or investigation) against the Company or any of its Subsidiaries, and none of the Company or any of its Subsidiaries has received any notice, warning letter or other communication from the FDA or any other governmental entity, which (i) contests the premarket clearance, licensure, registration, or approval of, the uses of, the distribution of, the manufacturing or packaging of, the testing of, the sale of, or the labeling and promotion of any Pharmaceutical Product, (ii) withdraws its approval of, requests the recall, suspension, or seizure of, or withdraws or orders the withdrawal of advertising or sales promotional materials relating to, any Pharmaceutical Product, (iii) imposes a clinical hold on any clinical investigation by the Company or any of its Subsidiaries, (iv) enjoins production at any facility of the Company or any of its Subsidiaries, (v) enters or proposes to enter into a consent decree of permanent injunction with the Company or any of its Subsidiaries, or (vi) otherwise alleges any violation of any laws, rules or regulations by the Company or any of its Subsidiaries, and which, either individually or in the aggregate, would have a Material Adverse Effect. The properties, business and operations of the Company have been and are being conducted in all material respects in accordance with all applicable laws, rules and regulations of the FDA. The Company has not been informed by the FDA that the FDA will prohibit the marketing, sale, license or use in the United States of any product proposed to be developed, produced or marketed by the Company nor has the FDA expressed any concern as to approving or clearing for marketing any product being developed or proposed to be developed by the Company.
(ff) Office of Foreign Assets Control. Neither the Company nor, to the Company’s knowledge, any director, officer, agent, employee or affiliate of the Company is currently subject to any U.S. sanctions administered by the Office of Foreign Assets Control of the U.S. Treasury Department.
(gg) U.S. Real Property Holding Corporation. The Company is not and has never been a “United States real property holding corporation” within the meaning of Section 897(c)(2) of the Internal Revenue Code of 1986, as amended (the “Code”), during the applicable period specified in Section 897(c)(1)(A) of the Code, and the Company shall so certify upon the Representative’s request.
(hh) Bank Holding Company Act. The Company is not subject to the Bank Holding Company Act of 1956, as amended (the “BHCA”) and to regulation by the Board of Governors of the Federal Reserve System (the “Federal Reserve”). Neither the Company nor any of its Affiliates owns or controls, directly or indirectly, five percent (5%) or more of the outstanding shares of any class of voting securities or twenty-five percent (25%) or more of the total equity of a bank or any entity that is subject to the BHCA and to regulation by the Federal Reserve. Neither the Company nor any of its Affiliates exercises a controlling influence over the management or policies of a bank or any entity that is subject to the BHCA and to regulation by the Federal Reserve.
(ii) Money Laundering. The operations of the Company are and have been conducted at all times in compliance with applicable financial record-keeping and reporting requirements of the Currency and Foreign Transactions Reporting Act of 1970, as amended, applicable money laundering statutes and applicable rules and regulations thereunder (collectively, the “Money Laundering Laws”), and no action, suit or proceeding by or before any court or governmental agency, authority or body or any arbitrator involving the Company with respect to the Money Laundering Laws is pending or, to the knowledge of the Company, threatened.
(jj) D&O Questionnaires. To the Company’s knowledge, all information contained in the questionnaires completed by each of the Company’s directors and officers prior to the Offering as well as in the Lock-Up Agreement provided to the Underwriters is true and correct in all material respects and the Company has not become aware of any information which would cause the information disclosed in such questionnaires become inaccurate and incorrect.
(kk) FINRA Affiliation. No officer, director or, to the knowledge of the Company, any beneficial owner of 10% or more of the Company’s unregistered securities has any direct or indirect affiliation or association with any FINRA member (as determined in accordance with the rules and regulations of FINRA) that is participating in the Offering. The Company will advise the Representative and Blank Rome if it learns that any officer, director or owner of 10% or more of the Company’s outstanding shares of Common Stock or Common Stock Equivalents is or becomes an affiliate or associated person of a FINRA member firm.
(ll) Officers’ Certificate. Any certificate signed by any duly authorized officer of the Company and delivered to the Representative or Blank Rome shall be deemed a representation and warranty by the Company to the Underwriters as to the matters covered thereby.
(mm) Board of Directors. The Board of Directors is comprised of the persons set forth under the heading of the Prospectus captioned “Management.” The qualifications of the persons serving as board members and the overall composition of the Board of Directors comply with the Sarbanes-Oxley Act of 2002 and the rules promulgated thereunder applicable to the Company and the rules of the Trading Market. At least one member of the Board of Directors qualifies as a “financial expert” as such term is defined under the Sarbanes-Oxley Act of 2002 and the rules promulgated thereunder and the rules of the Trading Market. In addition, at least a majority of the persons serving on the Board of Directors qualify as “independent” as defined under the rules of the Trading Market.
(nn) Compliance with Privacy Laws; Cybersecurity. Except as would not reasonably be expected to have a Material Adverse Effect, the Company (i) has operated its business in a manner compliant with all United States federal, state, and local, and non-United States, privacy, data security, and data protection laws and regulations applicable to the Company’s collection, use, transfer, protection, disposal, disclosure, handling, storage, and analysis of data that is defined as “personal data” under applicable law (“Personal Data”), (ii) has been and is in compliance with its internal policies and procedures designed to ensure the integrity and security of the Personal Data collected, handled or stored by the Company in connection with its business, (iii) has been and is in compliance with its internal policies and procedures designed to ensure compliance with, to the extent applicable to the Company, health care laws that govern privacy and data security and takes, and has taken, reasonably appropriate steps designed to assure its compliance with such policies and procedures. Except as would not reasonably be expected to have a Material Adverse Effect, the Company has taken reasonable steps to maintain the confidentiality of its Personal Data, protected health information, consumer information, and other confidential information of the Company and any third parties in the possession and control of the Company (“Sensitive Company Data”). Except as would not reasonably be expected to have a Material Adverse Effect, the Company’s tangible or digital information technology systems (including computers, screens, servers, workstations, routers, hubs, switches, networks, data communications lines, technical data and hardware), software, websites, applications and telecommunications systems (the “Company IT Assets”) are adequate in capacity and operation for, in accordance with their documentation and functional specifications, the business of the Company as now operated as described in the Registration Statement and the Prospectus, free and clear of all Trojan horses, time bombs, malware, and other bugs, errors, or defects that, in each case, create material risk of a security breach with respect to such Company IT Assets or Sensitive Company Data processed thereon. Except as would not reasonably be expected to have a Material Adverse Effect, the Company has used reasonable efforts to establish, and has established, commercially reasonable disaster recovery and security plans, procedures and facilities for the business of the Company consistent with industry standards and practices, including, without limitation, for the Company IT Assets and Sensitive Company Data held or used by the Company. Except as would not reasonably be expected to have a Material Adverse Effect, (i) to the Company’s knowledge, the Company has not suffered or incurred any security breaches, compromises or incidents with respect to any Company IT Asset or Sensitive Company Data and (ii) to the Company’s knowledge, there has been no unauthorized or illegal use of, or access to, any Company IT Asset or Sensitive Company Data by any unauthorized third party. Except as would not reasonably be expected to have a Material Adverse Effect, to the Company’s knowledge, the Company has not been required to notify any individual of any information security breach, compromise or incident involving Sensitive Company Data.
(oo) Environmental Laws. The Company and its Subsidiaries (i) are in compliance with all federal, state, local and foreign laws relating to pollution or protection of human health or the environment (including ambient air, surface water, groundwater, land surface or subsurface strata), including laws relating to emissions, discharges, releases or threatened releases of chemicals, pollutants, contaminants, or toxic or hazardous substances or wastes (collectively, “Hazardous Materials”) into the environment, or otherwise relating to the manufacture, processing, distribution, use, treatment, storage, disposal, transport or handling of Hazardous Materials, as well as all authorizations, codes, decrees, demands, or demand letters, injunctions, judgments, licenses, notices or notice letters, orders, permits, plans or regulations, issued, entered, promulgated or approved thereunder (“Environmental Laws”); (ii) have received all permits licenses or other approvals required of them under applicable Environmental Laws to conduct their respective businesses; and (iii) are in compliance with all terms and conditions of any such permit, license or approval where in each clause (i), (ii) and (iii), the failure to so comply could be reasonably expected to have, individually or in the aggregate, a Material Adverse Effect.
(pp) Consents and Permits. The Company and the Subsidiaries have made all filings, applications and submissions required by, and possess and are operating in compliance in all material respects with all approvals, licenses, certificates, certifications, clearances, consents, grants, exemptions, marks, notifications, orders, permits and other authorizations issued by, the appropriate federal, state or foreign Governmental Authority (including the FDA, the United States Drug Enforcement Administration, the Centers for Medicare & Medicaid Services (“CMS”), or any other foreign, federal, state, provincial or local Government Authorities engaged in the regulation of clinical trials, pharmaceuticals, biologics or biohazardous substances or materials) necessary for the ownership or lease of their respective properties or to conduct their businesses as described in the Registration Statement or the Prospectus (collectively, “Permits”), except for such Permits the failure of which to possess, obtain or file would not reasonably be expected to have, individually or in the aggregate, a Material Adverse Effect; the Company and the Subsidiaries are in compliance with the terms and conditions of all such Permits, except where the failure to be in compliance would not reasonably be expected to have, individually or in the aggregate, a Material Adverse Effect; all of the Permits are valid and in full force and effect, except where any invalidity would not reasonably be expected to have, individually or in the aggregate, a Material Adverse Effect; and neither the Company nor any of the Subsidiaries has received any notice relating to the limitation, revocation, cancellation, suspension, modification or non-renewal of any such Permit which, if the subject of an unfavorable decision, ruling or finding, would reasonably be expected to have, individually or in the aggregate, a Material Adverse Effect, or has any reason to believe that any such license, certificate, permit or authorization will not be renewed in the ordinary course. To the extent required by applicable Laws of the FDA, the Company or the applicable Subsidiary has submitted to the FDA an Investigational Device Exemption Application or amendment or supplement thereto for each clinical trial it has conducted or sponsored or is conducting or sponsoring; all such submissions were in material compliance with applicable Laws when submitted and no material deficiencies have been asserted by the FDA with respect to any such submissions. The Company and each Subsidiary possess such valid and current certificates, authorizations or permits issued by the appropriate state, federal or foreign regulatory agencies or bodies necessary to conduct their respective businesses, and neither the Company nor any Subsidiary has received, or has any reason to believe that it will receive, any notice of proceedings relating to the revocation or modification of, or non-compliance with, any such certificate, authorization or permit which, if the subject of an unfavorable decision, ruling or finding, would reasonably be expected to have, individually or in the aggregate, a Material Adverse Effect. The Company has not applied for “approved enterprise”, “benefited enterprise” or “preferred enterprise” status with respect to any of the Company’s facilities or operations.
(qq) Regulatory Filings. Neither the Company nor any of the Subsidiaries has failed to file with the applicable Governmental Authority (including the FDA, or any foreign, federal, state, provincial or local Governmental Authority performing functions similar to those performed by the FDA) any required filing, declaration, listing, registration, report or submission, except for such failures that would not reasonably be expected to have, individually or in the aggregate, a Material Adverse Effect. All such filings, declarations, listings, registrations, reports or submissions were in substantial compliance with applicable Laws when filed and no deficiencies have been asserted by any applicable regulatory authority with respect to any such filings, declarations, listings, registrations, reports or submissions, except for any deficiencies that would not reasonably be expected to have, individually or in the aggregate, a Material Adverse Effect. The Company has operated and currently is, in all material respects, in compliance with the United States Federal Food, Drug, and Cosmetic Act, and all applicable rules and regulations of the FDA and other federal, state, local and foreign Governmental Authority exercising comparable authority. The Company has no knowledge of any studies, tests or trials not described in the Registration Statement, and the Prospectus the results of which reasonably call into question in any material respect the results of the studies, tests and trials described in the Registration Statement or the Prospectus.
(rr) Health Care Laws. The Company and, to the Company’s knowledge, its directors, officers, employees, and agents (while acting in such capacity) are in compliance with all health care laws and regulations applicable to the Company or any of its product candidates or activities, including development and testing of medical devices and pharmaceutical products, kickbacks, recordkeeping, documentation requirements, the hiring of employees, quality, safety, privacy, security, licensure, accreditation or any other aspect of developing and testing medical devices or pharmaceutical products (collectively, “Health Care Laws”), except where such noncompliance would not, individually or in the aggregate, have a Material Adverse Effect. The Company has not received any notification, correspondence or any other written or oral communication, including notification of any pending or threatened claim, suit, proceeding, hearing, enforcement, investigation, arbitration or other action from any governmental or regulatory authority or third party, including, without limitation, the FDA, CMS, the U.S. Department of Health and Human Services Office of Inspector General or Officer of Civil Rights, of potential or actual non-compliance by, or liability of, the Company under any Health Care Laws, except for any such communication (i) that asserts any such non-compliance that would not reasonably be expected to have a Material Adverse Effect or (ii) that the Company believes has been resolved to the satisfaction of such governmental or regulatory authority or third party as of the date hereof. No such claim, action, suit, proceeding, hearing, enforcement, investigation, arbitration or other action is pending or, to the Company’s knowledge, threatened. The Company has not received any FDA Form 483, notice including a determination of adverse finding by the FDA, warning letter, untitled letter or other correspondence or notice from the FDA or any other Governmental Authority alleging or asserting noncompliance with any Health Care Laws or any licenses, certificates, approvals, clearances, authorizations, permits and supplements or amendments thereto required by any such Health Care Laws (“Authorizations”), except for any such letter or other correspondence or notice (A) that asserts any such non-compliance that would not reasonably be expected to have a Material Adverse Effect or (B) that the Company believes has been resolved to the satisfaction of the FDA or other such Governmental Authority as of the date hereof. The Company reasonably believes it is not a “covered entity”, as defined under the Health Insurance Portability and Accountability Act of 1996, as amended (“HIPAA”) and has not received any requests or demands from a covered entity or governmental authority to make available its internal practices, books, and/or records relating to its use and disclosure of health information for purposes of determining a covered entity’s or the Company’s compliance with HIPAA or with other applicable privacy law.
(ss) Clinical Studies. The preclinical studies and tests and clinical trials described in the Registration Statement or the Prospectus were, and, if still pending, are being conducted in all material respects in accordance with the experimental protocols, procedures and controls pursuant to, where applicable, accepted professional and scientific standards for products or product candidates comparable to those being developed by the Company; the descriptions of such studies, tests and trials, and the results thereof, contained in the Registration Statement or the Prospectus are accurate and complete in all material respects; the Company is not aware of any tests, studies or trials not described in the Registration Statement and the Prospectus, the results of which reasonably call into question in any material respect the results of the tests, studies and trials described in the Registration Statement or the Prospectus; and the Company has not received any written notice or correspondence from the FDA or any foreign, state or local Governmental Authority exercising comparable authority or any institutional review board or comparable authority requiring the termination, suspension, clinical hold or material modification of any tests, studies or trials described in the Registration Statement and/or the Prospectus.
ARTICLE IV.
OTHER AGREEMENTS OF THE PARTIES
4.1 Amendments to Registration Statement. The Company has delivered, or will as promptly as practicable deliver, or made available, to the Underwriters complete conformed copies of the Registration Statement and of each consent and certificate of experts, as applicable, filed as a part thereof, and conformed copies of the Registration Statement (without exhibits), the Prospectus and the Prospectus Supplement, if any, as amended or supplemented, in such quantities and at such places as an Underwriter reasonably requests. Neither the Company nor any of its directors and officers has distributed and none of them will distribute, prior to the Closing Date, any offering material in connection with the offering and sale of the Securities other than the Prospectus, the Prospectus Supplement, if any, the Registration Statement, any Permitted Free Writing Prospectus. The Company shall not file any such amendment or supplement to which the Representative shall reasonably object in writing.
4.2 Federal Securities Laws.
(a) Compliance. During the time when a Prospectus is required to be delivered under the Securities Act, the Company will use its best efforts to comply with all requirements imposed upon it by the Securities Act and the rules and regulations thereunder and the Exchange Act and the rules and regulations thereunder, as from time to time in force, so far as necessary to permit the continuance of sales of or dealings in the Securities in accordance with the provisions hereof and the Prospectus. If at any time when a Prospectus relating to the Securities is required to be delivered under the Securities Act, any event shall have occurred as a result of which, in the opinion of counsel for the Company or counsel for the Underwriters, the Prospectus, as then amended or supplemented, includes an untrue statement of a material fact or omits to state any material fact required to be stated therein or necessary to make the statements therein, in light of the circumstances under which they were made, not misleading, or if it is necessary at any time to amend the Prospectus to comply with the Securities Act, the Company will notify the Underwriters promptly and prepare and file with the Commission, subject to Section 4.1 hereof, an appropriate amendment or supplement in accordance with Section 10 of the Securities Act.
(b) Filing of Final Prospectus. The Company will file the final Prospectus (in form and substance satisfactory to the Representative) with the Commission pursuant to the requirements of Rule 424.
(c) Exchange Act Registration. For a period of three years from the Execution Date, the Company will use its best efforts to maintain the registration of the Common Stock under the Exchange Act. The Company will not deregister the Common Stock under the Exchange Act without the prior written consent of the Representative.
(d) Free Writing Prospectuses. The Company represents and agrees that it has not made and will not make any offer relating to the Securities that would constitute an issuer free writing prospectus, as defined in Rule 433 of the rules and regulations under the Securities Act, without the prior written consent of the Representative.
4.3 Delivery to the Underwriters of Prospectuses. The Company will deliver to the Underwriters, without charge, from time to time during the period when the Prospectus is required to be delivered under the Securities Act or the Exchange Act such number of copies of each Prospectus as the Underwriters may reasonably request and, as soon as the Registration Statement or any amendment or supplement thereto becomes effective, deliver to you two original executed Registration Statements, including exhibits, and all post-effective amendments thereto and copies of all exhibits filed therewith or incorporated therein by reference and all original executed consents of certified experts.
4.4 Effectiveness and Events Requiring Notice to the Underwriters. The Company will use its best efforts to cause the Registration Statement to remain effective with a current prospectus until the later of nine (9) months from the Execution Date and the date on which the Warrants are no longer outstanding, and will notify the Underwriters and holders of the Warrants immediately and confirm the notice in writing: (i) of the effectiveness of the Registration Statement and any amendment thereto, provided that the filing of an amendment to the Registration Statement on the Commission’s EDGAR system, and the posting of the notice of effectiveness on EDGAR, shall be deemed to be such notification; (ii) of the issuance by the Commission of any stop order or of the initiation, or the threatening, of any proceeding for that purpose; (iii) of the issuance by any state securities commission of any proceedings for the suspension of the qualification of the Securities for offering or sale in any jurisdiction or of the initiation, or the threatening, of any proceeding for that purpose; (iv) of the mailing and delivery to the Commission for filing of any amendment or supplement to the Registration Statement or Prospectus, provided that the filing of an amendment or supplement to the Registration Statement on the Commission’s EDGAR system shall be deemed to be such notification; (v) of the receipt of any comments or request for any additional information from the Commission with respect to the Registration Statement; and (vi) of the happening of any event during the period described in this Section 4.4 that, in the judgment of the Company, causes the Registration Statement or the Prospectus, as the case may be, to include an untrue statement of material fact or omit to state a material fact necessary in order to make the statements therein, under the circumstances in which they were made, not misleading. If the Commission or any state securities commission shall enter a stop order or suspend such qualification at any time, the Company will make every reasonable effort to obtain promptly the lifting of such order.
4.5 Expenses of the Offering.
(a) General Expenses Related to the Offering. The Company hereby agrees to pay on each of the Closing Date and each Option Closing Date, if any, to the extent not paid at the Closing Date, all expenses incident to the performance of the obligations of the Company under this Agreement, including, but not limited to: (a) all filing fees and communication expenses relating to the registration of the Securities to be sold in the Offering (including the Option Securities) with the Commission; (b) all FINRA Public Offering Filing System fees associated with the review of the Offering by FINRA; all fees and expenses relating to the listing of the Closing Shares, Option Shares, and Warrant Shares on the Trading Market and such other stock exchanges as the Company and the Representative together determine; (c) all fees, expenses and disbursements relating to the registration or qualification of such Securities under the “blue sky” securities laws of such states and other foreign jurisdictions as the Representative may reasonably designate; (d) the costs of all mailing and printing of the underwriting documents, Registration Statements, Prospectuses and all amendments, supplements and exhibits thereto and as many preliminary and final Prospectuses as the Representative may reasonably deem necessary; (e) the costs and expenses of the Company’s public relations firm; (f) the costs of preparing, printing and delivering the Securities; (g) fees and expenses of the Transfer Agent for the Securities (including, without limitation, any fees required for same-day processing of any instruction letter delivered by the Company), including, without limitation, fees and expenses of the Company’s warrant agent, including pursuant to the Warrant Agency Agreement; (h) the fees and expenses of the Company’s accountants; and (i) the fees and expenses of the Company’s legal counsel and other agents and representatives.
(b) Expenses. The Company further agrees that, in addition to the expenses payable pursuant to Section 4.5(a), on the Closing Date it will reimburse the Representative for its reasonable and documented out-of-pocket expenses related to the Offering in an amount up to $115,000 in the aggregate, consisting of pre-closing expenses of up to $30,000 (as further detailed in Section 7.1(b)) and closing expenses of up to $85,000, which shall be paid by deduction from the proceeds of the Offering contemplated herein. It is understood, however, that, except as provided in this Section, Section 7.1(b), and Article VI hereof, the Representative and the Underwriters will pay all of their own costs and expenses, including the fees of their counsel, and stock transfer taxes on resale of any of the Public Securities.
4.6 Application of Net Proceeds. The Company will apply the net proceeds from the Offering received by it in a manner consistent with the application described under the caption “Use of Proceeds” in the Prospectus.
4.7 Delivery of Earnings Statements to Security Holders. The Company will make generally available to its security holders as soon as practicable, but not later than the first day of the fifteenth full calendar month following the Execution Date, an earnings statement (which need not be certified by independent public or independent certified public accountants unless required by the Securities Act or the Rules and Regulations under the Securities Act, but which shall satisfy the provisions of Rule 158(a) under Section 11(a) of the Securities Act) covering a period of at least twelve consecutive months beginning after the Execution Date. For the avoidance of doubt, documents filed with the Commission pursuant to its EDGAR system that contain the earning statements required by this Section 4.7 shall be deemed to have been delivered to security holders and satisfy the requirements of this Section 4.7.
4.8 Stabilization. Neither the Company, nor, to its knowledge, any of its employees, directors or shareholders (without the consent of the Representative) has taken or will take, directly or indirectly, any action designed to or that has constituted or that might reasonably be expected to cause or result in, under the Exchange Act, or otherwise, stabilization or manipulation of the price of any security of the Company to facilitate the sale or resale of the Securities.
4.9 Internal Controls. The Company will maintain a system of internal accounting controls sufficient to provide reasonable assurances that: (i) transactions are executed in accordance with management’s general or specific authorization; (ii) transactions are recorded as necessary in order to permit preparation of financial statements in accordance with GAAP and to maintain accountability for assets; (iii) access to assets is permitted only in accordance with management’s general or specific authorization; and (iv) the recorded accountability for assets is compared with existing assets at reasonable intervals and appropriate action is taken with respect to any differences.
4.10 Accountants. The Company shall continue to retain a nationally recognized independent certified public accounting firm for a period of at least three years after the Execution Date. The Underwriters acknowledge that the Company Auditor is acceptable to the Underwriters.
4.11 FINRA. The Company shall advise the Underwriters (who shall make an appropriate filing with FINRA) if it is aware that any 5% or greater shareholder of the Company becomes an affiliate or associated person of an Underwriter.
4.12 No Fiduciary Duties. The Company acknowledges and agrees that the Underwriters’ responsibility to the Company is solely contractual and commercial in nature, based on arms-length negotiations and that neither the Underwriters nor their affiliates or any selected dealer shall be deemed to be acting in a fiduciary capacity, or otherwise owes any fiduciary duty to the Company or any of its affiliates in connection with the Offering and the other transactions contemplated by this Agreement. Notwithstanding anything in this Agreement to the contrary, the Company acknowledges that the Underwriters may have financial interests in the success of the Offering that are not limited to the difference between the price to the public and the purchase price paid to the Company by the Underwriters for the Securities and the Underwriters have no obligation to disclose, or account to the Company for, any of such additional financial interests. The Company hereby waives and releases, to the fullest extent permitted by law, any claims that the Company may have against the Underwriters with respect to any breach or alleged breach of fiduciary duty.
4.13 Warrant Shares. If all or any portion of a Warrant is exercised at a time when there is an effective registration statement to cover the issuance of the Warrant Shares, or if the Warrant is exercised via cashless exercise at a time when such Warrant Shares would be eligible for resale under Rule 144 by a non-affiliate of the Company, the Warrant Shares issued pursuant to any such exercise shall be issued free of all restrictive legends. If at any time following the date hereof, the Registration Statement (or any subsequent registration statement registering the sale or resale of the Warrant Shares) is not effective or is not otherwise available for the sale of the Warrant Shares, the Company shall immediately notify the holders of the Warrants in writing that such registration statement is not then effective and thereafter shall promptly notify such holders when the registration statement is effective again and available for the sale of the Warrant Shares (it being understood and agreed that the foregoing shall not limit the ability of the Company to issue, or any holder thereof to sell, any of the Warrant Shares in compliance with applicable federal and state securities laws).
4.14 Board Composition and Board Designations. The Company shall ensure that: (i) the qualifications of the persons serving as board members and the overall composition of the Board of Directors comply with the Sarbanes-Oxley Act of 2002 and the rules promulgated thereunder and with the listing requirements of the Trading Market and (ii) if applicable, at least one member of the Board of Directors qualifies as a “financial expert” as such term is defined under the Sarbanes-Oxley Act of 2002 and the rules promulgated thereunder.
4.15 Securities Laws Disclosure; Publicity. By 9:00 a.m. (New York City time) on the day following the Execution Date, the Company shall issue a press release disclosing the material terms of the Offering. The Company and the Representative shall consult with each other in issuing any other press releases with respect to the Offering, and neither the Company nor any Underwriter shall issue any such press release nor otherwise make any such public statement without the prior consent of the Company, with respect to any press release of such Underwriter, or without the prior consent of such Underwriter, with respect to any press release of the Company, which consent shall not unreasonably be withheld or delayed, except if such disclosure is required by law, in which case the disclosing party shall promptly provide the other party with prior notice of such public statement or communication.
4.16 Shareholder Rights Plan. No claim will be made or enforced by the Company or, with the consent of the Company, any other Person, that any Underwriter of the Securities is an “Acquiring Person” under any control share acquisition, business combination, poison pill (including any distribution under a rights agreement) or similar anti-takeover plan or arrangement in effect or hereafter adopted by the Company, or that any Underwriter of Securities could be deemed to trigger the provisions of any such plan or arrangement, by virtue of receiving Securities.
4.17 Reservation of Common Stock. As of the date hereof, the Company has reserved and the Company shall continue to reserve and keep available at all times, free of preemptive rights, a sufficient number of shares of Common Stock for the purpose of enabling the Company to issue the Option Securities pursuant to the Over-Allotment Option and the Warrant Shares pursuant to any exercise of any Warrant.
4.18 Listing of Common Stock. The Company hereby agrees to use best efforts to maintain the listing or quotation of the Common Stock on the Trading Market on which it is currently listed, and concurrently with the Closing, the Company shall apply to list or quote all of the Closing Shares, Option Shares and Warrant Shares on such Trading Market and promptly secure the listing of all of the Closing Shares, Option Shares and Warrant Shares on such Trading Market. The Company further agrees, if the Company applies to have the Common Stock traded on any other Trading Market, it will then include in such application all of the Closing Shares, Option Shares and Warrant Shares, and will take such other action as is necessary to cause all of the Closing Shares, Option Shares and Warrant Shares to be listed or quoted on such other Trading Market as promptly as possible. The Company will then take all action reasonably necessary to continue the listing and trading of its Common Stock on a Trading Market and will comply in all respects with the Company’s reporting, filing and other obligations under the bylaws or rules of the Trading Market.
4.19 Investment Company Act. The Company shall continue to conduct its business in a manner so that it will not become an “investment company” subject to registration under the Investment Company Act of 1940, as amended.
4.20 Subsequent Equity Sales Approval.
(a) From the date hereof until 90 days following the Closing Date, the Company shall not issue, enter into any agreement to issue or announce the issuance or proposed issuance of any shares of Common Stock or Common Stock Equivalents, except Exempt Issuances.
(b) From the date hereof until the one year anniversary of the Closing Date, the Company shall be prohibited from effecting or entering into an agreement to effect any issuance by the Company or any of its Subsidiaries of Common Stock or Common Stock Equivalents (or a combination of units thereof) involving a Variable Rate Transaction. “Variable Rate Transaction” means a transaction in which the Company (i) issues or sells any debt or equity securities that are convertible into, exchangeable or exercisable for, or include the right to receive, additional shares of Common Stock either (A) at a conversion price, exercise price or exchange rate or other price that is based upon, and/or varies with, the trading prices of or quotations for the shares of Common Stock at any time after the initial issuance of such debt or equity securities or (B) with a conversion, exercise or exchange price that is subject to being reset at some future date after the initial issuance of such debt or equity security or upon the occurrence of specified or contingent events directly or indirectly related to the business of the Company or the market for the Common Stock or (ii) enters into, or effects a transaction under, any agreement, including, but not limited to, an equity line of credit, whereby the Company may issue securities at a future determined price. Any Underwriter shall be entitled to obtain injunctive relief against the Company to preclude any such issuance, which remedy shall be in addition to any right to collect damages. Notwithstanding the foregoing, the Company shall be permitted to enter into, or effect a transaction under, an at-the-market offering after the 90-day anniversary of the Closing Date.
(c) Notwithstanding the foregoing, this Section 4.20 shall not apply in respect of an Exempt Issuance, except that no Variable Rate Transaction shall be an Exempt Issuance.
4.21 Research Independence. The Company acknowledges that each Underwriter’s research analysts and research departments, if any, are required to be independent from their respective investment banking divisions and are subject to certain regulations and internal policies, and that such Underwriter’s research analysts may hold and make statements or investment recommendations and/or publish research reports with respect to the Company and/or the offering that differ from the views of its investment bankers. The Company hereby waives and releases, to the fullest extent permitted by law, any claims that the Company may have against such Underwriter with respect to any conflict of interest that may arise from the fact that the views expressed by their independent research analysts and research departments may be different from or inconsistent with the views or advice communicated to the Company by such Underwriter’s investment banking divisions. The Company acknowledges that the Representative is a full-service securities firm and as such from time to time, subject to applicable securities laws, may effect transactions for its own account or the account of its customers and hold long or short position in debt or equity securities of the Company.
4.22 Right of First Refusal. The Company hereby grants to the Representative a right of first refusal for a period of nine (9) months following the closing of this offering the opportunity to participate as a sole bookrunner or exclusive placement agent or exclusive sales agent in respect of any future financing by the Company on terms and conditions mutually acceptable to the Company and the Representative. The Representative may decline such participation interest in its sole and absolute discretion and will notify the Company as to its decision as to whether to participate no later than the fifth business day following notification of such proposed financing. The terms of such engagements shall be set forth in separate agreements and may be subject to, among other things, satisfactory completion of due diligence by the Representative, market conditions, the absence of adverse change to the Company’s business or financial condition, approval of the Representative’s internal committee and any other conditions that the Representative may reasonably deem appropriate for transactions of such nature. The Company will notify the Representative in writing of its intention to pursue such further financing, and the Representative will advise the Company promptly of the Representative’s election to participate in such financing (but in no event no later than five (5) business days following the Company’s notice to the Representative). If such proposed financing is not accepted by the Representative, but later materially modified as to the scope and nature of the proposed financing, the Company will re-submit such then proposed financing in writing to the Representative and the Representative will be subject to the same five (5) business day notice provision to advise of its election to participate in the proposed financing. The Representative’s election not to participate with respect to a particular proposed financing will not adversely affect its rights hereunder with respect to any other proposed financing of the Company during the period referred to above.
4.23 Fee Obligation. Within nine (9) months after the date of termination or expiration of the engagement agreement between the Company and the Representative dated May 21, 2024, the securities or securities convertible into or exchangeable for the securities that are sold by the Company to investors contacted by the Representative, then the Company shall pay to the Representative, at the time of each such sale or transaction, the fees set forth in this Agreement with respect to any such sale.
ARTICLE V.
DEFAULT BY UNDERWRITERS
If on the Closing Date or any Option Closing Date, if any, any Underwriter shall fail to purchase and pay for the portion of the Closing Securities or Option Securities, as the case may be, which such Underwriter has agreed to purchase and pay for on such date (otherwise than by reason of any default on the part of the Company), the Representative, or if the Representative is the defaulting Underwriter, the non-defaulting Underwriters, shall use their reasonable efforts to procure within 36 hours thereafter one or more of the other Underwriters, or any others, to purchase from the Company such amounts as may be agreed upon and upon the terms set forth herein, the Closing Securities or Option Securities, as the case may be, which the defaulting Underwriter or Underwriters failed to purchase. If during such 36 hours the Representative shall not have procured such other Underwriters, or any others, to purchase the Closing Securities or Option Securities, as the case may be, agreed to be purchased by the defaulting Underwriter or Underwriters, then (a) if the aggregate number of Closing Securities or Option Securities, as the case may be, with respect to which such default shall occur does not exceed 10% of the Closing Securities or Option Securities, as the case may be, covered hereby, the other Underwriters shall be obligated, severally, in proportion to the respective numbers of Closing Securities or Option Securities, as the case may be, which they are obligated to purchase hereunder, to purchase the Closing Securities or Option Securities, as the case may be, which such defaulting Underwriter or Underwriters failed to purchase, or (b) if the aggregate number of Closing Securities or Option Securities, as the case may be, with respect to which such default shall occur exceeds 10% of the Closing Securities or Option Securities, as the case may be, covered hereby, the Company or the Representative will have the right to terminate this Agreement without liability on the part of the non-defaulting Underwriters or of the Company except to the extent provided in Article VI hereof. In the event of a default by any Underwriter or Underwriters, as set forth in this Article V, the applicable Closing Date may be postponed for such period, not exceeding seven days, as the Representative, or if the Representative is the defaulting Underwriter, the non-defaulting Underwriters, may determine in order that the required changes in the Prospectus or in any other documents or arrangements may be effected. The term “Underwriter” includes any person substituted for a defaulting Underwriter. Any action taken under this Section shall not relieve any defaulting Underwriter from liability in respect of any default of such Underwriter under this Agreement.
ARTICLE VI.
INDEMNIFICATION
6.1 Indemnification of the Underwriters. Subject to the conditions set forth below, the Company agrees to indemnify and hold harmless the Underwriters, and each dealer selected by each Underwriter that participates in the offer and sale of the Securities (each a “Selected Dealer”) and each of their respective directors, officers and employees and each Person, if any, who controls such Underwriter or any Selected Dealer (“Controlling Person”) within the meaning of Section 15 of the Securities Act or Section 20 of the Exchange Act, against any and all loss, liability, claim, damage and expense whatsoever (including but not limited to any and all legal or other expenses reasonably incurred in investigating, preparing or defending against any litigation, commenced or threatened, or any claim whatsoever, whether arising out of any action between such Underwriter and the Company or between such Underwriter and any third party or otherwise) to which they or any of them may become subject under the Securities Act, the Exchange Act or any other statute or at common law or otherwise or under the laws of foreign countries, arising out of or based upon any untrue statement or alleged untrue statement of a material fact contained in (i) any Preliminary Prospectus, if any, the Registration Statement or the Prospectus (as from time to time each may be amended and supplemented); (ii) any materials or information provided to investors by, or with the approval of, the Company in connection with the marketing of the offering of the Securities, including any “road show” or investor presentations made to investors by the Company (whether in person or electronically); or (iii) any application or other document or written communication (in this Article VI, collectively called “application”) executed by the Company or based upon written information furnished by the Company in any jurisdiction in order to qualify the Securities under the securities laws thereof or filed with the Commission, any state securities commission or agency, Trading Market or any securities exchange; or the omission or alleged omission therefrom of a material fact required to be stated therein or necessary to make the statements therein, in light of the circumstances under which they were made, not misleading, unless such statement or omission was made in reliance upon and in conformity with written information furnished to the Company with respect to the applicable Underwriter by or on behalf of such Underwriter expressly for use in any Preliminary Prospectus, if any, the Registration Statement or Prospectus, or any amendment or supplement thereto, or in any application, as the case may be; provided, that the foregoing indemnity agreement shall not apply to any loss, claim, damage, liability or expense to the extent, but only to the extent, arising out of or based upon any untrue statement or alleged untrue statement or omission or alleged omission made in reliance upon and in conformity with information relating to any Underwriter Information (as defined below). With respect to any untrue statement or omission or alleged untrue statement or omission made in the Preliminary Prospectus, if any, the indemnity agreement contained in this Section 6.1 shall not inure to the benefit of an Underwriter to the extent that any loss, liability, claim, damage or expense of such Underwriter results from the fact that a copy of the Prospectus was not given or sent to the Person asserting any such loss, liability, claim or damage at or prior to the written confirmation of sale of the Securities to such Person as required by the Securities Act and the rules and regulations thereunder, and if the untrue statement or omission has been corrected in the Prospectus, unless such failure to deliver the Prospectus was a result of non-compliance by the Company with its obligations under this Agreement. The Company agrees promptly to notify each Underwriter of the commencement of any litigation or proceedings against the Company or any of its officers, directors or Controlling Persons in connection with the issue and sale of the Securities or in connection with the Registration Statement or Prospectus.
6.2 Procedure. If any action is brought against an Underwriter, a Selected Dealer or a Controlling Person in respect of which indemnity may be sought against the Company pursuant to Section 6.1, such Underwriter, such Selected Dealer or Controlling Person, as the case may be, shall promptly notify the Company in writing of the institution of such action and the Company shall assume the defense of such action, including the employment and fees of counsel (subject to the reasonable approval of such Underwriter or such Selected Dealer, as the case may be) and payment of actual expenses. Such Underwriter, such Selected Dealer or Controlling Person shall have the right to employ its or their own counsel in any such case, but the fees and expenses of such counsel shall be at the expense of such Underwriter, such Selected Dealer or Controlling Person unless (i) the employment of such counsel at the expense of the Company shall have been authorized in writing by the Company in connection with the defense of such action, or (ii) the Company shall not have employed counsel to have charge of the defense of such action, or (iii) such indemnified party or parties shall have reasonably concluded that there may be defenses available to it or them which are different from or additional to those available to the Company (in which case the Company shall not have the right to direct the defense of such action on behalf of the indemnified party or parties), in any of which events the reasonable fees and expenses of not more than one additional firm of attorneys selected by such Underwriter (in addition to local counsel), Selected Dealer and/or Controlling Person shall be borne by the Company. Notwithstanding anything to the contrary contained herein, if any Underwriter, Selected Dealer or Controlling Person shall assume the defense of such action as provided above, the Company shall have the right to approve the terms of any settlement of such action which approval shall not be unreasonably withheld.
6.3 Indemnification of the Company. Each Underwriter severally and not jointly agrees to indemnify and hold harmless the Company, each of its directors, each of its officers who signed the Registration Statement, and each person, if any, who controls the Company within the meaning of Section 15 of the Securities Act or Section 20 of the Exchange Act against any and all loss, liability, claim, damage and expense described in the foregoing indemnity from the Company to such Underwriter, as incurred, but only with respect to untrue statements or omissions, or alleged untrue statements or omissions made in any Preliminary Prospectus, if any, the Registration Statement or Prospectus or any amendment or supplement thereto or in any application, in reliance upon, and in strict conformity with, written information furnished to the Company with respect to such Underwriter by or on behalf of such Underwriter expressly for use in such Preliminary Prospectus, if any, the Registration Statement or Prospectus or any amendment or supplement thereto or in any such application. The parties acknowledge and agree such information consists solely of the following disclosure in the “Underwriting” section of the Prospectus; the last sentence of the third paragraph, the sentence constituting the sixth paragraph and the disclosure under “Stabilization, Short Positions and Penalty Bids” (the “Underwriter Information”). In case any action shall be brought against the Company or any other Person so indemnified based on any Preliminary Prospectus, if any, the Registration Statement or Prospectus or any amendment or supplement thereto or any application, and in respect of which indemnity may be sought against such Underwriter, such Underwriter shall have the rights and duties given to the Company, and the Company and each other Person so indemnified shall have the rights and duties given to such Underwriter by the provisions of this Article VI. Notwithstanding the provisions of this Section 6.3, no Underwriter shall be required to indemnify the Company for any amount in excess of the underwriting discounts and commissions applicable to the Securities purchased by such Underwriter. The Underwriters’ obligations in this Section 6.3 to indemnify the Company are several in proportion to their respective underwriting obligations and not joint.
6.4 Contribution.
(a) Contribution Rights. In order to provide for just and equitable contribution under the Securities Act in any case in which (i) any Person entitled to indemnification under this Article VI makes a claim for indemnification pursuant hereto but it is judicially determined (by the entry of a final judgment or decree by a court of competent jurisdiction and the expiration of time to appeal or the denial of the last right of appeal) that such indemnification may not be enforced in such case notwithstanding the fact that this Article VI provides for indemnification in such case, or (ii) contribution under the Securities Act, the Exchange Act or otherwise may be required on the part of any such Person in circumstances for which indemnification is provided under this Article VI, then, and in each such case, the Company and each Underwriter, severally and not jointly, shall contribute to the aggregate losses, liabilities, claims, damages and expenses of the nature contemplated by said indemnity agreement incurred by the Company and such Underwriter, as incurred, in such proportions as is appropriate to reflect the relative benefits received by the Company, on the one hand, and the Underwriters, on the other hand, from the offering of the Securities pursuant to this Agreement or (ii) if the allocation provided by clause (i) above is not permitted by applicable law, in such proportion as is appropriate to reflect not only the relative benefits referred to in clause (i) above but also the relative fault of the Company, on the one hand, and the Underwriters, on the other hand, in connection with the statements or omissions which resulted in such losses, claims, damages, liabilities or expenses, as well as any other relevant equitable considerations. The relative benefits received by the Company, on the one hand, and the Underwriters, on the other hand, in connection with the offering of the Securities pursuant to this Agreement shall be deemed to be in the same respective proportions as the total proceeds from the offering of the Securities pursuant to this Agreement (before deducting expenses) received by the Company, and the total underwriting discounts and commissions received by the Underwriters, in each case as set forth on the front cover page of the Prospectus, bear to the aggregate initial public offering price of the Securities as set forth on such cover. The relative fault of the Company, on the one hand, and the Underwriters, on the other hand, shall be determined by reference to, among other things, whether any such untrue or alleged untrue statement of a material fact or omission or alleged omission to state a material fact relates to information supplied by the Company, on the one hand, or the Underwriters, on the other hand, and the parties’ relative intent, knowledge, access to information and opportunity to correct or prevent such statement or omission. Notwithstanding the foregoing, no Person liable of a fraudulent misrepresentation (within the meaning of Section 11(f) of the Securities Act) shall be entitled to contribution from any Person who was not liable of such fraudulent misrepresentation. For purposes of this Section, each director, officer and employee of such Underwriter or the Company, as applicable, and each Person, if any, who controls such Underwriter or the Company, as applicable, within the meaning of Section 15 of the Securities Act shall have the same rights to contribution as such Underwriter or the Company, as applicable. Notwithstanding the provisions of this Section 6.4, no Underwriter shall be required to contribute any amount in excess of the underwriting discounts and commissions applicable to the Securities purchased by such Underwriter. The Underwriters’ obligations in this Section 6.4 to contribute are several in proportion to their respective underwriting obligations and not joint.
ARTICLE VII.
MISCELLANEOUS
7.1 Termination.
(a) Termination Right. The Representative shall have the right to terminate this Agreement at any time prior to any Closing Date, by notice given to the Company: (i) if any domestic or international event or act or occurrence has materially disrupted, or in the Representative’s reasonable opinion will in the immediate future materially disrupt, general securities markets in the United States; or (ii) if trading on any Trading Market shall have been suspended or materially limited, or minimum or maximum prices for trading shall have been fixed, or maximum ranges for prices for securities shall have been required by FINRA or by order of the Commission or any other government authority having jurisdiction, or (iii) if the United States shall have become involved in a new war or a substantial increase in major hostilities, as in the Representative’s reasonable judgment is material and adverse and will make it impracticable to market the Securities in the manner and on the terms described in the Preliminary Prospectus or the Prospectus or to enforce contracts for the sale of securities, or (iv) if a general banking moratorium has been declared by a New York State or federal authority, (v) a moratorium on foreign exchange trading has been declared with materially adversely impacts the United States securities markets, (vi) if the Company shall have sustained a material loss by fire, flood, accident, hurricane, earthquake, theft, sabotage or other calamity or malicious act which, whether or not such loss shall have been insured, will, in the Representative’s reasonable opinion, make it inadvisable to proceed with the delivery of the Securities, (vii) if the Company is in material breach of any of its representations, warranties or covenants hereunder, or (viii) if in the reasonable judgment of the Representative there shall have occurred any Material Adverse Effect.
(b) Expenses. In the event this Agreement shall be terminated pursuant to Section 7.1(a), within the time specified herein or any extensions thereof pursuant to the terms herein, the Company shall be obligated to pay to the Representative its actual and accountable out of pocket expenses related to the transactions contemplated herein then due and payable, including the fees and disbursements of Blank Rome; provided, however, that such expenses shall not exceed $30,000 in the aggregate (provided, further, however, that such expense cap in no way limits or impairs the indemnification and contribution provisions of this Agreement).
(c) Indemnification. Notwithstanding any contrary provision contained in this Agreement, any election hereunder or any termination of this Agreement, and whether or not this Agreement is otherwise carried out, the provisions of Article VI shall not be in any way effected by such election or termination or failure to carry out the terms of this Agreement or any part hereof.
7.2 Entire Agreement. The Transaction Documents, together with the exhibits and schedules thereto, the Prospectus and the Prospectus Supplement, if any, contain the entire understanding of the parties with respect to the subject matter hereof and thereof and supersede all prior agreements and understandings, oral or written, with respect to such matters, which the parties acknowledge have been merged into such documents, exhibits and schedules. Notwithstanding anything herein to the contrary, the Investment Banking Agreement, dated December 30, 2021 (the “December Engagement”), between the Company and the Representative, shall continue to be effective during its term and the terms therein, including, without limitation, Section 4(b) and Section 5 with respect to the December Engagement with respect to any future offerings, shall continue to survive and be enforceable by the Representative in accordance with its terms, provided that, in the event of a conflict between the terms and conditions of this Agreement and the December Engagement, the terms and conditions of this Agreement shall control.
7.3 Notices. Any and all notices or other communications or deliveries required or permitted to be provided hereunder shall be in writing and shall be deemed given and effective on the earliest of: (a) the time of transmission, if such notice or communication is delivered via facsimile at the facsimile number or e-mail attachment at the e-mail address set forth on the signature pages attached hereto at or prior to 5:30 p.m. (New York City time) on a Trading Day, (b) the next Trading Day after the time of transmission, if such notice or communication is delivered via facsimile at the facsimile number or e-mail attachment at the e-mail address as set forth on the signature pages attached hereto on a day that is not a Trading Day or later than 5:30 p.m. (New York City time) on any Trading Day, (c) the second (2nd) Trading Day following the date of mailing, if sent by U.S. nationally recognized overnight courier service or (d) upon actual receipt by the party to whom such notice is required to be given. The address for such notices and communications shall be as set forth on the signature pages attached hereto.
7.4 Amendments; Waivers. No provision of this Agreement may be waived, modified, supplemented or amended except in a written instrument signed, in the case of an amendment, by the Company and the Representative. No waiver of any default with respect to any provision, condition or requirement of this Agreement shall be deemed to be a continuing waiver in the future or a waiver of any subsequent default or a waiver of any other provision, condition or requirement hereof, nor shall any delay or omission of any party to exercise any right hereunder in any manner impair the exercise of any such right.
7.5 Headings. The headings herein are for convenience only, do not constitute a part of this Agreement and shall not be deemed to limit or affect any of the provisions hereof.
7.6 Successors and Assigns. This Agreement shall be binding upon and inure to the benefit of the parties and their successors and permitted assigns.
7.7 Governing Law. All questions concerning the construction, validity, enforcement and interpretation of the Transaction Documents shall be governed by and construed and enforced in accordance with the internal laws of the State of New York, without regard to the principles of conflicts of law thereof. Each party agrees that all legal proceedings concerning the interpretations, enforcement and defense of the transactions contemplated by this Agreement and any other Transaction Documents (whether brought against a party hereto or its respective affiliates, directors, officers, shareholders, partners, members, employees or agents) shall be commenced exclusively in the state and federal courts sitting in the City of New York. Each party hereby irrevocably submits to the exclusive jurisdiction of the state and federal courts sitting in the City of New York, Borough of Manhattan for the adjudication of any dispute hereunder or in connection herewith or with any transaction contemplated hereby or discussed herein (including with respect to the enforcement of any of the Transaction Documents), and hereby irrevocably waives, and agrees not to assert in any action, suit or proceeding, any claim that it is not personally subject to the jurisdiction of any such court, that such suit, action or proceeding is improper or is an inconvenient venue for such proceeding. Each party hereby irrevocably waives personal service of process and consents to process being served in any such suit, action or proceeding by mailing a copy thereof via registered or certified mail or overnight delivery (with evidence of delivery) to such party at the address in effect for notices to it under this Agreement and agrees that such service shall constitute good and sufficient service of process and notice thereof. Nothing contained herein shall be deemed to limit in any way any right to serve process in any other manner permitted by law. If either party shall commence an action or proceeding to enforce any provisions of the Transaction Documents, then, in addition to the obligations of the Company under Article VI, the prevailing party in such action, suit or proceeding shall be reimbursed by the other party for its reasonable attorneys’ fees and other costs and expenses incurred with the investigation, preparation and prosecution of such action or proceeding.
7.8 Survival. The representations and warranties contained herein shall survive the Closing and the Option Closing, if any, and the delivery of the Securities.
7.9 Execution. This Agreement may be executed in two or more counterparts, all of which when taken together shall be considered one and the same agreement and shall become effective when counterparts have been signed by each party and delivered to each other party, it being understood that the parties need not sign the same counterpart. In the event that any signature is delivered by facsimile transmission or by e-mail delivery (including a “.pdf” format data file or any electronic signature complying with the U.S. federal ESIGN Act of 2000, e.g., www.docusign.com or www.echosign.com) or by other transmission method, such signature shall create a valid and binding obligation of the party executing (or on whose behalf such signature is executed) with the same force and effect as if such facsimile or “.pdf” signature page or electronic signature were an original thereof.
7.10 Severability. If any term, provision, covenant or restriction of this Agreement is held by a court of competent jurisdiction to be invalid, illegal, void or unenforceable, the remainder of the terms, provisions, covenants and restrictions set forth herein shall remain in full force and effect and shall in no way be affected, impaired or invalidated, and the parties hereto shall use their commercially reasonable efforts to find and employ an alternative means to achieve the same or substantially the same result as that contemplated by such term, provision, covenant or restriction. It is hereby stipulated and declared to be the intention of the parties that they would have executed the remaining terms, provisions, covenants and restrictions without including any of such that may be hereafter declared invalid, illegal, void or unenforceable.
7.11 Saturdays, Sundays, Holidays, etc. If the last or appointed day for the taking of any action or the expiration of any right required or granted herein shall not be a Business Day, then such action may be taken, or such right may be exercised on the next succeeding Business Day.
7.12 Construction. The parties agree that each of them and/or their respective counsel have reviewed and had an opportunity to revise the Transaction Documents and, therefore, the normal rule of construction to the effect that any ambiguities are to be resolved against the drafting party shall not be employed in the interpretation of the Transaction Documents or any amendments thereto. In addition, each and every reference to share prices and shares of Common Stock in any Transaction Document shall be subject to adjustment for reverse and forward stock splits, stock dividends, stock combinations and other similar transactions of the Common Stock that occur after the date of this Agreement.
7.13 WAIVER OF JURY TRIAL. IN ANY ACTION, SUIT, OR PROCEEDING IN ANY JURISDICTION BROUGHT BY ANY PARTY AGAINST ANY OTHER PARTY ARISING OUT OF OR RELATING TO THIS AGREEMENT OR THE TRANSACTIONS CONTEMPLATED HEREBY, THE PARTIES EACH KNOWINGLY AND INTENTIONALLY, TO THE GREATEST EXTENT PERMITTED BY APPLICABLE LAW, HEREBY ABSOLUTELY, UNCONDITIONALLY, IRREVOCABLY AND EXPRESSLY WAIVE FOREVER ANY RIGHT TO TRIAL BY JURY.
(Signature Pages Follow)
If the foregoing correctly sets forth the understanding between the Underwriters and the Company, please so indicate in the space provided below for that purpose, whereupon this letter shall constitute a binding agreement among the Company and the several Underwriters in accordance with its terms.
|
|
Very truly yours,
AUTONOMIX MEDICAL, INC. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
By: |
|
|
|
|
|
Name: Lori Bisson |
|
|
|
|
Title: Chief Executive Officer |
|
|
Accepted on the date first above written.
LADENBURG THALMANN & CO. INC. |
Address for Notice:
Autonomix Medical, Inc.
Copy to:
ArentFox Schiff LLP |
||
| By: | Ladenburg Thalmann & Co. Inc. | ||
| By: | |||
| Name: Nicholas Stergis | |||
| Title: Managing Director | |||
Address for Notice:
640 Fifth Avenue, 4th Floor
New York, NY 10019
Attn: General Counsel
Copy to:
Blank Rome LLP
1271 Avenue of the Americas
New York, NY 10020
E-mail: leslie.marlow@blankrome.com
Attention: Leslie Marlow
[Signature Page to Underwriting Agreement]
SCHEDULE I
SCHEDULE OF UNDERWRITERS
|
Underwriters |
Closing Shares |
Closing Pre-Funded |
Closing Warrants |
Closing Purchase Price |
||||
|
Ladenburg Thalmann & Co. Inc. |
[•] |
[•] |
[•] |
$[•] |
||||
|
Total |
[•] |
[•] |
[•] |
$[•] |
EXHIBIT A
Form of Lock-Up Agreement
[•], 2024
Ladenburg Thalmann & Co. Inc.,
acting as representative of the several underwriters
named in the Underwriting Agreement
|
Re: |
Underwriting Agreement, dated [•], 2024, by and between Autonomix Medical, Inc., and Ladenburg Thalmann & Co. Inc., acting as representative of the several underwriters |
Ladies and Gentlemen:
The undersigned irrevocably agrees with the Representative (as defined below) that, from the date hereof until 90 days following the date of the Underwriting Agreement (the “Underwriting Agreement”) entered into by and between Autonomix Medical, Inc., (the “Company”) and Ladenburg Thalmann & Co. Inc., acting as the representative (the “Representative”) of the underwriters listed on Schedule I thereto (the “Underwriters”) (such period, the “Restriction Period”), the undersigned will not offer, sell, contract to sell, hypothecate, pledge or otherwise dispose of (or enter into any transaction which is designed to, or might reasonably be expected to, result in the disposition (whether by actual disposition or effective economic disposition due to cash settlement or otherwise) by the undersigned or any Affiliate (as defined in the Underwriting Agreement) of the undersigned or any person in privity with the undersigned or any Affiliate of the undersigned), directly or indirectly, or establish or increase a put equivalent position or liquidate or decrease a call equivalent position within the meaning of Section 16 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), with respect to, any shares of common stock of the Company or securities convertible, exchangeable or exercisable into, shares of common stock of the Company beneficially owned, held or hereafter acquired by the undersigned (the “Securities”).
Beneficial ownership shall be calculated in accordance with Section 13(d) of the Exchange Act. In order to enforce this covenant, to the extent the Securities are not currently subject to a restrictive legend, the Company shall impose irrevocable stop-transfer instructions preventing the transfer agent of the Company from effecting any actions in violation of this letter agreement. The Representative may consent to an early release from the Restriction Period if, in its sole and absolute discretion, the market for the Securities would not be adversely impacted by sales and in cases of financial emergency. The restrictions contained in this letter agreement shall not apply to the Securities to be sold pursuant to the Underwriting Agreement on behalf of the undersigned, if any.
The foregoing restrictions shall not apply to:
(a) transfers of Securities (i) if the undersigned is a natural person, by bona fide gift, will or intestate succession; (ii) to the spouse, domestic partner, father, mother, siblings, aunts, uncles, nieces and nephews and lineal descendants and ancestors (each, an “immediate family member”) of the undersigned or to a trust formed for the benefit of an immediate family member; (iii) if the undersigned is a corporation, partnership or other business entity (x) to another corporation, partnership or other business entity that controls, is controlled by or is under common control with the undersigned, provided that such transfer is not for value, or (y) as part of a disposition, transfer or distribution to limited partners, stockholders, equity holders or members of the undersigned, provided that such transfer is not for value; or (iv) if the undersigned is a trust, to a trustee or beneficiary of the trust in a transaction not involving a disposition of value; provided that in the case of any transfer or distribution pursuant to this clause (a), (A) each recipient shall sign and deliver a lock-up letter substantially in the form of this letter and (B) no filing under Section 16(a) of the Exchange Act, reporting a reduction in beneficial ownership of Securities, shall be required or voluntarily made during the Restriction Period, other than reports on Form 5;
(b) the transfer of Securities to the Company upon a vesting event of the Securities or upon the exercise of Securities, in each case on a “cashless” or “net exercise” basis or to cover tax withholding obligations of the undersigned in connection with such vesting or exercise, so long as such “cashless exercise” or “net exercise” is effected solely by the surrender of Securities to the Company and the Company’s cancellation of all or a portion thereof to pay the exercise price and/or withholding tax and remittance obligations; provided that no filing under Section 16(a) of the Exchange Act or any other public filing or disclosure of such receipt or transfer, shall be voluntarily made by or on behalf of the undersigned and any filing required under Section 16(a) of the Exchange Act shall clearly indicate in the footnotes thereto that (i) the filing relates to the circumstances described in this clause (b), and (ii) no shares were sold by the reporting person;
(c) the exercise for cash of the Securities granted under a stock incentive plan or otherwise described in the prospectus relating to the public offering contemplated by the Underwriting Agreement (the “Public Offering”); provided, that the underlying Securities continue to be subject to the restrictions set forth in this letter agreement, and that no filing under Section 16(a) of the Exchange Act or any other public filing or disclosure of such receipt or transfer, shall be voluntarily made by or on behalf of the undersigned, and any filing required under Section 16(a) of the Exchange Act shall clearly indicate in the footnotes thereto that (i) the filing relates to the circumstances described in this clause (c), (ii) the shares received upon exercise of the option are subject to a lock-up agreement with the Underwriters of the Public Offering, and (iii) no shares were sold by the reporting person;
(d) the establishment of a trading plan pursuant to Rule 10b5-1 under the Exchange Act for the transfer of Securities; provided that (i) such plan does not provide for the transfer of Securities during the Restriction Period and (ii) to the extent a public announcement or filing under the Exchange Act, if any, is required of or voluntarily made by or on behalf of the undersigned or the Company regarding the establishment of such plan, such announcement or filing shall include a statement to the effect that no transfer of Securities may be made under such plan during the Restriction Period;
(e) the transfer of Securities to the Company, pursuant to agreements under which the Company has the option to repurchase such shares in connection with the undersigned’s termination of employment with the Company or a right of first refusal with respect to transfers of such shares, provided that any related filing, if any, under Section 16(a) of the Exchange Act shall clearly indicate in the footnotes thereto that the filing relates to the circumstances described in this clause (e);
(f) the transfer of Securities pursuant to a qualified domestic order, in connection with a divorce settlement, provided that each of the Company and the undersigned shall make best efforts to ensure that each transferee shall sign and deliver a lock up letter substantially in the form of this letter and no filing under Section 16(a) of the Exchange Act shall be made by or on behalf of the undersigned during the Restriction Period, unless such filing clearly indicates in the footnotes thereto that such transfer occurred by operation of law, pursuant to a qualified domestic order or in connection with a divorce settlement; or
(g) the transfer of Securities pursuant to a bona fide third party tender offer, merger, consolidation or other similar transaction made to all holders of the Securities involving a “change of control” (as defined below) of the Company occurring after the consummation of the Public Offering, that has been approved by the board of directors of the Company; provided, that if the tender offer, merger, consolidation or other such transaction is not completed, the Securities owned by the undersigned shall remain subject to the restrictions contained in this letter agreement. For purposes of this clause (g), “change of control” means the consummation of any bona fide third party tender offer, merger, consolidation or other similar transaction the result of which is that any “person” (as defined in Section 13(d)(3) of the Exchange Act), or group of persons, other than the Company, becomes the beneficial owner (as defined in Rules 13d-3 and 13d-5 of the Exchange Act) of more than 50% of total voting power of the voting stock of the Company.
The Representative hereby consents to receipt of this letter agreement in electronic form and understand and agree that this letter agreement may be signed electronically. If any signature is delivered by facsimile transmission, electronic mail or otherwise by electronic transmission evidencing an intent to sign this letter agreement (including any electronic signature complying with the U.S. federal ESIGN Act of 2000, e.g., www.docusign.com), such facsimile transmission, electronic mail or other electronic transmission shall create a valid and binding obligation of the undersigned with the same force and effect as if such signature were an original. Execution and delivery of this letter agreement by facsimile transmission, electronic mail or other electronic transmission is legal, valid and binding for all purposes.
The undersigned acknowledges that the execution, delivery and performance of this letter agreement is a material inducement to the Representative to perform under the Underwriting Agreement and that the Representative (which shall be a third party beneficiary of this letter agreement) and the Company shall be entitled to specific performance of the undersigned’s obligations hereunder. The undersigned hereby represents that the undersigned has the power and authority to execute, deliver and perform this letter agreement, that the undersigned has received adequate consideration therefor and that the undersigned will indirectly benefit from the closing of the transactions contemplated by the Underwriting Agreement.
Whether or not the Public Offering actually occurs depends on a number of factors, including market conditions. Any public offering will only be made pursuant to the Underwriting Agreement, the terms of which are subject to negotiation between the Company and the Representative.
This letter agreement may not be amended or otherwise modified in any respect without the written consent of each of the Company, the Representative and the undersigned. This letter agreement shall be construed and enforced in accordance with the laws of the State of New York without regard to the principles of conflict of laws. The undersigned hereby irrevocably submits to the exclusive jurisdiction of the United States District Court sitting in the Southern District of New York and the courts of the State of New York located in Manhattan, for the purposes of any suit, action or proceeding arising out of or relating to this letter agreement, and hereby waives, and agrees not to assert in any such suit, action or proceeding, any claim that (i) it is not personally subject to the jurisdiction of such court, (ii) the suit, action or proceeding is brought in an inconvenient forum, or (iii) the venue of the suit, action or proceeding is improper. The undersigned hereby irrevocably waives personal service of process and consents to process being served in any such suit, action or proceeding by receiving a copy thereof sent to the Company at the address in effect for notices to it under the Underwriting Agreement and agrees that such service shall constitute good and sufficient service of process and notice thereof. The undersigned hereby waives any right to a trial by jury. Nothing contained herein shall be deemed to limit in any way any right to serve process in any manner permitted by law. The undersigned agrees and understands that this letter agreement does not intend to create any relationship between the undersigned and each Underwriter and that no issuance or sale of the Securities is created or intended by virtue of this letter agreement.
In furtherance of the foregoing, the Company and its transfer agent and registrar are hereby authorized to (a) decline to make any transfer of Securities if such transfer would constitute a violation or breach of this Agreement and (b) place legends and stop transfer instructions on any such Securities owned or beneficially owned by the undersigned.
This letter agreement shall be binding on successors and assigns of the undersigned with respect to the Securities and any such successor or assign shall enter into a similar agreement for the benefit of the Underwriters.
*** SIGNATURE PAGE FOLLOWS***
This letter agreement may be executed in two or more counterparts, all of which when taken together may be considered one and the same agreement.
Signature: __________________
Print Name: _____________________
Position in Company, if any
Address for Notice:
____________________
____________________
____________________
____________________
Number of shares of Common Stock
Number of shares of Common Stock underlying subject to warrants, options, debentures or other convertible securities
By signing below, the Company agrees to enforce the restrictions on transfer set forth in this letter agreement.
|
AUTONOMIX MEDICAL, INC. |
||
|
By: |
||
|
Name: |
||
|
Title: |
||
EXHIBIT B
Form of Pre-Funded Warrant
EXHIBIT C
Form of Common Warrant
EXHIBIT D
Officers’ Certificate
EXHIBIT E
Secretary’s Certificate
EXHIBIT F
Form of Representative Warrant
EXHIBIT G
Form of Warrant Agency Agreement
Exhibit 4.3
PRE-FUNDED COMMON STOCK PURCHASE WARRANT
AUTONOMIX MEDICAL, INC.
|
Warrant Shares: ______ |
Issue Date: [●], 2024 |
THIS PRE-FUNDED COMMON STOCK PURCHASE WARRANT (the “Warrant”) certifies that, for value received, CEDE & CO. or its assigns (the “Holder”) is entitled, upon the terms and subject to the limitations on exercise and the conditions hereinafter set forth, at any time on or after the date hereof (the “Initial Exercise Date”) and until this Warrant is exercised in full (the “Termination Date”) but not thereafter, to subscribe for and purchase from AUTONOMIX MEDICAL, INC., a Delaware corporation (the “Company”), up to ______ shares (as subject to adjustment hereunder, the “Warrant Shares”) of the Company’s Common Stock. The purchase price of one share of Common Stock under this Warrant shall be equal to the Exercise Price, as defined in Section 2(b). This Warrant shall initially be issued and maintained in the form of a security held in book-entry form and the Depository Trust Company or its nominee (“DTC”) shall initially be the sole registered holder of this Warrant, subject to a Holder’s right to elect to receive a Warrant in certificated form pursuant to the terms of the Warrant Agency Agreement, in which case this sentence shall not apply.
Section 1. Definitions. Capitalized terms used and not otherwise defined herein shall have the meanings set forth in that certain Underwriting Agreement (the “Underwriting Agreement”), dated [●], 2024, by and between the Company and Ladenburg Thalmann & Co. Inc., as the Representative of the several underwriters named therein.
Section 2. Exercise.
|
a) |
Exercise of Warrant. Subject to the provisions of Section 2(e) herein, exercise of the purchase rights represented by this Warrant may be made, in whole or in part, at any time or times on or after the Initial Exercise Date and before the Termination Date, by delivery to the Company and Warrant Agent (or such other office or agency of the Company as it may designate by notice in writing to the registered Holder at the address of the Holder appearing on the books of the Company) of a duly executed PDF copy submitted by e-mail (or e-mail attachment) of the Notice of Exercise in the form annexed hereto as Annex A (the “Notice of Exercise”). The Holder shall deliver the aggregate Exercise Price for the Warrant Shares specified in the applicable Notice of Exercise by wire transfer of immediately available funds or cashier’s check drawn on a United States bank within the time period specified for the delivery of the Exercise Price by Section 2(d)(i) below unless the cashless exercise procedure specified in Section 2(c) below is specified in the applicable Notice of Exercise. Neither the Company nor the Warrant Agent shall have any obligation to inquire with respect to or otherwise confirm the authenticity of the signature(s) contained on any Notice of Exercise nor the authority of the person so executing the same. For the avoidance of doubt, any reference to cashless exercise herein shall include a reference to alternative cashless exercise (as defined herein). No ink-original Notice of Exercise shall be required, nor shall any medallion guarantee (or other type of guarantee or notarization) of any Notice of Exercise be required unless the Warrants are evidenced by definitive certificates. Notwithstanding anything herein to the contrary, the Holder shall not be required to physically surrender this Warrant to the Company or Warrant Agent until the Holder has purchased all of the Warrant Shares available hereunder and the Warrant has been exercised in full, in which case, the Holder shall surrender this Warrant to the Company or Warrant Agent for cancellation within three (3) Trading Days of the date on which the final Notice of Exercise is delivered to the Company. Partial exercises of this Warrant resulting in purchases of a portion of the total number of Warrant Shares available hereunder shall have the effect of lowering the outstanding number of Warrant Shares purchasable hereunder in an amount equal to the applicable number of Warrant Shares purchased. The Holder and the Warrant Agent shall maintain records showing the number of Warrant Shares purchased and the date of such purchases. The Company or Warrant Agent shall deliver any objection to any Notice of Exercise within one (1) Trading Day of receipt of such notice. The Holder and any assignee, by acceptance of this Warrant, acknowledge and agree that, by reason of the provisions of this paragraph, following the purchase of a portion of the Warrant Shares hereunder, the number of Warrant Shares available for purchase hereunder at any given time may be less than the amount stated on the face hereof. |
Notwithstanding the foregoing in this Section 2(a), a holder whose interest in this Warrant is a beneficial interest in certificate(s) representing this Warrant held in book-entry form through DTC (or another established clearing corporation performing similar functions), shall effect exercises made pursuant to this Section 2(a) by delivering to DTC (or such other clearing corporation, as applicable) the appropriate instruction form for exercise, complying with the procedures to effect exercise that are required by DTC (or such other clearing corporation, as applicable), subject to a Holder’s right to elect to receive a Warrant in certificated form pursuant to the terms of the Warrant Agency Agreement, in which case this sentence shall not apply. Notwithstanding anything to the contrary contained herein, a beneficial holder of the Warrant shall have all of the rights and remedies of the Holder hereunder.
|
b) |
Exercise Price. The aggregate exercise price of this Warrant, except for a nominal exercise price of $0.001 per Warrant Share, was pre-funded to the Company on or prior to the Initial Exercise Date and, consequently, no additional consideration (other than the nominal exercise price of $0.001 per Warrant Share) shall be required to be paid by the Holder to any Person to effect any exercise of this Warrant. The Holder shall not be entitled to the return or refund of all, or any portion, of such pre-paid aggregate exercise price under any circumstance or for any reason whatsoever, including in the event this Warrant shall not have been exercised prior to the Termination Date. The remaining unpaid exercise price per share of Common Stock under this Warrant shall be $0.001, subject to adjustment hereunder (the “Exercise Price”). |
|
c) |
Cashless Exercise. This Warrant may also be exercised, in whole or in part, at such time by means of a “cashless exercise” in which the Holder shall be entitled to receive a number of Warrant Shares equal to the quotient obtained by dividing [(A-B) (X)] by (A), where: |
|
(A) = |
as applicable: (i) the VWAP on the Trading Day immediately preceding the date of the applicable Notice of Exercise if such Notice of Exercise is (1) both executed and delivered pursuant to Section 2(a) hereof on a day that is not a Trading Day or (2) both executed and delivered pursuant to Section 2(a) hereof on a Trading Day prior to the opening of “regular trading hours” (as defined in Rule 600(b)(68) of Regulation NMS promulgated under the federal securities laws) on such Trading Day, (ii) the VWAP on the Trading Day immediately preceding the date of the applicable Notice of Exercise or (iii) the VWAP on the date of the applicable Notice of Exercise if the date of such Notice of Exercise is a Trading Day and such Notice of Exercise is both executed and delivered pursuant to Section 2(a) hereof after the close of “regular trading hours” on such Trading Day; |
|
(B) = |
the Exercise Price of this Warrant, as adjusted hereunder; and |
|
(X) = |
the number of Warrant Shares that would be issuable upon exercise of this Warrant in accordance with the terms of this Warrant if such exercise were by means of a cash exercise rather than a cashless exercise. |
“Bid Price” means, for any date, the price determined by the first of the following clauses that applies: (a) if the Common Stock is then listed or quoted on a Trading Market, the bid price of the Common Stock for the time in question (or the nearest preceding date) on the Trading Market on which the Common Stock is then listed or quoted as reported by Bloomberg L.P. (based on a Trading Day from 9:30 a.m. (New York City time) to 4:02 p.m. (New York City time)), (b) if the Common Stock is not then listed or quoted on a Trading Market and if prices for the Common Stock are then reported on OTCQB or OTCQX, as applicable, the volume weighted average price of the Common Stock for such date (or the nearest preceding date) on OTCQB or OTCQX, as applicable, (c) if the Common Stock is not then listed or quoted for trading on a Trading Market or on OTCQB or OTCQX and if prices for the Common Stock are then reported on The Pink Open Market (or a similar organization or agency succeeding to its functions of reporting prices), the most recent bid price per share of the Common Stock so reported, or (d) in all other cases, the fair market value of a share of Common Stock as determined by an independent appraiser selected in good faith by the Holders of a majority in interest of the Securities then outstanding and reasonably acceptable to the Company, the fees and expenses of which shall be paid by the Company.
“VWAP” means, for any date, the price determined by the first of the following clauses that applies: (a) if the Common Stock is then listed or quoted on a Trading Market, the daily volume weighted average price of the Common Stock for such date (or the nearest preceding date) on the Trading Market on which the Common Stock is then listed or quoted as reported by Bloomberg L.P. (based on a Trading Day from 9:30 a.m. (New York City time) to 4:02 p.m. (New York City time)), (b) if the Common Stock is not then listed or quoted on a Trading Market and if prices for the Common Stock are then reported on OTCQB or OTCQX, as applicable, the volume weighted average price of the Common Stock for such date (or the nearest preceding date) on OTCQB or OTCQX as applicable, (c) if the Common Stock is not then listed or quoted for trading on a Trading Market or on OTCQB or OTCQX and if prices for the Common Stock are then reported on The Pink Open Market (or a similar organization or agency succeeding to its functions of reporting prices), the most recent bid price per share of the Common Stock so reported, or (d) in all other cases, the fair market value of a share of Common Stock as determined by an independent appraiser selected in good faith by the Holders of a majority in interest of the Securities then outstanding and reasonably acceptable to the Company, the fees and expenses of which shall be paid by the Company.
If Warrant Shares are issued in such a cashless exercise, the parties acknowledge and agree that in accordance with Section 3(a)(9) of the Securities Act, the Warrant Shares shall take on the registered characteristics of the Warrants being exercised. The Company agrees not to take any position contrary to this Section 2(c).
|
d) |
Mechanics of Exercise. |
i. Delivery of Warrant Shares Upon Exercise. The Company shall cause the Warrant Shares purchased hereunder to be transmitted by the Transfer Agent to the Holder by crediting the account of the Holder’s or its designee’s balance account with The Depository Trust Company through its Deposit or Withdrawal at Custodian system (“DWAC”) if the Company is then a participant in such system and either (A) there is an effective registration statement permitting the issuance of the Warrant Shares or (B) this Warrant is being exercised via cashless exercise, and otherwise by physical delivery of a certificate, registered in the Company’s share register in the name of the Holder or its designee, for the number of Warrant Shares to which the Holder is entitled pursuant to such exercise to the address specified by the Holder in the Notice of Exercise by the date that is the earlier of (A) the earlier of (i) one (1) Trading Day and (ii) the number of days comprising the Standard Settlement Period, in each case after the delivery to the Company of the Notice of Exercise and (B) one (1) Trading Day after delivery of the aggregate Exercise Price to the Company (such date, the “Warrant Share Delivery Date”). Upon delivery of the Notice of Exercise, the Holder shall be deemed for all corporate purposes to have become the holder of record of the Warrant Shares with respect to which this Warrant has been exercised, irrespective of the date of delivery of the Warrant Shares, provided that payment of the aggregate Exercise Price (other than in the case of a cashless exercise) is received by the Warrant Share Delivery Date. If the Company fails for any reason to deliver to the Holder the Warrant Shares subject to a Notice of Exercise by the Warrant Share Delivery Date, provided that payment of the aggregate Exercise Price (other than in the instance of a cashless exercise) is received by the Company by such date, the Company shall pay to the Holder, in cash, as liquidated damages and not as a penalty, for each $1,000 of Warrant Shares subject to such exercise (based on the VWAP of the Common Stock on the date of the applicable Notice of Exercise), $10 per Trading Day (increasing to $20 per Trading Day on the fifth Trading Day after the Warrant Share Delivery Date) for each Trading Day after such Warrant Share Delivery Date until such Warrant Shares are delivered or Holder rescinds such exercise. Notwithstanding anything herein to the contrary, the Company shall not be responsible for the payment of liquidated damages resulting from the failure to issue or deliver Warrant Shares if such failure is caused by any action or inaction of the Holder. The Company agrees to maintain a transfer agent that is a participant in the FAST program so long as this Warrant remains outstanding and exercisable. As used herein, “Standard Settlement Period” means the standard settlement period, expressed in a number of Trading Days, on the Company’s primary Trading Market with respect to the Common Stock as in effect on the date of delivery of the Notice of Exercise. Notwithstanding the foregoing, with respect to any Notice(s) of Exercise delivered on or prior to 12:00 p.m. (New York City time) on the Initial Exercise Date, which may be delivered at any time after the time of execution of the Underwriting Agreement, the Company agrees to deliver the Warrant Shares subject to such notice(s) by 4:00 p.m. (New York City time) on the Initial Exercise Date and the Initial Exercise Date shall be the Warrant Share Delivery Date for purposes hereunder, provided that payment of the aggregate Exercise Price (other than in the case of a cashless exercise) is received by such Warrant Share Delivery Date.
ii. Delivery of New Warrants Upon Exercise. If this Warrant shall have been exercised in part, the Company shall, at the request of a Holder and upon surrender of this Warrant certificate, at the time of delivery of the Warrant Shares, deliver to the Holder a new Warrant evidencing the rights of the Holder to purchase the unpurchased Warrant Shares called for by this Warrant, which new Warrant shall in all other respects be identical with this Warrant.
iii. Rescission Rights. If the Company fails to cause the Transfer Agent to transmit to the Holder the Warrant Shares pursuant to Section 2(d)(i) by the Warrant Share Delivery Date, then the Holder will have the right to rescind such exercise.
iv. Compensation for Buy-In on Failure to Timely Deliver Warrant Shares Upon Exercise. In addition to any other rights available to the Holder, if the Company fails to cause the Transfer Agent to transmit to the Holder the Warrant Shares in accordance with the provisions of Section 2(d)(i) above pursuant to an exercise on or before the Warrant Share Delivery Date, and if after such date the Holder is required by its broker to purchase (in an open market transaction or otherwise) or the Holder’s brokerage firm otherwise purchases, shares of Common Stock to deliver in satisfaction of a sale by the Holder of the Warrant Shares which the Holder anticipated receiving upon such exercise (a “Buy-In”), then the Company shall (A) pay in cash to the Holder the amount, if any, by which (x) the Holder’s total purchase price (including brokerage commissions, if any) for the shares of Common Stock so purchased exceeds (y) the amount obtained by multiplying (1) the number of Warrant Shares that the Company was required to deliver to the Holder in connection with the exercise at issue times (2) the price at which the sell order giving rise to such purchase obligation was executed, and (B) at the option of the Holder, either reinstate the portion of the Warrant and equivalent number of Warrant Shares for which such exercise was not honored (in which case such exercise shall be deemed rescinded) or deliver to the Holder the number of shares of Common Stock that would have been issued had the Company timely complied with its exercise and delivery obligations hereunder. For example, if the Holder purchases Common Stock having a total purchase price of $11,000 to cover a Buy-In with respect to an attempted exercise of Warrants with an aggregate sale price giving rise to such purchase obligation of $10,000, under clause (A) of the immediately preceding sentence the Company shall be required to pay the Holder $1,000. The Holder shall provide the Company written notice indicating the amounts payable to the Holder in respect of the Buy-In and, upon request of the Company, evidence of the amount of such loss. Nothing herein shall limit a Holder’s right to pursue any other remedies available to it hereunder, at law or in equity including, without limitation, a decree of specific performance and/or injunctive relief with respect to the Company’s failure to timely deliver shares of Common Stock upon exercise of the Warrant as required pursuant to the terms hereof. Notwithstanding the forgoing, the Warrant Agent shall not, in any event, be subject to, or responsible for, Buy-In penalties contemplated by this Section 2(d)(iv).
v. No Fractional Shares or Scrip. No fractional shares or scrip representing fractional shares shall be issued upon the exercise of this Warrant. As to any fraction of a share which the Holder would otherwise be entitled to purchase upon such exercise, the Company shall, at its election, either pay a cash adjustment in respect of such final fraction in an amount equal to such fraction multiplied by the Exercise Price or round up to the next whole share.
vi. Charges, Taxes and Expenses. Issuance of Warrant Shares shall be made without charge to the Holder for any issue or transfer tax or other incidental expense in respect of the issuance of such Warrant Shares, all of which taxes and expenses shall be paid by the Company, and such Warrant Shares shall be issued in the name of the Holder or in such name or names as may be directed by the Holder; provided, however, that in the event that Warrant Shares are to be issued in a name other than the name of the Holder, this Warrant when surrendered for exercise shall be accompanied by the Assignment Form attached hereto duly executed by the Holder and the Company may require, as a condition thereto, the payment of a sum sufficient to reimburse it for any transfer tax incidental thereto. The Company shall pay all Transfer Agent fees required for same-day processing of any Notice of Exercise and all fees to the Depository Trust Company (or another established clearing corporation performing similar functions) required for same-day electronic delivery of the Warrant Shares.
vii. Closing of Books. The Company will not close its stockholder books or records in any manner which prevents the timely exercise of this Warrant, pursuant to the terms hereof.
|
e) |
Holder’s Exercise Limitations. The Company shall not effect any exercise of this Warrant, and a Holder shall not have the right to exercise any portion of this Warrant, pursuant to Section 2 or otherwise, to the extent that after giving effect to such issuance after exercise as set forth on the applicable Notice of Exercise, the Holder (together with the Holder’s Affiliates, and any other Persons acting as a group together with the Holder or any of the Holder’s Affiliates (such Persons, “Attribution Parties”)), would beneficially own in excess of the Beneficial Ownership Limitation (as defined below). For purposes of the foregoing sentence, the number of shares of Common Stock beneficially owned by the Holder and its Affiliates and Attribution Parties shall include the number of shares of Common Stock issuable upon exercise of this Warrant with respect to which such determination is being made, but shall exclude the number of shares of Common Stock which would be issuable upon (i) exercise of the remaining, nonexercised portion of this Warrant beneficially owned by the Holder or any of its Affiliates or Attribution Parties and (ii) exercise or conversion of the unexercised or nonconverted portion of any other securities of the Company (including, without limitation, any other Common Stock Equivalents) subject to a limitation on conversion or exercise analogous to the limitation contained herein beneficially owned by the Holder or any of its Affiliates or Attribution Parties. Except as set forth in the preceding sentence, for purposes of this Section 2(e), beneficial ownership shall be calculated in accordance with Section 13(d) of the Exchange Act and the rules and regulations promulgated thereunder, it being acknowledged by the Holder that the Company is not representing to the Holder that such calculation is in compliance with Section 13(d) of the Exchange Act and the Holder is solely responsible for any schedules required to be filed in accordance therewith. To the extent that the limitation contained in this Section 2(e) applies, the determination of whether this Warrant is exercisable (in relation to other securities owned by the Holder together with any Affiliates and Attribution Parties) and of which portion of this Warrant is exercisable shall be in the sole discretion of the Holder, and the submission of a Notice of Exercise shall be deemed to be the Holder’s determination of whether this Warrant is exercisable (in relation to other securities owned by the Holder together with any Affiliates and Attribution Parties) and of which portion of this Warrant is exercisable, in each case subject to the Beneficial Ownership Limitation, and the Company shall have no obligation to verify or confirm the accuracy of such determination and shall have no liability for exercises of the Warrant that are not in compliance with the Beneficial Ownership Limitation, except to the extent the Holder relies on a number of outstanding shares of Common Stock that was provided by the Company. In addition, a determination as to any group status as contemplated above shall be determined in accordance with Section 13(d) of the Exchange Act and the rules and regulations promulgated thereunder, and the Company shall have no obligation to verify or confirm the accuracy of such determination and shall have no liability for exercises of the Warrant that are not in compliance with the Beneficial Ownership Limitation, except to the extent the Holder relies on the number of outstanding shares of Common Stock that was provided by the Company . For purposes of this Section 2(e), in determining the number of outstanding shares of Common Stock, a Holder may rely on the number of outstanding shares of Common Stock as reflected in (A) the Company’s most recent periodic or annual report filed with the Commission, as the case may be, (B) a more recent public announcement by the Company or (C) a more recent written notice by the Company or the Transfer Agent setting forth the number of shares of Common Stock outstanding. Upon the written request of a Holder, the Company shall within one (1) Trading Day confirm orally and in writing to the Holder the number of shares of Common Stock then outstanding. In any case, the number of outstanding shares of Common Stock shall be determined after giving effect to the conversion or exercise of securities of the Company, including this Warrant, by the Holder or its Affiliates or Attribution Parties since the date as of which such number of outstanding shares of Common Stock was reported. The “Beneficial Ownership Limitation” shall be [4.99/9.99]% of the number of shares of the Common Stock outstanding immediately after giving effect to the issuance of shares of Common Stock issuable upon exercise of this Warrant. The Holder, upon written notice to the Company, may increase or decrease the Beneficial Ownership Limitation provisions of this Section 2(e), provided that the Beneficial Ownership Limitation in no event exceeds 9.99% of the number of shares of the Common Stock outstanding immediately after giving effect to the issuance of shares of Common Stock upon exercise of this Warrant held by the Holder and the provisions of this Section 2(e) shall continue to apply. Any increase in the Beneficial Ownership Limitation will not be effective until the 61st day after such notice is delivered to the Company. The provisions of this paragraph shall be construed and implemented in a manner otherwise than in strict conformity with the terms of this Section 2(e) to correct this paragraph (or any portion hereof) which may be defective or inconsistent with the intended Beneficial Ownership Limitation herein contained or to make changes or supplements necessary or desirable to properly give effect to such limitation. The limitations contained in this paragraph shall apply to a successor holder of this Warrant. If the Warrant is unexercisable as a result of the Holder’s Beneficial Ownership Limitation, no alternate consideration is owing to the Holder. |
Section 3. Certain Adjustments.
a) Stock Dividends and Splits. If the Company, at any time while this Warrant is outstanding: (i) pays a stock dividend or otherwise makes a distribution or distributions on shares of its Common Stock or any other equity or equity equivalent securities payable in shares of Common Stock (which, for avoidance of doubt, shall not include any shares of Common Stock issued by the Company upon exercise of this Warrant), (ii) subdivides outstanding shares of Common Stock into a larger number of shares, (iii) combines (including by way of reverse stock split) outstanding shares of Common Stock into a smaller number of shares, or (iv) issues by reclassification of shares of the Common Stock any shares of capital stock of the Company, then in each case the Exercise Price shall be multiplied by a fraction of which the numerator shall be the number of shares of Common Stock (excluding treasury shares, if any) outstanding immediately before such event and of which the denominator shall be the number of shares of Common Stock outstanding immediately after such event, and the number of shares issuable upon exercise of this Warrant shall be proportionately adjusted such that the aggregate Exercise Price of this Warrant shall remain unchanged. Any adjustment made pursuant to this Section 3(a) shall become effective immediately after the record date for the determination of stockholders entitled to receive such dividend or distribution and shall become effective immediately after the effective date in the case of a subdivision, combination or re‑classification.
b) Subsequent Rights Offerings. In addition to any adjustments pursuant to Section 3(a) above, if at any time the Company grants, issues or sells any Common Stock Equivalents or rights to purchase stock, warrants, securities or other property pro rata to the record holders of any class of shares of Common Stock (the “Purchase Rights”), then the Holder will be entitled to acquire, upon the terms applicable to such Purchase Rights, the aggregate Purchase Rights which the Holder could have acquired if the Holder had held the number of shares of Common Stock acquirable upon complete exercise of this Warrant (without regard to any limitations on exercise hereof, including without limitation, the Beneficial Ownership Limitation) immediately before the date on which a record is taken for the grant, issuance or sale of such Purchase Rights, or, if no such record is taken, the date as of which the record holders of shares of Common Stock are to be determined for the grant, issue or sale of such Purchase Rights (provided, however, that to the extent that the Holder’s right to participate in any such Purchase Right would result in the Holder exceeding the Beneficial Ownership Limitation, then the Holder shall not be entitled to participate in such Purchase Right to such extent (or beneficial ownership of such shares of Common Stock as a result of such Purchase Right to such extent)and such Purchase Right to such extent shall be held in abeyance for the Holder until such time, if ever, as its right thereto would not result in the Holder exceeding the Beneficial Ownership Limitation).
c) Pro Rata Distributions. During such time as this Warrant is outstanding, if the Company shall declare or make any dividend or other distribution of its assets (or rights to acquire its assets) to holders of shares of Common Stock, by way of return of capital or otherwise (including, without limitation, any distribution of cash, stock or other securities, property or options by way of a dividend, spin off, reclassification, corporate rearrangement, scheme of arrangement or other similar transaction) (a “Distribution”), at any time after the issuance of this Warrant, then, in each such case, the Holder shall be entitled to participate in such Distribution to the same extent that the Holder would have participated therein if the Holder had held the number of shares of Common Stock acquirable upon complete exercise of this Warrant (without regard to any limitations on exercise hereof, including without limitation, the Beneficial Ownership Limitation) immediately before the date of which a record is taken for such Distribution, or, if no such record is taken, the date as of which the record holders of shares of Common Stock are to be determined for the participation in such Distribution (provided, however, that to the extent that the Holder’s right to participate in any such Distribution would result in the Holder exceeding the Beneficial Ownership Limitation, then the Holder shall not be entitled to participate in such Distribution to such extent (or in the beneficial ownership of any shares of Common Stock as a result of such Distribution to such extent) and the portion of such Distribution shall be held in abeyance for the benefit of the Holder until such time, if ever, as its right thereto would not result in the Holder exceeding the Beneficial Ownership Limitation).
d) Fundamental Transaction. If, at any time while this Warrant is outstanding, (i) the Company, directly or indirectly, in one or more related transactions effects any merger or consolidation of the Company with or into another Person, (ii) the Company (or any Subsidiary), directly or indirectly, effects any sale, lease, license, assignment, transfer, conveyance or other disposition of all or substantially all of the Company’s assets in one or a series of related transactions, (iii) any, direct or indirect, purchase offer, tender offer or exchange offer (whether by the Company or another Person) is completed pursuant to which holders of Common Stock are permitted to sell, tender or exchange their shares for other securities, cash or property and has been accepted by the holders of greater than 50% of the voting power of the outstanding common and preferred stock of the Company, (iv) the Company, directly or indirectly, in one or more related transactions effects any reclassification, reorganization or recapitalization of the Common Stock or any compulsory share exchange pursuant to which the Common Stock is effectively converted into or exchanged for other securities, cash or property, or (v) the Company, directly or indirectly, in one or more related transactions consummates a stock or share purchase agreement or other business combination (including, without limitation, a reorganization, recapitalization, spin-off, merger or scheme of arrangement) with another Person or group of Persons whereby such other Person or group acquires greater than 50% of the voting power of the outstanding common and preferred stock of the Company (each a “Fundamental Transaction”), then, upon any subsequent exercise of this Warrant, the Holder shall have the right to receive, for each Warrant Share that would have been issuable upon such exercise immediately prior to the occurrence of such Fundamental Transaction, at the option of the Holder (without regard to any limitation in Section 2(e) on the exercise of this Warrant), the number of shares of Common Stock of the successor or acquiring corporation or of the Company, if it is the surviving corporation, and any additional consideration (the “Alternate Consideration”) receivable as a result of such Fundamental Transaction by a holder of the number of shares of Common Stock for which this Warrant is exercisable immediately prior to such Fundamental Transaction (without regard to any limitation in Section 2(e) on the exercise of this Warrant). For purposes of any such exercise, the determination of the Exercise Price shall be appropriately adjusted to apply to such Alternate Consideration based on the amount of Alternate Consideration issuable in respect of one share of Common Stock in such Fundamental Transaction, and the Company shall apportion the Exercise Price among the Alternate Consideration in a reasonable manner reflecting the relative value of any different components of the Alternate Consideration. If holders of Common Stock are given any choice as to the securities, cash or property to be received in a Fundamental Transaction, then the Holder shall be given the same choice as to the Alternate Consideration it receives upon any exercise of this Warrant following such Fundamental Transaction. The Company shall cause any successor entity in a Fundamental Transaction in which the Company is not the survivor (the “Successor Entity”) to assume in writing all of the obligations of the Company under this Warrant and the other Transaction Documents in accordance with the provisions of this Section 3(d) pursuant to written agreements in form and substance reasonably satisfactory to the Holder and approved by the Holder (without unreasonable delay) prior to such Fundamental Transaction and shall, at the option of the Holder, deliver to the Holder in exchange for this Warrant a security of the Successor Entity evidenced by a written instrument substantially similar in form and substance to this Warrant which is exercisable for a corresponding number of shares of capital stock of such Successor Entity (or its parent entity) equivalent to the shares of Common Stock acquirable and receivable upon exercise of this Warrant (without regard to any limitations on the exercise of this Warrant) prior to such Fundamental Transaction, and with an exercise price which applies the exercise price hereunder to such shares of capital stock (but taking into account the relative value of the shares of Common Stock pursuant to such Fundamental Transaction and the value of such shares of capital stock, such number of shares of capital stock and such exercise price being for the purpose of protecting the economic value of this Warrant immediately prior to the consummation of such Fundamental Transaction), and which is reasonably satisfactory in form and substance to the Holder. Upon the occurrence of any such Fundamental Transaction, the Successor Entity shall be added to the term “Company” under this Warrant (so that from and after the occurrence or consummation of such Fundamental Transaction, the provisions of this Warrant and the other Transaction Documents referring to the “Company” shall refer instead to each of the Company and the Successor Entity, or Successor Entities, jointly and severally), and the Successor Entity or Successor Entities, jointly and severally with the Company, may exercise every right and power of the Company prior thereto, and the Successor Entity or Successor Entities shall assume all of the obligations of the Company prior thereto under this Warrant and the other Transaction Documents with the same effect as if the Company and such Successor Entity or Successor Entities, jointly and severally, had been named as the Company herein. For the avoidance of doubt, the Holder shall be entitled to the benefits of the provisions of this Section 3(d) regardless of (i) whether the Company has sufficient authorized shares of Common Stock for the issuance of Warrant Shares and/or (ii) whether a Change of Control occurs prior to the Initial Exercise Date.
e) Calculations. All calculations under this Section 3 shall be made to the nearest cent or the nearest 1/100th of a share, as the case may be. For purposes of this Section 3, the number of shares of Common Stock deemed to be issued and outstanding as of a given date shall be the sum of the number of shares of Common Stock (excluding treasury shares, if any) issued and outstanding.
f) Notice to Holder.
|
i. |
Adjustment to Exercise Price. Whenever the Exercise Price is adjusted pursuant to any provision of this Section 3, the Company shall promptly deliver to the Holder and the Warrant Agent by facsimile or email a notice setting forth the Exercise Price after such adjustment and any resulting adjustment to the number of Warrant Shares and setting forth a brief statement of the facts requiring such adjustment. |
|
ii. |
Notice to Allow Exercise by Holder. If (A) the Company shall declare a dividend (or any other distribution in whatever form other than a stock split) on the Common Stock, (B) the Company shall declare a special nonrecurring cash dividend on or a redemption of the Common Stock, (C) the Company shall authorize the granting to all holders of the Common Stock rights or warrants to subscribe for or purchase any shares of capital stock of any class or of any rights, (D) the approval of any stockholders of the Company shall be required in connection with any reclassification of the Common Stock, any consolidation or merger to which the Company is a party, any sale or transfer of all or substantially all of the assets of the Company, or any compulsory share exchange whereby the Common Stock is converted into other securities, cash or property, or (E) the Company shall authorize the voluntary or involuntary dissolution, liquidation or winding up of the affairs of the Company, then, in each case, the Company shall cause to be delivered by facsimile or email to the Holder at its last facsimile number or email address as it shall appear upon the Warrant Register of the Company, at least 20 calendar days prior to the applicable record or effective date hereinafter specified, a notice stating (x) the date on which a record is to be taken for the purpose of such dividend, distribution, redemption, rights or warrants, or if a record is not to be taken, the date as of which the holders of the Common Stock of record to be entitled to such dividend, distributions, redemption, rights or warrants are to be determined or (y) the date on which such reclassification, consolidation, merger, sale, transfer or share exchange is expected to become effective or close, and the date as of which it is expected that holders of the Common Stock of record shall be entitled to exchange their shares of the Common Stock for securities, cash or other property deliverable upon such reclassification, consolidation, merger, sale, transfer or share exchange; provided that the failure to deliver such notice or any defect therein or in the delivery thereof shall not affect the validity of the corporate action required to be specified in such notice. To the extent that any notice provided in this Warrant constitutes, or contains, material, non-public information regarding the Company or any of the Company’s subsidiaries, the Company shall simultaneously file such notice with the Commission pursuant to a Current Report on Form 8-K. The Holder shall remain entitled to exercise this Warrant during the period commencing on the date of such notice to the effective date of the event triggering such notice except as may otherwise be expressly set forth herein. |
|
iii. |
Voluntary Adjustment by the Company. Subject to the rules and regulations of the Trading Market, the Company may at any time during the term of this Warrant, subject to the prior written consent of the Holder, reduce the then current Exercise Price to any amount and for any period of time deemed appropriate by the board of directors of the Company. |
Section 4. Transfer of Warrant.
a) Transferability. This Warrant and all rights hereunder (including, without limitation, any registration rights) are transferable, in whole or in part, upon surrender of this Warrant at the office of the Warrant Agent designated for such purpose, together with a written assignment of this Warrant substantially in the form attached hereto properly completed and duly executed by the Holder or its agent or attorney and funds sufficient to pay any transfer taxes payable upon the making of such transfer accompanied by reasonable evidence of authority of the party making such request that may be required by the Warrant Agent including but not limited to, the signature guarantee of a guarantor institution which is a participant in a signature guarantee program approved by the Securities Transfer Association. Upon such surrender and, if required, such payment, the Company shall execute and deliver a new Warrant or Warrants in the name of the assignee or assignees, as applicable, and in the denomination or denominations specified in such instrument of assignment, and shall issue to the assignor a new Warrant evidencing the portion of this Warrant not so assigned, and this Warrant shall promptly be cancelled. Notwithstanding anything herein to the contrary, the Holder shall not be required to physically surrender this Warrant to the Company unless the Holder has assigned this Warrant in full, in which case, the Holder shall surrender this Warrant to the Company or the Warrant Agent within three (3) Trading Days of the date on which the Holder delivers an assignment form to the Company assigning this Warrant in full. The Warrant, if properly assigned in accordance herewith, may be exercised by a new holder for the purchase of Warrant Shares without having a new Warrant issued.
b) New Warrants. This Warrant may be divided or combined with other Warrants upon presentation hereof at the aforesaid office of the Company, together with a written notice specifying the names and denominations in which new Warrants are to be issued, signed by the Holder or its agent or attorney. Subject to compliance with Section 4(a), as to any transfer which may be involved in such division or combination, the Company shall execute and deliver a new Warrant or Warrants in exchange for the Warrant or Warrants to be divided or combined in accordance with such notice. All Warrants issued on transfers or exchanges shall be dated the initial issuance date of this Warrant and shall be identical with this Warrant except as to the number of Warrant Shares issuable pursuant thereto.
c) Warrant Register. The Warrant Agent shall register this Warrant, upon records to be maintained by the Warrant Agent for that purpose (the “Warrant Register”), in the name of the record Holder hereof from time to time. The Company and the Warrant Agent may deem and treat the registered Holder of this Warrant as the absolute owner hereof for the purpose of any exercise hereof or any distribution to the Holder, and for all other purposes, absent actual notice to the contrary. Notwithstanding the foregoing, nothing herein shall prevent the Warrant Agent or any agent of the Warrant Agent from giving effect to any written certification, proxy or other authorization furnished by DTC or any other depository governing the exercise of the rights of a holder of a beneficial interest in any Warrant. The rights of beneficial owners in a Warrant held in global shall be exercised by the Holder or a Participant (as defined in the Warrant Agency Agreement) through the depository’s system, except to the extent expressly set forth in the Warrant Agency Agreement.
Section 5. Miscellaneous.
a) No Rights as Stockholder Until Exercise; No Settlement in Cash. This Warrant does not entitle the Holder to any voting rights, dividends or other rights as a stockholder of the Company prior to the exercise hereof as set forth in Section 2(d)(i), except as expressly set forth in Section 3. Without limiting the rights of a Holder to receive Warrant Shares on a “cashless exercise” only as permitted in Section 2(c), and to receive the cash payments contemplated pursuant to Sections 2(d)(i) and 2(d)(iv), in no event will the Company be required to net cash settle an exercise of this Warrant.
b) Loss, Theft, Destruction or Mutilation of Warrant. The Company covenants that upon receipt by the Company and the Warrant Agent of evidence reasonably satisfactory to them of the loss, theft, destruction or mutilation of this Warrant or any stock certificate relating to the Warrant Shares, and in case of loss, theft or destruction, of indemnity or security reasonably satisfactory to them (which, in the case of the Warrant held in global form through DTC, shall not include the posting of any bond), and upon surrender and cancellation of such Warrant or stock certificate, if mutilated, the Company will make and deliver a new Warrant or stock certificate of like tenor and dated as of such cancellation, in lieu of such Warrant or stock certificate.
c) Saturdays, Sundays, Holidays, etc. If the last or appointed day for the taking of any action or the expiration of any right required or granted herein shall not be a Trading Day, then, such action may be taken or such right may be exercised on the next succeeding Trading Day.
d) Authorized Shares.The Company covenants that, at all times during the period the Warrant is outstanding, it will reserve from its authorized and unissued Common Stock a sufficient number of shares to provide for the issuance of the Warrant Shares upon the exercise of any purchase rights under this Warrant. The Company further covenants that its issuance of this Warrant shall constitute full authority to its officers who are charged with the duty of issuing the necessary Warrant Shares upon the exercise of the purchase rights under this Warrant. The Company will take all such reasonable action as may be necessary to assure that such Warrant Shares may be issued as provided herein without violation of any applicable law or regulation, or of any requirements of the Trading Market upon which the Common Stock may be listed. The Company covenants that all Warrant Shares which may be issued upon the exercise of the purchase rights represented by this Warrant will, upon exercise of the purchase rights represented by this Warrant and payment for such Warrant Shares in accordance herewith, be duly authorized, validly issued, fully paid and nonassessable and free from all taxes, liens and charges created by the Company in respect of the issue thereof (other than taxes in respect of any transfer occurring contemporaneously with such issue).
Except and to the extent as waived or consented to by the Holder, the Company shall not by any action, including, without limitation, amending its certificate of incorporation or through any reorganization, transfer of assets, consolidation, merger, dissolution, issue or sale of securities or any other voluntary action, avoid or seek to avoid the observance or performance of any of the terms of this Warrant, but will at all times in good faith assist in the carrying out of all such terms and in the taking of all such actions as may be necessary or appropriate to protect the rights of Holder as set forth in this Warrant against impairment (it being understood that this Warrant shall not in any case prevent the Company from effecting any such amendment, reorganization, transfer, consolidation, merger, dissolution, issuance or sale). Without limiting the generality of the foregoing, the Company will (i) not increase the par value of any Warrant Shares above the amount payable therefor upon such exercise immediately prior to such increase in par value, (ii) take all such action as may be necessary or appropriate in order that the Company may validly and legally issue fully paid and nonassessable Warrant Shares upon the exercise of this Warrant and (iii) use commercially reasonable efforts to obtain all such authorizations, exemptions or consents from any public regulatory body having jurisdiction thereof, as may be, necessary to enable the Company to perform its obligations under this Warrant.
Before taking any action, which would result in an adjustment in the number of Warrant Shares for which this Warrant is exercisable or in the Exercise Price, the Company shall obtain all such authorizations or exemptions thereof, or consents thereto, as may be necessary from any public regulatory body or bodies having jurisdiction thereof.
e) Jurisdiction. All questions concerning the construction, validity, enforcement and interpretation of this Warrant shall be governed by and construed and enforced in accordance with the internal laws of the State of New York, without regard to the principles of conflicts of law thereof. Each party agrees that all legal proceedings concerning the interpretations, enforcement and defense of the transactions contemplated by this Warrant (whether brought against a party hereto or their respective affiliates, directors, officers, shareholders, partners, members, employees or agents) shall be commenced exclusively in the state and federal courts sitting in the City of New York. Each party hereby irrevocably submits to the exclusive jurisdiction of the state and federal courts sitting in the City of New York, Borough of Manhattan for the adjudication of any dispute hereunder or in connection herewith or with any transaction contemplated hereby or discussed herein, and hereby irrevocably waives, and agrees not to assert in any suit, action or proceeding, any claim that it is not personally subject to the jurisdiction of any such court, that such suit, action or proceeding is improper or is an inconvenient venue for such proceeding. As between the Company and Holder, (i) each party hereby irrevocably waives personal service of process and consents to process being served in any such suit, action or proceeding by mailing a copy thereof via registered or certified mail or overnight delivery (with evidence of delivery) to such party at the address in effect for notices to it under this Warrant and agrees that such service shall constitute good and sufficient service of process and notice thereof (nothing contained herein shall be deemed to limit in any way any right to serve process in any other manner permitted by law), and (ii) if either party shall commence an action, suit or proceeding to enforce any provisions of this Warrant, the prevailing party in such action, suit or proceeding shall be reimbursed by the other party for their reasonable attorneys’ fees and other costs and expenses incurred with the investigation, preparation and prosecution of such action or proceeding.
f) Restrictions. The Holder acknowledges that the Warrant Shares acquired upon the exercise of this Warrant, if not registered, and the Holder does not utilize cashless exercise, will have restrictions upon resale imposed by state and federal securities laws.
g) Nonwaiver and Expenses. No course of dealing or any delay or failure to exercise any right hereunder on the part of Holder shall operate as a waiver of such right or otherwise prejudice the Holder’s rights, powers or remedies, notwithstanding the fact that the right to exercise this Warrant terminates on the Termination Date. Without limiting any other provision of this Warrant, if the Company willfully and knowingly fails to comply with any provision of this Warrant, which results in any material damages to the Holder, the Company shall pay to the Holder such amounts as shall be sufficient to cover any costs and expenses including, but not limited to, reasonable attorneys’ fees, including those of appellate proceedings, incurred by the Holder in collecting any amounts due pursuant hereto or in otherwise enforcing any of its rights, powers or remedies hereunder.
h) Notices. Any and all notices or other communications or deliveries to be provided by the Holders hereunder including, without limitation, any Notice of Exercise, shall be in writing and delivered personally, or e-mail (other than to the Warrant Agent), or sent by a nationally recognized overnight courier service, addressed:
If to the Company, to:
Autonomix Medical, Inc.
21 Waterway Avenue, Suite 300
The Woodlands, Texas 77380
Attention: Chief Financial Officer
If to the Warrant Agent, to:
Equity Stock Transfer LLC
[●]
Attention: _________
or such other facsimile number, email address or address as the Company or the Warrant Agent may specify for such purposes by notice to the Holders. Any and all notices or other communications or deliveries to be provided by the Company hereunder shall be in writing and delivered personally, by facsimile or e-mail, or sent by a nationally recognized overnight courier service addressed to each Holder at the facsimile number, e-mail address or address of such Holder appearing on the books of the Warrant Agent. Any notice or other communication or deliveries hereunder shall be deemed given and effective on the earliest of (i) the date of transmission, if such notice or communication is delivered via facsimile at the facsimile number or via e-mail at the e-mail address set forth in this Section prior to 5:30 p.m. (New York City time) on any date, (ii) the next Trading Day after the date of transmission, if such notice or communication is delivered via facsimile at the facsimile number or via e-mail at the e-mail address set forth in this Section on a day that is not a Trading Day or later than 5:30 p.m. (New York City time) on any Trading Day, (iii) the second Trading Day following the date of mailing, if sent by U.S. nationally recognized overnight courier service, or (iv) upon actual receipt by the party to whom such notice is required to be given. Notwithstanding any other provision of this Warrant, where this Warrant provides for notice of any event to the Holder, if this Warrant is held in global form by DTC (or any successor depositary), such notice shall be sufficiently given if given to DTC (or any successor depositary) pursuant to the procedures of DTC (or such successor depositary), subject to a Holder’s right to elect to receive a Warrant in certificated form pursuant to the terms of the Warrant Agent Agreement, in which case this sentence shall not apply. To the extent that any notice provided hereunder constitutes, or contains, material, non-public information regarding the Company or any subsidiaries, the Company shall simultaneously file such notice with the Commission pursuant to a Current Report on Form 8-K.
i) Limitation of Liability. No provision hereof, in the absence of any affirmative action by the Holder to exercise this Warrant to purchase Warrant Shares, and no enumeration herein of the rights or privileges of the Holder, shall give rise to any liability of the Holder for the purchase price of any Common Stock or as a stockholder of the Company, whether such liability is asserted by the Company or by creditors of the Company.
j) Remedies. The Holder, in addition to being entitled to exercise all rights granted by law, including recovery of damages, will be entitled to specific performance of its rights under this Warrant. The Company agrees that monetary damages would not be adequate compensation for any loss incurred by reason of a breach by it of the provisions of this Warrant and hereby agrees to waive and not to assert the defense in any action for specific performance that a remedy at law would be adequate.
k) Successors and Assigns. Subject to applicable securities laws, this Warrant and the rights and obligations evidenced hereby shall inure to the benefit of and be binding upon the successors and permitted assigns of the Company and the successors and permitted assigns of Holder. The provisions of this Warrant are intended to be for the benefit of any Holder from time to time of this Warrant and shall be enforceable by the Holder or holder of Warrant Shares.
l) Amendment. This Warrant may be modified or amended or the provisions hereof waived with the written consent of the Company, on the one hand, and the Holders of this Warrant, on the other hand.
m) Severability. Wherever possible, each provision of this Warrant shall be interpreted in such manner as to be effective and valid under applicable law, but if any provision of this Warrant shall be prohibited by or invalid under applicable law, such provision shall be ineffective to the extent of such prohibition or invalidity, without invalidating the remainder of such provisions or the remaining provisions of this Warrant.
n) Headings. The headings used in this Warrant are for the convenience of reference only and shall not, for any purpose, be deemed a part of this Warrant.
o) Warrant Agency Agreement. This Warrant is issued subject to the Warrant Agency Agreement. To the extent any provision of this Warrant conflicts with the express provisions of the Warrant Agency Agreement, the provisions of this Warrant shall govern and be controlling; provided, however, that all provisions with respect to the rights, duties, obligations, protections, immunities and liability of the Warrant Agent only shall be determined and interpreted solely by the provisions of the Warrant Agency Agreement.
********************
(Signature Page Follows)
IN WITNESS WHEREOF, the Company has caused this Warrant to be executed by its officer thereunto duly authorized as of the date first above indicated.
|
AUTONOMIX MEDICAL, INC. |
|||
|
By: |
|||
|
Name: |
|||
|
Title: |
|||
[SIGNATURE PAGE TO PRE-FUNDED COMMON STOCK PURCHASE WARRANT,
AUTONOMIX MEDICAL, INC.]
EXHIBIT A
NOTICE OF EXERCISE
TO: AUTONOMIX MEDICAL, INC.
(1) The undersigned hereby elects to purchase ________ Warrant Shares of the Company pursuant to the terms of the attached Warrant (only if exercised in full), and tenders herewith payment of the exercise price in full, together with all applicable transfer taxes, if any.
(2) Payment shall take the form of (check applicable box):
[ ] in lawful money of the United States; or
[ ] if permitted the cancellation of such number of Warrant Shares as is necessary, in accordance with the formula set forth in subsection 2(c), to exercise this Warrant with respect to the maximum number of Warrant Shares purchasable pursuant to the cashless exercise procedure set forth in subsection 2(c).
(3) Please issue said Warrant Shares in the name of the undersigned or in such other name as is specified below:
_______________________________
The Warrant Shares shall be delivered to the following DWAC Account Number:
_______________________________
_______________________________
_______________________________
[SIGNATURE OF HOLDER]
Name of Investing Entity: ______________________________________________________
Signature of Authorized Signatory of Investing Entity: _________________________________
Name of Authorized Signatory: ___________________________________________________
Title of Authorized Signatory: ____________________________________________________
Date: ________________________________________________________________________
EXHIBIT B
ASSIGNMENT FORM
(To assign the foregoing Warrant, execute this form and supply required information. Do not use this form to exercise the Warrant to purchase shares.)
FOR VALUE RECEIVED, the foregoing Warrant and all rights evidenced thereby are hereby assigned to
|
Name: |
|||
|
(Please Print) |
|||
|
Address: |
|||
|
(Please Print) |
|||
|
Phone Number: |
|||
|
Email Address: |
|||
|
Dated: _______________ __, ______ |
|||
|
Holder’s Signature: |
|||
|
Holder’s Address: |
|||
Exhibit 4.4
SERIES A COMMON STOCK PURCHASE WARRANT
AUTONOMIX MEDICAL, INC.
|
Warrant Shares: ______ |
Issue Date: [●], 2024 |
THIS SERIES A COMMON STOCK PURCHASE WARRANT (the “Warrant”) certifies that, for value received, CEDE & CO. or its assigns (the “Holder”) is entitled, upon the terms and subject to the limitations on exercise and the conditions hereinafter set forth, at any time on or after the date hereof (the “Initial Exercise Date”) and on or prior to 5:00 p.m. (New York City time) on the date that is the five (5) year anniversary of the Initial Exercise Date, provided that, if such date is not a Trading Day, the date that is the immediately following Trading Day (the “Termination Date”) but not thereafter, to subscribe for and purchase from AUTONOMIX MEDICAL, INC., a Delaware corporation (the “Company”), up to ______ shares (as subject to adjustment hereunder, the “Warrant Shares”) of the Company’s Common Stock. The purchase price of one share of Common Stock under this Warrant shall be equal to the Exercise Price, as defined in Section 2(b). This Warrant shall initially be issued and maintained in the form of a security held in book-entry form and the Depository Trust Company or its nominee (“DTC”) shall initially be the sole registered holder of this Warrant, subject to a Holder’s right to elect to receive a Warrant in certificated form pursuant to the terms of the Warrant Agency Agreement, in which case this sentence shall not apply.
Section 1. Definitions. Capitalized terms used and not otherwise defined herein shall have the meanings set forth in that certain Underwriting Agreement (the “Underwriting Agreement”), dated [●], 2024, by and between the Company and Ladenburg Thalmann & Co. Inc., as the Representative of the several underwriters named therein.
Section 2. Exercise.
|
a) |
Exercise of Warrant. Subject to the provisions of Section 2(e) herein, exercise of the purchase rights represented by this Warrant may be made, in whole or in part, at any time or times on or after the Initial Exercise Date and before 5:00 p.m. New York City Time on or before the Termination Date, by delivery to the Company and Warrant Agent (or such other office or agency of the Company as it may designate by notice in writing to the registered Holder at the address of the Holder appearing on the books of the Company) of a duly executed PDF copy submitted by e-mail (or e-mail attachment) of the Notice of Exercise in the form annexed hereto as Annex A (the “Notice of Exercise”). The Holder shall deliver the aggregate Exercise Price for the Warrant Shares specified in the applicable Notice of Exercise by wire transfer of immediately available funds or cashier’s check drawn on a United States bank within the time period specified for the delivery of the Exercise Price by Section 2(d)(i) below unless the cashless exercise procedure specified in Section 2(c) below is specified in the applicable Notice of Exercise. Neither the Company nor the Warrant Agent shall have any obligation to inquire with respect to or otherwise confirm the authenticity of the signature(s) contained on any Notice of Exercise nor the authority of the person so executing the same. For the avoidance of doubt, any reference to cashless exercise herein shall include a reference to alternative cashless exercise (as defined herein). No ink-original Notice of Exercise shall be required, nor shall any medallion guarantee (or other type of guarantee or notarization) of any Notice of Exercise be required unless the Warrants are evidenced by definitive certificates. Notwithstanding anything herein to the contrary, the Holder shall not be required to physically surrender this Warrant to the Company or Warrant Agent until the Holder has purchased all of the Warrant Shares available hereunder and the Warrant has been exercised in full, in which case, the Holder shall surrender this Warrant to the Company or Warrant Agent for cancellation within three (3) Trading Days of the date on which the final Notice of Exercise is delivered to the Company. Partial exercises of this Warrant resulting in purchases of a portion of the total number of Warrant Shares available hereunder shall have the effect of lowering the outstanding number of Warrant Shares purchasable hereunder in an amount equal to the applicable number of Warrant Shares purchased. The Holder and the Warrant Agent shall maintain records showing the number of Warrant Shares purchased and the date of such purchases. The Company or Warrant Agent shall deliver any objection to any Notice of Exercise within one (1) Trading Day of receipt of such notice. The Holder and any assignee, by acceptance of this Warrant, acknowledge and agree that, by reason of the provisions of this paragraph, following the purchase of a portion of the Warrant Shares hereunder, the number of Warrant Shares available for purchase hereunder at any given time may be less than the amount stated on the face hereof. |
Notwithstanding the foregoing in this Section 2(a), a holder whose interest in this Warrant is a beneficial interest in certificate(s) representing this Warrant held in book-entry form through DTC (or another established clearing corporation performing similar functions), shall effect exercises made pursuant to this Section 2(a) by delivering to DTC (or such other clearing corporation, as applicable) the appropriate instruction form for exercise, complying with the procedures to effect exercise that are required by DTC (or such other clearing corporation, as applicable), subject to a Holder’s right to elect to receive a Warrant in certificated form pursuant to the terms of the Warrant Agency Agreement, in which case this sentence shall not apply. Notwithstanding anything to the contrary contained herein, a beneficial holder of the Warrant shall have all of the rights and remedies of the Holder hereunder.
|
b) |
Exercise Price. The exercise price per share of Common Stock under this Warrant shall be $[●], subject to adjustment hereunder (the “Exercise Price”). |
|
c) |
Cashless Exercise. This Warrant may also be exercised, in whole or in part, at such time by means of a “cashless exercise” in which the Holder shall be entitled to receive a number of Warrant Shares equal to the quotient obtained by dividing [(A-B) (X)] by (A), where: |
|
(A) = |
as applicable: (i) the VWAP on the Trading Day immediately preceding the date of the applicable Notice of Exercise if such Notice of Exercise is (1) both executed and delivered pursuant to Section 2(a) hereof on a day that is not a Trading Day or (2) both executed and delivered pursuant to Section 2(a) hereof on a Trading Day prior to the opening of “regular trading hours” (as defined in Rule 600(b)(68) of Regulation NMS promulgated under the federal securities laws) on such Trading Day, (ii) the VWAP on the Trading Day immediately preceding the date of the applicable Notice of Exercise or (iii) the VWAP on the date of the applicable Notice of Exercise if the date of such Notice of Exercise is a Trading Day and such Notice of Exercise is both executed and delivered pursuant to Section 2(a) hereof after the close of “regular trading hours” on such Trading Day; |
|
(B) = |
the Exercise Price of this Warrant, as adjusted hereunder; and |
|
(X) = |
the number of Warrant Shares that would be issuable upon exercise of this Warrant in accordance with the terms of this Warrant if such exercise were by means of a cash exercise rather than a cashless exercise. |
“Bid Price” means, for any date, the price determined by the first of the following clauses that applies: (a) if the Common Stock is then listed or quoted on a Trading Market, the bid price of the Common Stock for the time in question (or the nearest preceding date) on the Trading Market on which the Common Stock is then listed or quoted as reported by Bloomberg L.P. (based on a Trading Day from 9:30 a.m. (New York City time) to 4:02 p.m. (New York City time)), (b) if the Common Stock is not then listed or quoted on a Trading Market and if prices for the Common Stock are then reported on OTCQB or OTCQX, as applicable, the volume weighted average price of the Common Stock for such date (or the nearest preceding date) on OTCQB or OTCQX, as applicable, (c) if the Common Stock is not then listed or quoted for trading on a Trading Market or on OTCQB or OTCQX and if prices for the Common Stock are then reported on The Pink Open Market (or a similar organization or agency succeeding to its functions of reporting prices), the most recent bid price per share of the Common Stock so reported, or (d) in all other cases, the fair market value of a share of Common Stock as determined by an independent appraiser selected in good faith by the Holders of a majority in interest of the Securities then outstanding and reasonably acceptable to the Company, the fees and expenses of which shall be paid by the Company.
“VWAP” means, for any date, the price determined by the first of the following clauses that applies: (a) if the Common Stock is then listed or quoted on a Trading Market, the daily volume weighted average price of the Common Stock for such date (or the nearest preceding date) on the Trading Market on which the Common Stock is then listed or quoted as reported by Bloomberg L.P. (based on a Trading Day from 9:30 a.m. (New York City time) to 4:02 p.m. (New York City time)), (b) if the Common Stock is not then listed or quoted on a Trading Market and if prices for the Common Stock are then reported on OTCQB or OTCQX, as applicable, the volume weighted average price of the Common Stock for such date (or the nearest preceding date) on OTCQB or OTCQX as applicable, (c) if the Common Stock is not then listed or quoted for trading on a Trading Market or on OTCQB or OTCQX and if prices for the Common Stock are then reported on The Pink Open Market (or a similar organization or agency succeeding to its functions of reporting prices), the most recent bid price per share of the Common Stock so reported, or (d) in all other cases, the fair market value of a share of Common Stock as determined by an independent appraiser selected in good faith by the Holders of a majority in interest of the Securities then outstanding and reasonably acceptable to the Company, the fees and expenses of which shall be paid by the Company.
If Warrant Shares are issued in such a cashless exercise, the parties acknowledge and agree that in accordance with Section 3(a)(9) of the Securities Act, the Warrant Shares shall take on the registered characteristics of the Warrants being exercised. The Company agrees not to take any position contrary to this Section 2(c).
|
d) |
Mechanics of Exercise. |
i. Delivery of Warrant Shares Upon Exercise. The Company shall cause the Warrant Shares purchased hereunder to be transmitted by the Transfer Agent to the Holder by crediting the account of the Holder’s or its designee’s balance account with The Depository Trust Company through its Deposit or Withdrawal at Custodian system (“DWAC”) if the Company is then a participant in such system and either (A) there is an effective registration statement permitting the issuance of the Warrant Shares or (B) this Warrant is being exercised via cashless exercise, and otherwise by physical delivery of a certificate, registered in the Company’s share register in the name of the Holder or its designee, for the number of Warrant Shares to which the Holder is entitled pursuant to such exercise to the address specified by the Holder in the Notice of Exercise by the date that is the earlier of (A) the earlier of (i) one (1) Trading Day and (ii) the number of days comprising the Standard Settlement Period, in each case after the delivery to the Company of the Notice of Exercise and (B) one (1) Trading Day after delivery of the aggregate Exercise Price to the Company (such date, the “Warrant Share Delivery Date”). Upon delivery of the Notice of Exercise, the Holder shall be deemed for all corporate purposes to have become the holder of record of the Warrant Shares with respect to which this Warrant has been exercised, irrespective of the date of delivery of the Warrant Shares, provided that payment of the aggregate Exercise Price (other than in the case of a cashless exercise) is received by the Warrant Share Delivery Date. If the Company fails for any reason to deliver to the Holder the Warrant Shares subject to a Notice of Exercise by the Warrant Share Delivery Date, provided that payment of the aggregate Exercise Price (other than in the instance of a cashless exercise) is received by the Company by such date, the Company shall pay to the Holder, in cash, as liquidated damages and not as a penalty, for each $1,000 of Warrant Shares subject to such exercise (based on the VWAP of the Common Stock on the date of the applicable Notice of Exercise), $10 per Trading Day (increasing to $20 per Trading Day on the fifth Trading Day after the Warrant Share Delivery Date) for each Trading Day after such Warrant Share Delivery Date until such Warrant Shares are delivered or Holder rescinds such exercise. Notwithstanding anything herein to the contrary, the Company shall not be responsible for the payment of liquidated damages resulting from the failure to issue or deliver Warrant Shares if such failure is caused by any action or inaction of the Holder. The Company agrees to maintain a transfer agent that is a participant in the FAST program so long as this Warrant remains outstanding and exercisable. As used herein, “Standard Settlement Period” means the standard settlement period, expressed in a number of Trading Days, on the Company’s primary Trading Market with respect to the Common Stock as in effect on the date of delivery of the Notice of Exercise. Notwithstanding the foregoing, with respect to any Notice(s) of Exercise delivered on or prior to 12:00 p.m. (New York City time) on the Initial Exercise Date, which may be delivered at any time after the time of execution of the Underwriting Agreement, the Company agrees to deliver the Warrant Shares subject to such notice(s) by 4:00 p.m. (New York City time) on the Initial Exercise Date and the Initial Exercise Date shall be the Warrant Share Delivery Date for purposes hereunder, provided that payment of the aggregate Exercise Price (other than in the case of a cashless exercise) is received by such Warrant Share Delivery Date.
ii. Delivery of New Warrants Upon Exercise. If this Warrant shall have been exercised in part, the Company shall, at the request of a Holder and upon surrender of this Warrant certificate, at the time of delivery of the Warrant Shares, deliver to the Holder a new Warrant evidencing the rights of the Holder to purchase the unpurchased Warrant Shares called for by this Warrant, which new Warrant shall in all other respects be identical with this Warrant.
iii. Rescission Rights. If the Company fails to cause the Transfer Agent to transmit to the Holder the Warrant Shares pursuant to Section 2(d)(i) by the Warrant Share Delivery Date, then the Holder will have the right to rescind such exercise.
iv. Compensation for Buy-In on Failure to Timely Deliver Warrant Shares Upon Exercise. In addition to any other rights available to the Holder, if the Company fails to cause the Transfer Agent to transmit to the Holder the Warrant Shares in accordance with the provisions of Section 2(d)(i) above pursuant to an exercise on or before the Warrant Share Delivery Date, and if after such date the Holder is required by its broker to purchase (in an open market transaction or otherwise) or the Holder’s brokerage firm otherwise purchases, shares of Common Stock to deliver in satisfaction of a sale by the Holder of the Warrant Shares which the Holder anticipated receiving upon such exercise (a “Buy-In”), then the Company shall (A) pay in cash to the Holder the amount, if any, by which (x) the Holder’s total purchase price (including brokerage commissions, if any) for the shares of Common Stock so purchased exceeds (y) the amount obtained by multiplying (1) the number of Warrant Shares that the Company was required to deliver to the Holder in connection with the exercise at issue times (2) the price at which the sell order giving rise to such purchase obligation was executed, and (B) at the option of the Holder, either reinstate the portion of the Warrant and equivalent number of Warrant Shares for which such exercise was not honored (in which case such exercise shall be deemed rescinded) or deliver to the Holder the number of shares of Common Stock that would have been issued had the Company timely complied with its exercise and delivery obligations hereunder. For example, if the Holder purchases Common Stock having a total purchase price of $11,000 to cover a Buy-In with respect to an attempted exercise of Warrants with an aggregate sale price giving rise to such purchase obligation of $10,000, under clause (A) of the immediately preceding sentence the Company shall be required to pay the Holder $1,000. The Holder shall provide the Company written notice indicating the amounts payable to the Holder in respect of the Buy-In and, upon request of the Company, evidence of the amount of such loss. Nothing herein shall limit a Holder’s right to pursue any other remedies available to it hereunder, at law or in equity including, without limitation, a decree of specific performance and/or injunctive relief with respect to the Company’s failure to timely deliver shares of Common Stock upon exercise of the Warrant as required pursuant to the terms hereof. Notwithstanding the forgoing, the Warrant Agent shall not, in any event, be subject to, or responsible for, Buy-In penalties contemplated by this Section 2(d)(iv).
v. No Fractional Shares or Scrip. No fractional shares or scrip representing fractional shares shall be issued upon the exercise of this Warrant. As to any fraction of a share which the Holder would otherwise be entitled to purchase upon such exercise, the Company shall, at its election, either pay a cash adjustment in respect of such final fraction in an amount equal to such fraction multiplied by the Exercise Price or round up to the next whole share.
vi. Charges, Taxes and Expenses. Issuance of Warrant Shares shall be made without charge to the Holder for any issue or transfer tax or other incidental expense in respect of the issuance of such Warrant Shares, all of which taxes and expenses shall be paid by the Company, and such Warrant Shares shall be issued in the name of the Holder or in such name or names as may be directed by the Holder; provided, however, that in the event that Warrant Shares are to be issued in a name other than the name of the Holder, this Warrant when surrendered for exercise shall be accompanied by the Assignment Form attached hereto duly executed by the Holder and the Company may require, as a condition thereto, the payment of a sum sufficient to reimburse it for any transfer tax incidental thereto. The Company shall pay all Transfer Agent fees required for same-day processing of any Notice of Exercise and all fees to the Depository Trust Company (or another established clearing corporation performing similar functions) required for same-day electronic delivery of the Warrant Shares.
vii. Closing of Books. The Company will not close its stockholder books or records in any manner which prevents the timely exercise of this Warrant, pursuant to the terms hereof.
|
e) |
Holder’s Exercise Limitations. The Company shall not effect any exercise of this Warrant, and a Holder shall not have the right to exercise any portion of this Warrant, pursuant to Section 2 or otherwise, to the extent that after giving effect to such issuance after exercise as set forth on the applicable Notice of Exercise, the Holder (together with the Holder’s Affiliates, and any other Persons acting as a group together with the Holder or any of the Holder’s Affiliates (such Persons, “Attribution Parties”)), would beneficially own in excess of the Beneficial Ownership Limitation (as defined below). For purposes of the foregoing sentence, the number of shares of Common Stock beneficially owned by the Holder and its Affiliates and Attribution Parties shall include the number of shares of Common Stock issuable upon exercise of this Warrant with respect to which such determination is being made, but shall exclude the number of shares of Common Stock which would be issuable upon (i) exercise of the remaining, nonexercised portion of this Warrant beneficially owned by the Holder or any of its Affiliates or Attribution Parties and (ii) exercise or conversion of the unexercised or nonconverted portion of any other securities of the Company (including, without limitation, any other Common Stock Equivalents) subject to a limitation on conversion or exercise analogous to the limitation contained herein beneficially owned by the Holder or any of its Affiliates or Attribution Parties. Except as set forth in the preceding sentence, for purposes of this Section 2(e), beneficial ownership shall be calculated in accordance with Section 13(d) of the Exchange Act and the rules and regulations promulgated thereunder, it being acknowledged by the Holder that the Company is not representing to the Holder that such calculation is in compliance with Section 13(d) of the Exchange Act and the Holder is solely responsible for any schedules required to be filed in accordance therewith. To the extent that the limitation contained in this Section 2(e) applies, the determination of whether this Warrant is exercisable (in relation to other securities owned by the Holder together with any Affiliates and Attribution Parties) and of which portion of this Warrant is exercisable shall be in the sole discretion of the Holder, and the submission of a Notice of Exercise shall be deemed to be the Holder’s determination of whether this Warrant is exercisable (in relation to other securities owned by the Holder together with any Affiliates and Attribution Parties) and of which portion of this Warrant is exercisable, in each case subject to the Beneficial Ownership Limitation, and the Company shall have no obligation to verify or confirm the accuracy of such determination and shall have no liability for exercises of the Warrant that are not in compliance with the Beneficial Ownership Limitation, except to the extent the Holder relies on a number of outstanding shares of Common Stock that was provided by the Company. In addition, a determination as to any group status as contemplated above shall be determined in accordance with Section 13(d) of the Exchange Act and the rules and regulations promulgated thereunder, and the Company shall have no obligation to verify or confirm the accuracy of such determination and shall have no liability for exercises of the Warrant that are not in compliance with the Beneficial Ownership Limitation, except to the extent the Holder relies on the number of outstanding shares of Common Stock that was provided by the Company. For purposes of this Section 2(e), in determining the number of outstanding shares of Common Stock, a Holder may rely on the number of outstanding shares of Common Stock as reflected in (A) the Company’s most recent periodic or annual report filed with the Commission, as the case may be, (B) a more recent public announcement by the Company or (C) a more recent written notice by the Company or the Transfer Agent setting forth the number of shares of Common Stock outstanding. Upon the written request of a Holder, the Company shall within one (1) Trading Day confirm orally and in writing to the Holder the number of shares of Common Stock then outstanding. In any case, the number of outstanding shares of Common Stock shall be determined after giving effect to the conversion or exercise of securities of the Company, including this Warrant, by the Holder or its Affiliates or Attribution Parties since the date as of which such number of outstanding shares of Common Stock was reported. The “Beneficial Ownership Limitation” shall be [4.99/9.99]% of the number of shares of the Common Stock outstanding immediately after giving effect to the issuance of shares of Common Stock issuable upon exercise of this Warrant. The Holder, upon written notice to the Company, may increase or decrease the Beneficial Ownership Limitation provisions of this Section 2(e), provided that the Beneficial Ownership Limitation in no event exceeds 9.99% of the number of shares of the Common Stock outstanding immediately after giving effect to the issuance of shares of Common Stock upon exercise of this Warrant held by the Holder and the provisions of this Section 2(e) shall continue to apply. Any increase in the Beneficial Ownership Limitation will not be effective until the 61st day after such notice is delivered to the Company. The provisions of this paragraph shall be construed and implemented in a manner otherwise than in strict conformity with the terms of this Section 2(e) to correct this paragraph (or any portion hereof) which may be defective or inconsistent with the intended Beneficial Ownership Limitation herein contained or to make changes or supplements necessary or desirable to properly give effect to such limitation. The limitations contained in this paragraph shall apply to a successor holder of this Warrant. If the Warrant is unexercisable as a result of the Holder’s Beneficial Ownership Limitation, no alternate consideration is owing to the Holder. |
Section 3. Certain Adjustments.
a) Stock Dividends and Splits. If the Company, at any time while this Warrant is outstanding: (i) pays a stock dividend or otherwise makes a distribution or distributions on shares of its Common Stock or any other equity or equity equivalent securities payable in shares of Common Stock (which, for avoidance of doubt, shall not include any shares of Common Stock issued by the Company upon exercise of this Warrant), (ii) subdivides outstanding shares of Common Stock into a larger number of shares, (iii) combines (including by way of reverse stock split) outstanding shares of Common Stock into a smaller number of shares, or (iv) issues by reclassification of shares of the Common Stock any shares of capital stock of the Company, then in each case the Exercise Price shall be multiplied by a fraction of which the numerator shall be the number of shares of Common Stock (excluding treasury shares, if any) outstanding immediately before such event and of which the denominator shall be the number of shares of Common Stock outstanding immediately after such event, and the number of shares issuable upon exercise of this Warrant shall be proportionately adjusted such that the aggregate Exercise Price of this Warrant shall remain unchanged. Any adjustment made pursuant to this Section 3(a) shall become effective immediately after the record date for the determination of stockholders entitled to receive such dividend or distribution and shall become effective immediately after the effective date in the case of a subdivision, combination or re‑classification.
b) Subsequent Rights Offerings. In addition to any adjustments pursuant to Section 3(a) above, if at any time the Company grants, issues or sells any Common Stock Equivalents or rights to purchase stock, warrants, securities or other property pro rata to the record holders of any class of shares of Common Stock (the “Purchase Rights”), then the Holder will be entitled to acquire, upon the terms applicable to such Purchase Rights, the aggregate Purchase Rights which the Holder could have acquired if the Holder had held the number of shares of Common Stock acquirable upon complete exercise of this Warrant (without regard to any limitations on exercise hereof, including without limitation, the Beneficial Ownership Limitation) immediately before the date on which a record is taken for the grant, issuance or sale of such Purchase Rights, or, if no such record is taken, the date as of which the record holders of shares of Common Stock are to be determined for the grant, issue or sale of such Purchase Rights (provided, however, that to the extent that the Holder’s right to participate in any such Purchase Right would result in the Holder exceeding the Beneficial Ownership Limitation, then the Holder shall not be entitled to participate in such Purchase Right to such extent (or beneficial ownership of such shares of Common Stock as a result of such Purchase Right to such extent)and such Purchase Right to such extent shall be held in abeyance for the Holder until such time, if ever, as its right thereto would not result in the Holder exceeding the Beneficial Ownership Limitation).
c) Pro Rata Distributions. During such time as this Warrant is outstanding, if the Company shall declare or make any dividend or other distribution of its assets (or rights to acquire its assets) to holders of shares of Common Stock, by way of return of capital or otherwise (including, without limitation, any distribution of cash, stock or other securities, property or options by way of a dividend, spin off, reclassification, corporate rearrangement, scheme of arrangement or other similar transaction) (a “Distribution”), at any time after the issuance of this Warrant, then, in each such case, the Holder shall be entitled to participate in such Distribution to the same extent that the Holder would have participated therein if the Holder had held the number of shares of Common Stock acquirable upon complete exercise of this Warrant (without regard to any limitations on exercise hereof, including without limitation, the Beneficial Ownership Limitation) immediately before the date of which a record is taken for such Distribution, or, if no such record is taken, the date as of which the record holders of shares of Common Stock are to be determined for the participation in such Distribution (provided, however, that to the extent that the Holder’s right to participate in any such Distribution would result in the Holder exceeding the Beneficial Ownership Limitation, then the Holder shall not be entitled to participate in such Distribution to such extent (or in the beneficial ownership of any shares of Common Stock as a result of such Distribution to such extent) and the portion of such Distribution shall be held in abeyance for the benefit of the Holder until such time, if ever, as its right thereto would not result in the Holder exceeding the Beneficial Ownership Limitation).
d) Fundamental Transaction; Change of Control. If, at any time while this Warrant is outstanding, (i) the Company, directly or indirectly, in one or more related transactions effects any merger or consolidation of the Company with or into another Person, (ii) the Company (or any Subsidiary), directly or indirectly, effects any sale, lease, license, assignment, transfer, conveyance or other disposition of all or substantially all of the assets of the Company in one or a series of related transactions, (iii) any, direct or indirect, purchase offer, tender offer or exchange offer (whether by the Company or another Person) is completed pursuant to which holders of Common Stock are permitted to sell, tender or exchange their shares for other securities, cash or property and has been accepted by the holders of 50% or more of the outstanding Common Stock or 50% or more of the voting power of the common equity of the Company, (iv) the Company, directly or indirectly, in one or more related transactions effects any reclassification, reorganization or recapitalization of the Common Stock or any compulsory share exchange pursuant to which the Common Stock is effectively converted into or exchanged for other securities, cash or property, or (v) the Company, directly or indirectly, in one or more related transactions consummates a stock or share purchase agreement or other business combination (including, without limitation, a reorganization, recapitalization, spin-off, merger or scheme of arrangement) with another Person or group of Persons whereby such other Person or group acquires 50% or more of the outstanding shares of Common Stock or 50% or more of the voting power of the common equity of the Company (each a “Fundamental Transaction”), then, upon any subsequent exercise of this Warrant, the Holder shall have the right to receive, for each Warrant Share that would have been issuable upon such exercise immediately prior to the occurrence of such Fundamental Transaction, at the option of the Holder (without regard to any limitation in Section 2(e) on the exercise of this Warrant), the number of shares of Common Stock of the successor or acquiring corporation or of the Company, if it is the surviving corporation, and any additional consideration (the “Alternate Consideration”) receivable as a result of such Fundamental Transaction by a holder of the number of shares of Common Stock for which this Warrant is exercisable immediately prior to such Fundamental Transaction (without regard to any limitation in Section 2(e) on the exercise of this Warrant). For purposes of any such exercise, the determination of the Exercise Price shall be appropriately adjusted to apply to such Alternate Consideration based on the amount of Alternate Consideration issuable in respect of one share of Common Stock in such Fundamental Transaction, and the Company shall apportion the Exercise Price among the Alternate Consideration in a reasonable manner reflecting the relative value of any different components of the Alternate Consideration. If holders of Common Stock are given any choice as to the securities, cash or property to be received in a Fundamental Transaction, then the Holder shall be given the same choice as to the Alternate Consideration it receives upon any exercise of this Warrant following such Fundamental Transaction.
Notwithstanding anything to the contrary, in the event of a Change of Control, the Company or any Successor Entity (as defined below) shall, at the Holder’s option, exercisable at any time concurrently with, or within 30 days after, the consummation of the Change of Control (or, if later, the date of the public announcement of the applicable Change of Control), purchase this Warrant from the Holder by paying to the Holder an amount of cash equal to the Black Scholes Value (as defined below) of the remaining unexercised portion of this Warrant on the date of the consummation of such Fundamental Transaction; provided, however, that, if the Change of Control is not within the Company’s control, including not approved by the Company’s Board of Directors, the Holder shall only be entitled to receive from the Company or any Successor Entity the same type or form of consideration (and in the same proportion), at the Black Scholes Value of the unexercised portion of this Warrant, that is being offered and paid to the holders of Common Stock of the Company in connection with the Change of Control, whether that consideration be in the form of cash, stock or any combination thereof, or whether the holders of Common Stock are given the choice to receive from among alternative forms of consideration in connection with the Change of Control; provided, further, that if holders of Common Stock of the Company are not offered or paid any consideration in such Change of Control, such holders of Common Stock will be deemed to have received common stock of the Successor Entity (which Entity may be the Company following such Change of Control) in such Change of Control. “Black Scholes Value” means the value of this Warrant based on the Black-Scholes Option Pricing Model obtained from the “OV” function on Bloomberg determined as of the day of consummation of the applicable contemplated Change of Control for pricing purposes and reflecting (A) a risk-free interest rate corresponding to the U.S. Treasury rate for a period equal to the time between the date of the public announcement of the applicable contemplated Change of Control and the Termination Date, (B) an expected volatility equal to the greater of 100% and the 100 day volatility obtained from the HVT function on Bloomberg (determined utilizing a 365 day annualization factor) as of the Trading Day immediately following the public announcement of the applicable contemplated Change of Control, (C) the underlying price per share used in such calculation shall be the highest VWAP during the period beginning on the Trading Day immediately preceding the public announcement of the applicable contemplated Change of Control (or the consummation of the applicable Change of Control, if earlier) and ending on the Trading Day immediately prior to the consummation of such Change of Control, (D) a remaining option time equal to the time between the date of the public announcement of the applicable contemplated Change of Control and the Termination Date and (E) a zero cost of borrow. The payment of the Black Scholes Value will be made by wire transfer of immediately available funds (or such other consideration) within the later of (i) five Business Days of the Holder’s election and (ii) the date of consummation of the Change of Control. The Company shall cause any successor entity in a Change of Control in which the Company is not the survivor (the “Successor Entity”) to assume in writing all of the obligations of the Company under this Warrant in accordance with the provisions of this Section 3(d) pursuant to written agreements in form and substance reasonably satisfactory to the Holder and approved by the Holder (without unreasonable delay) prior to such Fundamental Transaction and shall, at the option of the Holder, deliver to the Holder in exchange for this Warrant a security of the Successor Entity evidenced by a written instrument substantially similar in form and substance to this Warrant which is exercisable for a corresponding number of shares of capital stock of such Successor Entity (or its parent entity) equivalent to the shares of Common Stock acquirable and receivable upon exercise of this Warrant (without regard to any limitations on the exercise of this Warrant) prior to such Fundamental Transaction, and with an exercise price which applies the exercise price hereunder to such shares of capital stock (but taking into account the relative value of the shares of Common Stock pursuant to such Fundamental Transaction and the value of such shares of capital stock, such number of shares of capital stock and such exercise price being for the purpose of protecting the economic value of this Warrant immediately prior to the consummation of such Change of Control), and which is reasonably satisfactory in form and substance to the Holder. Upon the occurrence of any such Change of Control, the Successor Entity shall be added to the term “Company” under this Warrant (so that from and after the occurrence or consummation of such Change of Control, each and every provision of this Warrant and the other Transaction Documents referring to the “Company” shall refer instead to each of the Company and the Successor Entity or Successor Entities, jointly and severally), and the Successor Entity or Successor Entities, jointly and severally with the Company, may exercise every right and power of the Company prior thereto and the Successor Entity or Successor Entities shall assume all of the obligations of the Company prior thereto under this Warrant and the other Transaction Documents with the same effect as if the Company and such Successor Entity or Successor Entities, jointly and severally, had been named as the Company herein. For the avoidance of doubt, the Holder shall be entitled to the benefits of the provisions of this Section 3(d) regardless of (i) whether the Company has sufficient authorized shares of Common Stock for the issuance of Warrant Shares and/or (ii) whether a Change of Control occurs prior to the Initial Exercise Date.
For purposes of this Section 3(d), “Change of Control” means any Fundamental Transaction other than (i) any reorganization, recapitalization or reclassification of the Common Stock in which holders of the Company’s voting power immediately prior to such reorganization, recapitalization or reclassification continue after such reorganization, recapitalization or reclassification to hold publicly traded securities and, directly or indirectly, are, in all material respect, the holders of the voting power of the surviving entity (or entities with the authority or voting power to elect the members of the board of directors (or their equivalent if other than a corporation) of such entity or entities) after such reorganization, recapitalization or reclassification, (ii) pursuant to a migratory merger effected solely for the purpose of changing the jurisdiction of incorporation of the Company or (iii) a merger in connection with a bona fide acquisition by the Company of any Person in which (x) the gross consideration paid, directly or indirectly, by the Company in such acquisition is not greater than 50% of the Company’s market capitalization as calculated on the date of the consummation of such merger and (y) such merger does not contemplate a change to the identity of a majority of the board of directors of the Company. Notwithstanding anything herein to the contrary, any transaction or series of transactions that, directly or indirectly, results in the Company or the Successor Entity not having Common Stock or common stock, as applicable, registered under the Securities Exchange Act of 1934, as amended, and listed on a Trading Market shall be deemed a Change of Control.
e) Calculations. All calculations under this Section 3 shall be made to the nearest cent or the nearest 1/100th of a share, as the case may be. For purposes of this Section 3, the number of shares of Common Stock deemed to be issued and outstanding as of a given date shall be the sum of the number of shares of Common Stock (excluding treasury shares, if any) issued and outstanding.
f) Notice to Holder.
|
i. |
Adjustment to Exercise Price. Whenever the Exercise Price is adjusted pursuant to any provision of this Section 3, the Company shall promptly deliver to the Holder and the Warrant Agent by facsimile or email a notice setting forth the Exercise Price after such adjustment and any resulting adjustment to the number of Warrant Shares and setting forth a brief statement of the facts requiring such adjustment. |
|
ii. |
Notice to Allow Exercise by Holder. If (A) the Company shall declare a dividend (or any other distribution in whatever form other than a stock split) on the Common Stock, (B) the Company shall declare a special nonrecurring cash dividend on or a redemption of the Common Stock, (C) the Company shall authorize the granting to all holders of the Common Stock rights or warrants to subscribe for or purchase any shares of capital stock of any class or of any rights, (D) the approval of any stockholders of the Company shall be required in connection with any reclassification of the Common Stock, any consolidation or merger to which the Company is a party, any sale or transfer of all or substantially all of the assets of the Company, or any compulsory share exchange whereby the Common Stock is converted into other securities, cash or property, or (E) the Company shall authorize the voluntary or involuntary dissolution, liquidation or winding up of the affairs of the Company, then, in each case, the Company shall cause to be delivered by facsimile or email to the Holder at its last facsimile number or email address as it shall appear upon the Warrant Register of the Company, at least 20 calendar days prior to the applicable record or effective date hereinafter specified, a notice stating (x) the date on which a record is to be taken for the purpose of such dividend, distribution, redemption, rights or warrants, or if a record is not to be taken, the date as of which the holders of the Common Stock of record to be entitled to such dividend, distributions, redemption, rights or warrants are to be determined or (y) the date on which such reclassification, consolidation, merger, sale, transfer or share exchange is expected to become effective or close, and the date as of which it is expected that holders of the Common Stock of record shall be entitled to exchange their shares of the Common Stock for securities, cash or other property deliverable upon such reclassification, consolidation, merger, sale, transfer or share exchange; provided that the failure to deliver such notice or any defect therein or in the delivery thereof shall not affect the validity of the corporate action required to be specified in such notice. To the extent that any notice provided in this Warrant constitutes, or contains, material, non-public information regarding the Company or any of the Company’s subsidiaries, the Company shall simultaneously file such notice with the Commission pursuant to a Current Report on Form 8-K. The Holder shall remain entitled to exercise this Warrant during the period commencing on the date of such notice to the effective date of the event triggering such notice except as may otherwise be expressly set forth herein. |
|
iii. |
Voluntary Adjustment by the Company. Subject to the rules and regulations of the Trading Market, the Company may at any time during the term of this Warrant, subject to the prior written consent of the Holder, reduce the then current Exercise Price to any amount and for any period of time deemed appropriate by the board of directors of the Company. |
Section 4. Transfer of Warrant.
a) Transferability. This Warrant and all rights hereunder (including, without limitation, any registration rights) are transferable, in whole or in part, upon surrender of this Warrant at the office of the Warrant Agent designated for such purpose, together with a written assignment of this Warrant substantially in the form attached hereto properly completed and duly executed by the Holder or its agent or attorney and funds sufficient to pay any transfer taxes payable upon the making of such transfer accompanied by reasonable evidence of authority of the party making such request that may be required by the Warrant Agent including but not limited to, the signature guarantee of a guarantor institution which is a participant in a signature guarantee program approved by the Securities Transfer Association. Upon such surrender and, if required, such payment, the Company shall execute and deliver a new Warrant or Warrants in the name of the assignee or assignees, as applicable, and in the denomination or denominations specified in such instrument of assignment, and shall issue to the assignor a new Warrant evidencing the portion of this Warrant not so assigned, and this Warrant shall promptly be cancelled. Notwithstanding anything herein to the contrary, the Holder shall not be required to physically surrender this Warrant to the Company unless the Holder has assigned this Warrant in full, in which case, the Holder shall surrender this Warrant to the Company or the Warrant Agent within three (3) Trading Days of the date on which the Holder delivers an assignment form to the Company assigning this Warrant in full. The Warrant, if properly assigned in accordance herewith, may be exercised by a new holder for the purchase of Warrant Shares without having a new Warrant issued.
b) New Warrants. If this Warrant is not held in global form through DTC (or any successor depositary), this Warrant may be divided or combined with other Warrants upon presentation hereof at the aforesaid office of the Company, together with a written notice specifying the names and denominations in which new Warrants are to be issued, signed by the Holder or its agent or attorney. Subject to compliance with Section 4(a), as to any transfer which may be involved in such division or combination, the Company shall execute and deliver a new Warrant or Warrants in exchange for the Warrant or Warrants to be divided or combined in accordance with such notice. All Warrants issued on transfers or exchanges shall be dated the initial issuance date of this Warrant and shall be identical with this Warrant except as to the number of Warrant Shares issuable pursuant thereto.
c) Warrant Register. The Warrant Agent shall register this Warrant, upon records to be maintained by the Warrant Agent for that purpose (the “Warrant Register”), in the name of the record Holder hereof from time to time. The Company and the Warrant Agent may deem and treat the registered Holder of this Warrant as the absolute owner hereof for the purpose of any exercise hereof or any distribution to the Holder, and for all other purposes, absent actual notice to the contrary. Notwithstanding the foregoing, nothing herein shall prevent the Warrant Agent or any agent of the Warrant Agent from giving effect to any written certification, proxy or other authorization furnished by DTC or any other depository governing the exercise of the rights of a holder of a beneficial interest in any Warrant. The rights of beneficial owners in a Warrant held in global shall be exercised by the Holder or a Participant (as defined in the Warrant Agency Agreement) through the depository’s system, except to the extent expressly set forth in the Warrant Agency Agreement.
Section 5. Miscellaneous.
a) No Rights as Stockholder Until Exercise; No Settlement in Cash. This Warrant does not entitle the Holder to any voting rights, dividends or other rights as a stockholder of the Company prior to the exercise hereof as set forth in Section 2(d)(i), except as expressly set forth in Section 3. Without limiting the rights of a Holder to receive Warrant Shares on a “cashless exercise” only as permitted in Section 2(c), and to receive the cash payments contemplated pursuant to Sections 2(d)(i) and 2(d)(iv), in no event will the Company be required to net cash settle an exercise of this Warrant.
b) Loss, Theft, Destruction or Mutilation of Warrant. The Company covenants that upon receipt by the Company and the Warrant Agent of evidence reasonably satisfactory to them of the loss, theft, destruction or mutilation of this Warrant or any stock certificate relating to the Warrant Shares, and in case of loss, theft or destruction, of indemnity or security reasonably satisfactory to them (which, in the case of the Warrant held in global form through DTC, shall not include the posting of any bond), and upon surrender and cancellation of such Warrant or stock certificate, if mutilated, the Company will make and deliver a new Warrant or stock certificate of like tenor and dated as of such cancellation, in lieu of such Warrant or stock certificate.
c) Saturdays, Sundays, Holidays, etc. If the last or appointed day for the taking of any action or the expiration of any right required or granted herein shall not be a Trading Day, then, such action may be taken or such right may be exercised on the next succeeding Trading Day.
d) Authorized Shares. The Company covenants that, at all times during the period the Warrant is outstanding, it will reserve from its authorized and unissued Common Stock a sufficient number of shares to provide for the issuance of the Warrant Shares upon the exercise of any purchase rights under this Warrant. The Company further covenants that its issuance of this Warrant shall constitute full authority to its officers who are charged with the duty of issuing the necessary Warrant Shares upon the exercise of the purchase rights under this Warrant. The Company will take all such reasonable action as may be necessary to assure that such Warrant Shares may be issued as provided herein without violation of any applicable law or regulation, or of any requirements of the Trading Market upon which the Common Stock may be listed. The Company covenants that all Warrant Shares which may be issued upon the exercise of the purchase rights represented by this Warrant will, upon exercise of the purchase rights represented by this Warrant and payment for such Warrant Shares in accordance herewith, be duly authorized, validly issued, fully paid and nonassessable and free from all taxes, liens and charges created by the Company in respect of the issue thereof (other than taxes in respect of any transfer occurring contemporaneously with such issue).
Except and to the extent as waived or consented to by the Holder, the Company shall not by any action, including, without limitation, amending its certificate of incorporation or through any reorganization, transfer of assets, consolidation, merger, dissolution, issue or sale of securities or any other voluntary action, avoid or seek to avoid the observance or performance of any of the terms of this Warrant, but will at all times in good faith assist in the carrying out of all such terms and in the taking of all such actions as may be necessary or appropriate to protect the rights of Holder as set forth in this Warrant against impairment (it being understood that this Warrant shall not in any case prevent the Company from effecting any such amendment, reorganization, transfer, consolidation, merger, dissolution, issuance or sale). Without limiting the generality of the foregoing, the Company will (i) not increase the par value of any Warrant Shares above the amount payable therefor upon such exercise immediately prior to such increase in par value, (ii) take all such action as may be necessary or appropriate in order that the Company may validly and legally issue fully paid and nonassessable Warrant Shares upon the exercise of this Warrant and (iii) use commercially reasonable efforts to obtain all such authorizations, exemptions or consents from any public regulatory body having jurisdiction thereof, as may be, necessary to enable the Company to perform its obligations under this Warrant.
Before taking any action, which would result in an adjustment in the number of Warrant Shares for which this Warrant is exercisable or in the Exercise Price, the Company shall obtain all such authorizations or exemptions thereof, or consents thereto, as may be necessary from any public regulatory body or bodies having jurisdiction thereof.
e) Jurisdiction. All questions concerning the construction, validity, enforcement and interpretation of this Warrant shall be governed by and construed and enforced in accordance with the internal laws of the State of New York, without regard to the principles of conflicts of law thereof. Each party agrees that all legal proceedings concerning the interpretations, enforcement and defense of the transactions contemplated by this Warrant (whether brought against a party hereto or their respective affiliates, directors, officers, shareholders, partners, members, employees or agents) shall be commenced exclusively in the state and federal courts sitting in the City of New York. Each party hereby irrevocably submits to the exclusive jurisdiction of the state and federal courts sitting in the City of New York, Borough of Manhattan for the adjudication of any dispute hereunder or in connection herewith or with any transaction contemplated hereby or discussed herein, and hereby irrevocably waives, and agrees not to assert in any suit, action or proceeding, any claim that it is not personally subject to the jurisdiction of any such court, that such suit, action or proceeding is improper or is an inconvenient venue for such proceeding. As between the Company and Holder, (i) each party hereby irrevocably waives personal service of process and consents to process being served in any such suit, action or proceeding by mailing a copy thereof via registered or certified mail or overnight delivery (with evidence of delivery) to such party at the address in effect for notices to it under this Warrant and agrees that such service shall constitute good and sufficient service of process and notice thereof (nothing contained herein shall be deemed to limit in any way any right to serve process in any other manner permitted by law), and (ii) if either party shall commence an action, suit or proceeding to enforce any provisions of this Warrant, the prevailing party in such action, suit or proceeding shall be reimbursed by the other party for their reasonable attorneys’ fees and other costs and expenses incurred with the investigation, preparation and prosecution of such action or proceeding.
f) Restrictions. The Holder acknowledges that the Warrant Shares acquired upon the exercise of this Warrant, if not registered, and the Holder does not utilize cashless exercise, will have restrictions upon resale imposed by state and federal securities laws.
g) Nonwaiver and Expenses. No course of dealing or any delay or failure to exercise any right hereunder on the part of Holder shall operate as a waiver of such right or otherwise prejudice the Holder’s rights, powers or remedies, notwithstanding the fact that the right to exercise this Warrant terminates on the Termination Date. Without limiting any other provision of this Warrant, if the Company willfully and knowingly fails to comply with any provision of this Warrant, which results in any material damages to the Holder, the Company shall pay to the Holder such amounts as shall be sufficient to cover any costs and expenses including, but not limited to, reasonable attorneys’ fees, including those of appellate proceedings, incurred by the Holder in collecting any amounts due pursuant hereto or in otherwise enforcing any of its rights, powers or remedies hereunder.
h) Notices. Any and all notices or other communications or deliveries to be provided by the Holders hereunder including, without limitation, any Notice of Exercise, shall be in writing and delivered personally, or e-mail (other than to the Warrant Agent), or sent by a nationally recognized overnight courier service, addressed:
If to the Company, to:
Autonomix Medical, Inc.
21 Waterway Avenue, Suite 300
The Woodlands, Texas 77380
Attention: Chief Financial Officer
If to the Warrant Agent, to:
Equity Stock Transfer LLC
[●]
Attention: _________
or such other facsimile number, email address or address as the Company or the Warrant Agent may specify for such purposes by notice to the Holders. Any and all notices or other communications or deliveries to be provided by the Company hereunder shall be in writing and delivered personally, by facsimile or e-mail, or sent by a nationally recognized overnight courier service addressed to each Holder at the facsimile number, e-mail address or address of such Holder appearing on the books of the Warrant Agent. Any notice or other communication or deliveries hereunder shall be deemed given and effective on the earliest of (i) the date of transmission, if such notice or communication is delivered via facsimile at the facsimile number or via e-mail at the e-mail address set forth in this Section prior to 5:30 p.m. (New York City time) on any date, (ii) the next Trading Day after the date of transmission, if such notice or communication is delivered via facsimile at the facsimile number or via e-mail at the e-mail address set forth in this Section on a day that is not a Trading Day or later than 5:30 p.m. (New York City time) on any Trading Day, (iii) the second Trading Day following the date of mailing, if sent by U.S. nationally recognized overnight courier service, or (iv) upon actual receipt by the party to whom such notice is required to be given. Notwithstanding any other provision of this Warrant, where this Warrant provides for notice of any event to the Holder, if this Warrant is held in global form by DTC (or any successor depositary), such notice shall be sufficiently given if given to DTC (or any successor depositary) pursuant to the procedures of DTC (or such successor depositary), subject to a Holder’s right to elect to receive a Warrant in certificated form pursuant to the terms of the Warrant Agent Agreement, in which case this sentence shall not apply. To the extent that any notice provided hereunder constitutes, or contains, material, non-public information regarding the Company or any subsidiaries, the Company shall simultaneously file such notice with the Commission pursuant to a Current Report on Form 8-K.
i) Limitation of Liability. No provision hereof, in the absence of any affirmative action by the Holder to exercise this Warrant to purchase Warrant Shares, and no enumeration herein of the rights or privileges of the Holder, shall give rise to any liability of the Holder for the purchase price of any Common Stock or as a stockholder of the Company, whether such liability is asserted by the Company or by creditors of the Company.
j) Remedies. The Holder, in addition to being entitled to exercise all rights granted by law, including recovery of damages, will be entitled to specific performance of its rights under this Warrant. The Company agrees that monetary damages would not be adequate compensation for any loss incurred by reason of a breach by it of the provisions of this Warrant and hereby agrees to waive and not to assert the defense in any action for specific performance that a remedy at law would be adequate.
k) Successors and Assigns. Subject to applicable securities laws, this Warrant and the rights and obligations evidenced hereby shall inure to the benefit of and be binding upon the successors and permitted assigns of the Company and the successors and permitted assigns of Holder. The provisions of this Warrant are intended to be for the benefit of any Holder from time to time of this Warrant and shall be enforceable by the Holder or holder of Warrant Shares.
l) Amendment. This Warrant may be modified or amended or the provisions hereof waived with the written consent of the Company, on the one hand, and the Holders of this Warrant, on the other hand.
m) Severability. Wherever possible, each provision of this Warrant shall be interpreted in such manner as to be effective and valid under applicable law, but if any provision of this Warrant shall be prohibited by or invalid under applicable law, such provision shall be ineffective to the extent of such prohibition or invalidity, without invalidating the remainder of such provisions or the remaining provisions of this Warrant.
n) Headings. The headings used in this Warrant are for the convenience of reference only and shall not, for any purpose, be deemed a part of this Warrant.
o) Warrant Agency Agreement. This Warrant is issued subject to the Warrant Agency Agreement. To the extent any provision of this Warrant conflicts with the express provisions of the Warrant Agency Agreement, the provisions of this Warrant shall govern and be controlling; provided, however, that all provisions with respect to the rights, duties, obligations, protections, immunities and liability of the Warrant Agent only shall be determined and interpreted solely by the provisions of the Warrant Agency Agreement.
********************
(Signature Page Follows)
IN WITNESS WHEREOF, the Company has caused this Warrant to be executed by its officer thereunto duly authorized as of the date first above indicated.
|
AUTONOMIX MEDICAL, INC. |
||
|
By: |
||
|
Name: |
||
|
Title: |
||
[SIGNATURE PAGE TO COMMON STOCK PURCHASE WARRANT,
AUTONOMIX MEDICAL, INC.]
EXHIBIT A
NOTICE OF EXERCISE
TO: AUTONOMIX MEDICAL, INC.
(1) The undersigned hereby elects to purchase ________ Warrant Shares of the Company pursuant to the terms of the attached Warrant (only if exercised in full), and tenders herewith payment of the exercise price in full, together with all applicable transfer taxes, if any.
(2) Payment shall take the form of (check applicable box):
[ ] in lawful money of the United States; or
[ ] if permitted the cancellation of such number of Warrant Shares as is necessary, in accordance with the formula set forth in subsection 2(c), to exercise this Warrant with respect to the maximum number of Warrant Shares purchasable pursuant to the cashless exercise procedure set forth in subsection 2(c).
(3) Please issue said Warrant Shares in the name of the undersigned or in such other name as is specified below:
_______________________________
The Warrant Shares shall be delivered to the following DWAC Account Number:
_______________________________
_______________________________
_______________________________
[SIGNATURE OF HOLDER]
Name of Investing Entity: ______________________________________________________
Signature of Authorized Signatory of Investing Entity: _________________________________
Name of Authorized Signatory: ___________________________________________________
Title of Authorized Signatory: ____________________________________________________
Date: ________________________________________________________________________
EXHIBIT B
ASSIGNMENT FORM
(To assign the foregoing Warrant, execute this form and supply required information. Do not use this form to exercise the Warrant to purchase shares.)
FOR VALUE RECEIVED, the foregoing Warrant and all rights evidenced thereby are hereby assigned to
|
Name: |
|||
|
(Please Print) |
|||
|
Address: |
|||
|
(Please Print) |
|||
|
Phone Number: |
|||
|
Email Address: |
|||
|
Dated: _______________ __, ______ |
|||
|
Holder’s Signature: |
|||
|
Holder’s Address: |
|||
Exhibit 4.5
REPRESENTATIVE WARRANT
THE REGISTERED HOLDER OF THIS PURCHASE WARRANT BY ITS ACCEPTANCE HEREOF, AGREES THAT IT WILL NOT SELL, TRANSFER, ASSIGN, PLEDGE OR HYPOTHECATE, OR BE THE SUBJECT OF ANY HEDGING, SHORT SALE, DERIVATIVE, PUT, OR CALL TRANSACTION THAT WOULD RESULT IN THE EFFECTIVE ECONOMIC DISPOSITION OF THIS PURCHASE WARRANT OR THE UNDERLYING SECURITIES FOR A PERIOD OF ONE HUNDRED EIGHTY (180) DAYS IMMEDIATELY FOLLOWING THE COMMENCEMENT DATE (DEFINED BELOW) EXCEPT AS HEREIN PROVIDED AND THE REGISTERED HOLDER OF THIS PURCHASE WARRANT AGREES THAT IT WILL NOT SELL, TRANSFER, ASSIGN, PLEDGE OR HYPOTHECATE, OR BE THE SUBJECT OF ANY HEDGING, SHORT SALE, DERIVATIVE, PUT, OR CALL TRANSACTION THAT WOULD RESULT IN THE EFFECTIVE ECONOMIC DISPOSITION OF, THIS PURCHASE WARRANT OR THE UNDERLYING SECURITIES FOR A PERIOD OF ONE HUNDRED EIGHTY (180) DAYS IMMEDIATELY FOLLOWING THE COMMENCEMENT DATE TO ANYONE OTHER THAN (I) LADENBEURG THALMANN & CO. INC OR ANY UNDERWRITER OR A SELECTED DEALER IN CONNECTION WITH THE OFFERING, OR (II) A BONA FIDE OFFICER OR PARTNER OF LADENBURG THALMANN & CO. INC., OR OF ANY SUCH UNDERWRITER OR SELECTED DEALER.
THIS PURCHASE WARRANT IS NOT EXERCISABLE PRIOR TO [________________] [DATE THAT IS 180 DAYS FROM THE COMMENCEMENT DATE OF THE OFFERING]. VOID AFTER 5:00 P.M., EASTERN TIME, [___________________] [DATE THAT IS FIVE YEARS FROM THE COMMENCEMENT DATE OF THE OFFERING].
AUTONOMIX MEDICAL, INC.
|
Warrant Shares: ______ |
Issue Date: [●], 2024 |
| Initial Exercise Date: [●], 2025 |
THIS COMMON STOCK PURCHASE WARRANT (the “Warrant”) certifies that, for value received, _____________ , or its assigns (the “Holder”) is entitled, upon the terms and subject to the limitations on exercise and the conditions hereinafter set forth, at any time on or after [_____], 2025, which is one hundred eighty (180) days following the Commencement Date (the “Initial Exercise Date”) and, in accordance with FINRA Rule 5110(g)(8)(A), prior to 5:00 p.m. (New York time) on [_____], 2029, the date that is five (5) years following the Commencement Date (the “Termination Date”), but not thereafter, to subscribe for and purchase from AUTONOMIX MEDICAL, INC., a Delaware corporation (the “Company”), up to ______1 shares of Common Stock, par value $0.001 per share (the “Common Stock”), of the Company (the “Warrant Shares”), as subject to adjustment hereunder. The purchase price of one share of Common Stock under this Warrant shall be equal to the Exercise Price, as defined in Section 2(b).
“Commencement Date” means [____], 2024, the date on which sales of the securities issued in the Offering commenced.
Section 1. Definitions. Capitalized terms used and not otherwise defined herein shall have the meanings set forth in that certain Underwriting Agreement (the “Underwriting Agreement”), dated [●], 2024, by and between the Company and Ladenburg Thalmann & Co. Inc., as the Representative of the several underwriters named therein.
1 6.0% of the aggregate number of shares of common stock and pre-funded warrants sold in the Offering inclusive of the over-allotment option.
Section 2. Exercise.
|
a) |
Exercise of Warrant. Subject to the terms and conditions hereof, exercise of the purchase rights represented by this Warrant may be made, in whole or in part, at any time or times on or after the Initial Exercise Date and on or before the Termination Date by delivery to the Company of a duly executed facsimile copy or PDF copy submitted by e-mail (or e-mail attachment) of the Notice of Exercise in the form attached hereto as Exhibit A (the “Notice of Exercise”). Within the earlier of (i) one (1) Trading Day and (ii) the number of Trading Days comprising the Standard Settlement Period (as defined in Section 2(d)(i) herein) following the date of exercise as aforesaid, the Holder shall deliver the aggregate Exercise Price for the Warrant Shares specified in the applicable Notice of Exercise by wire transfer or cashier’s check drawn on a United States bank unless the cashless exercise procedure specified in Section 2(c) below is applicable and specified in the attached Notice of Exercise. No ink-original Notice of Exercise shall be required, nor shall any medallion guarantee (or other type of guarantee or notarization) of any Notice of Exercise be required. Notwithstanding anything herein to the contrary, the Holder shall not be required to physically surrender this Warrant to the Company until the Holder has purchased all of the Warrant Shares available hereunder and the Warrant has been exercised in full, in which case, the Holder shall surrender this Warrant to the Company for cancellation within three (3) Trading Days of the date on which the final Notice of Exercise is delivered to the Company. Partial exercises of this Warrant resulting in purchases of a portion of the total number of Warrant Shares available hereunder shall have the effect of lowering the outstanding number of Warrant Shares purchasable hereunder in an amount equal to the applicable number of Warrant Shares purchased. The Holder and the Company shall maintain records showing the number of Warrant Shares purchased and the date of such purchases. The Company shall deliver any objection to any Notice of Exercise within one (1) Trading Day of receipt of such notice. The Holder and any assignee, by acceptance of this Warrant, acknowledge and agree that, by reason of the provisions of this paragraph, following the purchase of a portion of the Warrant Shares hereunder, the number of Warrant Shares available for purchase hereunder at any given time may be less than the amount stated on the face hereof. |
|
b) |
Exercise Price. The exercise price per share of the Common Stock under this Warrant shall be $______2, subject to adjustment hereunder (the “Exercise Price”). |
|
c) |
Cashless Exercise. This Warrant may also be exercised, in whole or in part, at such time by means of a “cashless exercise” in which the Holder shall be entitled to receive a number of Warrant Shares equal to the quotient obtained by dividing [(A-B) (X)] by (A), where: |
|
(A) = |
as applicable: (i) the VWAP on the Trading Day immediately preceding the date of the applicable Notice of Exercise if such Notice of Exercise is (1) both executed and delivered pursuant to Section 2(a) hereof on a day that is not a Trading Day or (2) both executed and delivered pursuant to Section 2(a) hereof on a Trading Day prior to the opening of “regular trading hours” (as defined in Rule 600(b)(68) of Regulation NMS promulgated under the federal securities laws) on such Trading Day, (ii) the VWAP on the Trading Day immediately preceding the date of the applicable Notice of Exercise or (iii) the VWAP on the date of the applicable Notice of Exercise if the date of such Notice of Exercise is a Trading Day and such Notice of Exercise is both executed and delivered pursuant to Section 2(a) hereof after the close of “regular trading hours” on such Trading Day; |
2 155% of the public offering price per share of common stock in the Offering.
|
(B) = |
the Exercise Price of this Warrant, as adjusted hereunder; and |
|
(X) = |
the number of Warrant Shares that would be issuable upon exercise of this Warrant in accordance with the terms of this Warrant if such exercise were by means of a cash exercise rather than a cashless exercise. |
“Bid Price” means, for any date, the price determined by the first of the following clauses that applies: (a) if the Common Stock is then listed or quoted on a Trading Market, the bid price of the Common Stock for the time in question (or the nearest preceding date) on the Trading Market on which the Common Stock is then listed or quoted as reported by Bloomberg L.P. (based on a Trading Day from 9:30 a.m. (New York City time) to 4:02 p.m. (New York City time)), (b) if the Common Stock is not then listed or quoted on a Trading Market and if prices for the Common Stock are then reported on OTCQB or OTCQX, as applicable, the volume weighted average price of the Common Stock for such date (or the nearest preceding date) on OTCQB or OTCQX, as applicable, (c) if the Common Stock is not then listed or quoted for trading on a Trading Market or on OTCQB or OTCQX and if prices for the Common Stock are then reported on The Pink Open Market (or a similar organization or agency succeeding to its functions of reporting prices), the most recent bid price per share of the Common Stock so reported, or (d) in all other cases, the fair market value of a share of Common Stock as determined by an independent appraiser selected in good faith by the Holders of a majority in interest of the Securities then outstanding and reasonably acceptable to the Company, the fees and expenses of which shall be paid by the Company.
“VWAP” means, for any date, the price determined by the first of the following clauses that applies: (a) if the Common Stock is then listed or quoted on a Trading Market, the daily volume weighted average price of the Common Stock for such date (or the nearest preceding date) on the Trading Market on which the Common Stock is then listed or quoted as reported by Bloomberg L.P. (based on a Trading Day from 9:30 a.m. (New York City time) to 4:02 p.m. (New York City time)), (b) if the Common Stock is not then listed or quoted on a Trading Market and if prices for the Common Stock are then reported on OTCQB or OTCQX, as applicable, the volume weighted average price of the Common Stock for such date (or the nearest preceding date) on OTCQB or OTCQX as applicable, (c) if the Common Stock is not then listed or quoted for trading on a Trading Market or on OTCQB or OTCQX and if prices for the Common Stock are then reported on The Pink Open Market (or a similar organization or agency succeeding to its functions of reporting prices), the most recent bid price per share of the Common Stock so reported, or (d) in all other cases, the fair market value of a share of Common Stock as determined by an independent appraiser selected in good faith by the Holders of a majority in interest of the Securities then outstanding and reasonably acceptable to the Company, the fees and expenses of which shall be paid by the Company.
If Warrant Shares are issued in such a cashless exercise, the parties acknowledge and agree that in accordance with Section 3(a)(9) of the Securities Act, the Warrant Shares shall take on the registered characteristics of the Warrants being exercised. The Company agrees not to take any position contrary to this Section 2(c).
|
d) |
Mechanics of Exercise. |
i. Delivery of Warrant Shares Upon Exercise. The Company shall cause the Warrant Shares purchased hereunder to be transmitted by the Transfer Agent to the Holder by crediting the account of the Holder’s or its designee’s balance account with The Depository Trust Company through its Deposit or Withdrawal at Custodian system (“DWAC”) if the Company is then a participant in such system and either (A) there is an effective registration statement permitting the issuance of the Warrant Shares or (B) this Warrant is being exercised via cashless exercise, and otherwise by physical delivery of a certificate, registered in the Company’s share register in the name of the Holder or its designee, for the number of Warrant Shares to which the Holder is entitled pursuant to such exercise to the address specified by the Holder in the Notice of Exercise by the date that is the earlier of (A) the earlier of (i) one (1) Trading Day and (ii) the number of days comprising the Standard Settlement Period, in each case after the delivery to the Company of the Notice of Exercise and (B) one (1) Trading Day after delivery of the aggregate Exercise Price to the Company (such date, the “Warrant Share Delivery Date”). Upon delivery of the Notice of Exercise, the Holder shall be deemed for all corporate purposes to have become the holder of record of the Warrant Shares with respect to which this Warrant has been exercised, irrespective of the date of delivery of the Warrant Shares, provided that payment of the aggregate Exercise Price (other than in the case of a cashless exercise) is received by the Warrant Share Delivery Date. If the Company fails for any reason to deliver to the Holder the Warrant Shares subject to a Notice of Exercise by the Warrant Share Delivery Date, provided that payment of the aggregate Exercise Price (other than in the instance of a cashless exercise) is received by the Company by such date, the Company shall pay to the Holder, in cash, as liquidated damages and not as a penalty, for each $1,000 of Warrant Shares subject to such exercise (based on the VWAP of the Common Stock on the date of the applicable Notice of Exercise), $10 per Trading Day (increasing to $20 per Trading Day on the fifth Trading Day after the Warrant Share Delivery Date) for each Trading Day after such Warrant Share Delivery Date until such Warrant Shares are delivered or Holder rescinds such exercise. Notwithstanding anything herein to the contrary, the Company shall not be responsible for the payment of liquidated damages resulting from the failure to issue or deliver Warrant Shares if such failure is caused by any action or inaction of the Holder. The Company agrees to maintain a transfer agent that is a participant in the FAST program so long as this Warrant remains outstanding and exercisable. As used herein, “Standard Settlement Period” means the standard settlement period, expressed in a number of Trading Days, on the Company’s primary Trading Market with respect to the Common Stock as in effect on the date of delivery of the Notice of Exercise. Notwithstanding the foregoing, with respect to any Notice(s) of Exercise delivered on or prior to 12:00 p.m. (New York City time) on the Initial Exercise Date, which may be delivered at any time after the time of execution of the Underwriting Agreement, the Company agrees to deliver the Warrant Shares subject to such notice(s) by 4:00 p.m. (New York City time) on the Initial Exercise Date and the Initial Exercise Date shall be the Warrant Share Delivery Date for purposes hereunder, provided that payment of the aggregate Exercise Price (other than in the case of a cashless exercise) is received by such Warrant Share Delivery Date.
ii. Delivery of New Warrants Upon Exercise. If this Warrant shall have been exercised in part, the Company shall, at the request of a Holder and upon surrender of this Warrant certificate, at the time of delivery of the Warrant Shares, deliver to the Holder a new Warrant evidencing the rights of the Holder to purchase the unpurchased Warrant Shares called for by this Warrant, which new Warrant shall in all other respects be identical with this Warrant.
iii. Rescission Rights. If the Company fails to cause the Transfer Agent to transmit to the Holder the Warrant Shares pursuant to Section 2(d)(i) by the Warrant Share Delivery Date, then the Holder will have the right to rescind such exercise.
iv. Compensation for Buy-In on Failure to Timely Deliver Warrant Shares Upon Exercise. In addition to any other rights available to the Holder, if the Company fails to cause the Transfer Agent to transmit to the Holder the Warrant Shares in accordance with the provisions of Section 2(d)(i) above pursuant to an exercise on or before the Warrant Share Delivery Date, and if after such date the Holder is required by its broker to purchase (in an open market transaction or otherwise) or the Holder’s brokerage firm otherwise purchases, shares of Common Stock to deliver in satisfaction of a sale by the Holder of the Warrant Shares which the Holder anticipated receiving upon such exercise (a “Buy-In”), then the Company shall (A) pay in cash to the Holder the amount, if any, by which (x) the Holder’s total purchase price (including brokerage commissions, if any) for the shares of Common Stock so purchased exceeds (y) the amount obtained by multiplying (1) the number of Warrant Shares that the Company was required to deliver to the Holder in connection with the exercise at issue times (2) the price at which the sell order giving rise to such purchase obligation was executed, and (B) at the option of the Holder, either reinstate the portion of the Warrant and equivalent number of Warrant Shares for which such exercise was not honored (in which case such exercise shall be deemed rescinded) or deliver to the Holder the number of shares of Common Stock that would have been issued had the Company timely complied with its exercise and delivery obligations hereunder. For example, if the Holder purchases Common Stock having a total purchase price of $11,000 to cover a Buy-In with respect to an attempted exercise of Warrants with an aggregate sale price giving rise to such purchase obligation of $10,000, under clause (A) of the immediately preceding sentence the Company shall be required to pay the Holder $1,000. The Holder shall provide the Company written notice indicating the amounts payable to the Holder in respect of the Buy-In and, upon request of the Company, evidence of the amount of such loss. Nothing herein shall limit a Holder’s right to pursue any other remedies available to it hereunder, at law or in equity including, without limitation, a decree of specific performance and/or injunctive relief with respect to the Company’s failure to timely deliver shares of Common Stock upon exercise of the Warrant as required pursuant to the terms hereof.
v. No Fractional Shares or Scrip. No fractional shares or scrip representing fractional shares shall be issued upon the exercise of this Warrant. As to any fraction of a share which the Holder would otherwise be entitled to purchase upon such exercise, the Company shall, at its election, either pay a cash adjustment in respect of such final fraction in an amount equal to such fraction multiplied by the Exercise Price or round up to the next whole share.
vi. Charges, Taxes and Expenses. Issuance of Warrant Shares shall be made without charge to the Holder for any issue or transfer tax or other incidental expense in respect of the issuance of such Warrant Shares, all of which taxes and expenses shall be paid by the Company, and such Warrant Shares shall be issued in the name of the Holder or in such name or names as may be directed by the Holder; provided, however, that in the event that Warrant Shares are to be issued in a name other than the name of the Holder, this Warrant when surrendered for exercise shall be accompanied by the Assignment Form attached hereto duly executed by the Holder and the Company may require, as a condition thereto, the payment of a sum sufficient to reimburse it for any transfer tax incidental thereto. The Company shall pay all Transfer Agent fees required for same-day processing of any Notice of Exercise and all fees to the Depository Trust Company (or another established clearing corporation performing similar functions) required for same-day electronic delivery of the Warrant Shares.
vii. Closing of Books. The Company will not close its stockholder books or records in any manner which prevents the timely exercise of this Warrant, pursuant to the terms hereof.
|
e) |
Holder’s Exercise Limitations. The Company shall not effect any exercise of this Warrant, and a Holder shall not have the right to exercise any portion of this Warrant, pursuant to Section 2 or otherwise, to the extent that after giving effect to such issuance after exercise as set forth on the applicable Notice of Exercise, the Holder (together with the Holder’s Affiliates, and any other Persons acting as a group together with the Holder or any of the Holder’s Affiliates (such Persons, “Attribution Parties”)), would beneficially own in excess of the Beneficial Ownership Limitation (as defined below). For purposes of the foregoing sentence, the number of shares of Common Stock beneficially owned by the Holder and its Affiliates and Attribution Parties shall include the number of shares of Common Stock issuable upon exercise of this Warrant with respect to which such determination is being made, but shall exclude the number of shares of Common Stock which would be issuable upon (i) exercise of the remaining, nonexercised portion of this Warrant beneficially owned by the Holder or any of its Affiliates or Attribution Parties and (ii) exercise or conversion of the unexercised or nonconverted portion of any other securities of the Company (including, without limitation, any other Common Stock Equivalents) subject to a limitation on conversion or exercise analogous to the limitation contained herein beneficially owned by the Holder or any of its Affiliates or Attribution Parties. Except as set forth in the preceding sentence, for purposes of this Section 2(e), beneficial ownership shall be calculated in accordance with Section 13(d) of the Exchange Act and the rules and regulations promulgated thereunder, it being acknowledged by the Holder that the Company is not representing to the Holder that such calculation is in compliance with Section 13(d) of the Exchange Act and the Holder is solely responsible for any schedules required to be filed in accordance therewith. To the extent that the limitation contained in this Section 2(e) applies, the determination of whether this Warrant is exercisable (in relation to other securities owned by the Holder together with any Affiliates and Attribution Parties) and of which portion of this Warrant is exercisable shall be in the sole discretion of the Holder, and the submission of a Notice of Exercise shall be deemed to be the Holder’s determination of whether this Warrant is exercisable (in relation to other securities owned by the Holder together with any Affiliates and Attribution Parties) and of which portion of this Warrant is exercisable, in each case subject to the Beneficial Ownership Limitation, and the Company shall have no obligation to verify or confirm the accuracy of such determination and shall have no liability for exercises of the Warrant that are not in compliance with the Beneficial Ownership Limitation, except to the extent the Holder relies on a number of outstanding shares of Common Stock that was provided by the Company. In addition, a determination as to any group status as contemplated above shall be determined in accordance with Section 13(d) of the Exchange Act and the rules and regulations promulgated thereunder, and the Company shall have no obligation to verify or confirm the accuracy of such determination and shall have no liability for exercises of the Warrant that are not in compliance with the Beneficial Ownership Limitation, except to the extent the Holder relies on the number of outstanding shares of Common Stock that was provided by the Company . For purposes of this Section 2(e), in determining the number of outstanding shares of Common Stock, a Holder may rely on the number of outstanding shares of Common Stock as reflected in (A) the Company’s most recent periodic or annual report filed with the Commission, as the case may be, (B) a more recent public announcement by the Company or (C) a more recent written notice by the Company or the Transfer Agent setting forth the number of shares of Common Stock outstanding. Upon the written request of a Holder, the Company shall within one (1) Trading Day confirm orally and in writing to the Holder the number of shares of Common Stock then outstanding. In any case, the number of outstanding shares of Common Stock shall be determined after giving effect to the conversion or exercise of securities of the Company, including this Warrant, by the Holder or its Affiliates or Attribution Parties since the date as of which such number of outstanding shares of Common Stock was reported. The “Beneficial Ownership Limitation” shall be [4.99/9.99]% of the number of shares of the Common Stock outstanding immediately after giving effect to the issuance of shares of Common Stock issuable upon exercise of this Warrant. The Holder, upon written notice to the Company, may increase or decrease the Beneficial Ownership Limitation provisions of this Section 2(e), provided that the Beneficial Ownership Limitation in no event exceeds 9.99% of the number of shares of the Common Stock outstanding immediately after giving effect to the issuance of shares of Common Stock upon exercise of this Warrant held by the Holder and the provisions of this Section 2(e) shall continue to apply. Any increase in the Beneficial Ownership Limitation will not be effective until the 61st day after such notice is delivered to the Company. The provisions of this paragraph shall be construed and implemented in a manner otherwise than in strict conformity with the terms of this Section 2(e) to correct this paragraph (or any portion hereof) which may be defective or inconsistent with the intended Beneficial Ownership Limitation herein contained or to make changes or supplements necessary or desirable to properly give effect to such limitation. The limitations contained in this paragraph shall apply to a successor holder of this Warrant. If the Warrant is unexercisable as a result of the Holder’s Beneficial Ownership Limitation, no alternate consideration is owing to the Holder. |
Section 3. Certain Adjustments.
a) Stock Dividends and Splits. If the Company, at any time while this Warrant is outstanding: (i) pays a stock dividend or otherwise makes a distribution or distributions on shares of its Common Stock or any other equity or equity equivalent securities payable in shares of Common Stock (which, for avoidance of doubt, shall not include any shares of Common Stock issued by the Company upon exercise of this Warrant), (ii) subdivides outstanding shares of Common Stock into a larger number of shares, (iii) combines (including by way of reverse stock split) outstanding shares of Common Stock into a smaller number of shares, or (iv) issues by reclassification of shares of the Common Stock any shares of capital stock of the Company, then in each case the Exercise Price shall be multiplied by a fraction of which the numerator shall be the number of shares of Common Stock (excluding treasury shares, if any) outstanding immediately before such event and of which the denominator shall be the number of shares of Common Stock outstanding immediately after such event, and the number of shares issuable upon exercise of this Warrant shall be proportionately adjusted such that the aggregate Exercise Price of this Warrant shall remain unchanged. Any adjustment made pursuant to this Section 3(a) shall become effective immediately after the record date for the determination of stockholders entitled to receive such dividend or distribution and shall become effective immediately after the effective date in the case of a subdivision, combination or re‑classification.
b) Subsequent Rights Offerings. In addition to any adjustments pursuant to Section 3(a) above, if at any time the Company grants, issues or sells any Common Stock Equivalents or rights to purchase stock, warrants, securities or other property pro rata to the record holders of any class of shares of Common Stock (the “Purchase Rights”), then the Holder will be entitled to acquire, upon the terms applicable to such Purchase Rights, the aggregate Purchase Rights which the Holder could have acquired if the Holder had held the number of shares of Common Stock acquirable upon complete exercise of this Warrant (without regard to any limitations on exercise hereof, including without limitation, the Beneficial Ownership Limitation) immediately before the date on which a record is taken for the grant, issuance or sale of such Purchase Rights, or, if no such record is taken, the date as of which the record holders of shares of Common Stock are to be determined for the grant, issue or sale of such Purchase Rights (provided, however, that to the extent that the Holder’s right to participate in any such Purchase Right would result in the Holder exceeding the Beneficial Ownership Limitation, then the Holder shall not be entitled to participate in such Purchase Right to such extent (or beneficial ownership of such shares of Common Stock as a result of such Purchase Right to such extent)and such Purchase Right to such extent shall be held in abeyance for the Holder until such time, if ever, as its right thereto would not result in the Holder exceeding the Beneficial Ownership Limitation).
c) Pro Rata Distributions. During such time as this Warrant is outstanding, if the Company shall declare or make any dividend or other distribution of its assets (or rights to acquire its assets) to holders of shares of Common Stock, by way of return of capital or otherwise (including, without limitation, any distribution of cash, stock or other securities, property or options by way of a dividend, spin off, reclassification, corporate rearrangement, scheme of arrangement or other similar transaction) (a “Distribution”), at any time after the issuance of this Warrant, then, in each such case, the Holder shall be entitled to participate in such Distribution to the same extent that the Holder would have participated therein if the Holder had held the number of shares of Common Stock acquirable upon complete exercise of this Warrant (without regard to any limitations on exercise hereof, including without limitation, the Beneficial Ownership Limitation) immediately before the date of which a record is taken for such Distribution, or, if no such record is taken, the date as of which the record holders of shares of Common Stock are to be determined for the participation in such Distribution (provided, however, that to the extent that the Holder’s right to participate in any such Distribution would result in the Holder exceeding the Beneficial Ownership Limitation, then the Holder shall not be entitled to participate in such Distribution to such extent (or in the beneficial ownership of any shares of Common Stock as a result of such Distribution to such extent) and the portion of such Distribution shall be held in abeyance for the benefit of the Holder until such time, if ever, as its right thereto would not result in the Holder exceeding the Beneficial Ownership Limitation).
d) Fundamental Transaction. If, at any time while this Warrant is outstanding, (i) the Company, directly or indirectly, in one or more related transactions effects any merger or consolidation of the Company with or into another Person, (ii) the Company (or any Subsidiary), directly or indirectly, effects any sale, lease, license, assignment, transfer, conveyance or other disposition of all or substantially all of the Company’s assets in one or a series of related transactions, (iii) any, direct or indirect, purchase offer, tender offer or exchange offer (whether by the Company or another Person) is completed pursuant to which holders of Common Stock are permitted to sell, tender or exchange their shares for other securities, cash or property and has been accepted by the holders of greater than 50% of the voting power of the outstanding common and preferred stock of the Company, (iv) the Company, directly or indirectly, in one or more related transactions effects any reclassification, reorganization or recapitalization of the Common Stock or any compulsory share exchange pursuant to which the Common Stock is effectively converted into or exchanged for other securities, cash or property, or (v) the Company, directly or indirectly, in one or more related transactions consummates a stock or share purchase agreement or other business combination (including, without limitation, a reorganization, recapitalization, spin-off, merger or scheme of arrangement) with another Person or group of Persons whereby such other Person or group acquires greater than 50% of the voting power of the outstanding common and preferred stock of the Company (each a “Fundamental Transaction”), then, upon any subsequent exercise of this Warrant, the Holder shall have the right to receive, for each Warrant Share that would have been issuable upon such exercise immediately prior to the occurrence of such Fundamental Transaction, at the option of the Holder (without regard to any limitation in Section 2(e) on the exercise of this Warrant), the number of shares of Common Stock of the successor or acquiring corporation or of the Company, if it is the surviving corporation, and any additional consideration (the “Alternate Consideration”) receivable as a result of such Fundamental Transaction by a holder of the number of shares of Common Stock for which this Warrant is exercisable immediately prior to such Fundamental Transaction (without regard to any limitation in Section 2(e) on the exercise of this Warrant). For purposes of any such exercise, the determination of the Exercise Price shall be appropriately adjusted to apply to such Alternate Consideration based on the amount of Alternate Consideration issuable in respect of one share of Common Stock in such Fundamental Transaction, and the Company shall apportion the Exercise Price among the Alternate Consideration in a reasonable manner reflecting the relative value of any different components of the Alternate Consideration. If holders of Common Stock are given any choice as to the securities, cash or property to be received in a Fundamental Transaction, then the Holder shall be given the same choice as to the Alternate Consideration it receives upon any exercise of this Warrant following such Fundamental Transaction. Notwithstanding anything to the contrary, in the event of a Fundamental Transaction, the Company or any Successor Entity (as defined below) shall, at the Holder’s option, exercisable at any time concurrently with, or within 30 days after, the consummation of the Fundamental Transaction (or, if later, the date of the public announcement of the applicable Fundamental Transaction), purchase this Warrant from the Holder by paying to the Holder an amount of cash equal to the Black Scholes Value (as defined below) of the remaining unexercised portion of this Warrant on the date of the consummation of such Fundamental Transaction; provided, however, that, if the Fundamental Transaction is not within the Company’s control, including not approved by the Company’s board of directors, the Holder shall only be entitled to receive from the Company or any Successor Entity the same type or form of consideration (and in the same proportion), at the Black Scholes Value of the unexercised portion of this Warrant, that is being offered and paid to the holders of shares of Common Stock of the Company in connection with the Fundamental Transaction, whether that consideration be in the form of cash, shares or any combination thereof, or whether the holders of shares of Common Stock are given the choice to receive from among alternative forms of consideration in connection with the Fundamental Transaction; provided, further, that if holders of shares of Common Stock of the Company are not offered or paid any consideration in such Fundamental Transaction, such holders of shares of Common Stock will be deemed to have received shares of common stock of the Successor Entity (which entity may be the Company following such Fundamental Transaction) in such Fundamental Transaction. “Black Scholes Value” means the value of this Warrant based on the Black-Scholes Option Pricing Model obtained from the “OV” function on Bloomberg determined as of the day of consummation of the applicable Fundamental Transaction for pricing purposes and reflecting (A) a risk-free interest rate corresponding to the U.S. Treasury rate for a period equal to the time between the date of the public announcement of the applicable contemplated Fundamental Transaction and the Termination Date, (B) an expected volatility equal to the greater of 100% and the 100 day volatility obtained from the HVT function on Bloomberg (determined utilizing a 365 day annualization factor) as of the Trading Day immediately following the public announcement of the applicable contemplated Fundamental Transaction, (C) the underlying price per share used in such calculation shall be the greater of (i) the sum of the price per share being offered in cash, if any, plus the value of any non-cash consideration, if any, being offered in such Fundamental Transaction and (ii) the highest VWAP during the period beginning on the Trading Day immediately preceding the public announcement of the applicable contemplated Fundamental Transaction (or the consummation of the applicable Fundamental Transaction, if earlier) and ending on the Trading Day of the Holder’s request pursuant to this Section 3(d) and (D) a remaining option time equal to the time between the date of the public announcement of the applicable contemplated Fundamental Transaction and the Termination Date and (E) a zero cost of borrow. The payment of the Black Scholes Value will be made by wire transfer of immediately available funds (or such other consideration) within the later of (i) five Business Days of the Holder’s election and (ii) the date of consummation of the Fundamental Transaction. The Company shall cause any successor entity in a Fundamental Transaction in which the Company is not the survivor (the “Successor Entity”) to assume in writing all of the obligations of the Company under this Warrant and the other Transaction Documents in accordance with the provisions of this Section 3(d) pursuant to written agreements in form and substance reasonably satisfactory to the Holder and approved by the Holder (without unreasonable delay) prior to such Fundamental Transaction and shall, at the option of the Holder, deliver to the Holder in exchange for this Warrant a security of the Successor Entity evidenced by a written instrument substantially similar in form and substance to this Warrant which is exercisable for a corresponding number of shares of capital stock of such Successor Entity (or its parent entity) equivalent to the shares of Common Stock acquirable and receivable upon exercise of this Warrant (without regard to any limitations on the exercise of this Warrant) prior to such Fundamental Transaction, and with an exercise price which applies the exercise price hereunder to such shares of capital stock (but taking into account the relative value of the shares of Common Stock pursuant to such Fundamental Transaction and the value of such shares of capital stock, such number of shares of capital stock and such exercise price being for the purpose of protecting the economic value of this Warrant immediately prior to the consummation of such Fundamental Transaction), and which is reasonably satisfactory in form and substance to the Holder. Upon the occurrence of any such Fundamental Transaction, the Successor Entity shall be added to the term “Company” under this Warrant (so that from and after the occurrence or consummation of such Fundamental Transaction, the provisions of this Warrant and the other Transaction Documents referring to the “Company” shall refer instead to each of the Company and the Successor Entity, or Successor Entities, jointly and severally), and the Successor Entity or Successor Entities, jointly and severally with the Company, may exercise every right and power of the Company prior thereto, and the Successor Entity or Successor Entities shall assume all of the obligations of the Company prior thereto under this Warrant and the other Transaction Documents with the same effect as if the Company and such Successor Entity or Successor Entities, jointly and severally, had been named as the Company herein.
e) Calculations. All calculations under this Section 3 shall be made to the nearest cent or the nearest 1/100th of a share, as the case may be. For purposes of this Section 3, the number of shares of Common Stock deemed to be issued and outstanding as of a given date shall be the sum of the number of shares of Common Stock (excluding treasury shares, if any) issued and outstanding.
|
f) |
Notice to Holder. |
|
i. |
Adjustment to Exercise Price. Whenever the Exercise Price is adjusted pursuant to any provision of this Section 3, the Company shall promptly deliver to the Holder by facsimile or email a notice setting forth the Exercise Price after such adjustment and any resulting adjustment to the number of Warrant Shares and setting forth a brief statement of the facts requiring such adjustment; . |
|
ii. |
Notice to Allow Exercise by Holder. If (A) the Company shall declare a dividend (or any other distribution in whatever form other than a stock split) on the Common Stock, (B) the Company shall declare a special nonrecurring cash dividend on or a redemption of the Common Stock, (C) the Company shall authorize the granting to all holders of the Common Stock rights or warrants to subscribe for or purchase any shares of capital stock of any class or of any rights, (D) the approval of any stockholders of the Company shall be required in connection with any reclassification of the Common Stock, any consolidation or merger to which the Company is a party, any sale or transfer of all or substantially all of the assets of the Company, or any compulsory share exchange whereby the Common Stock is converted into other securities, cash or property, or (E) the Company shall authorize the voluntary or involuntary dissolution, liquidation or winding up of the affairs of the Company, then, in each case, the Company shall cause to be delivered by facsimile or email to the Holder at its last facsimile number or email address as it shall appear upon the Warrant Register of the Company, at least 20 calendar days prior to the applicable record or effective date hereinafter specified, a notice stating (x) the date on which a record is to be taken for the purpose of such dividend, distribution, redemption, rights or warrants, or if a record is not to be taken, the date as of which the holders of the Common Stock of record to be entitled to such dividend, distributions, redemption, rights or warrants are to be determined or (y) the date on which such reclassification, consolidation, merger, sale, transfer or share exchange is expected to become effective or close, and the date as of which it is expected that holders of the Common Stock of record shall be entitled to exchange their shares of the Common Stock for securities, cash or other property deliverable upon such reclassification, consolidation, merger, sale, transfer or share exchange; provided that the failure to deliver such notice or any defect therein or in the delivery thereof shall not affect the validity of the corporate action required to be specified in such notice. To the extent that any notice provided in this Warrant constitutes, or contains, material, non-public information regarding the Company or any of the Company’s subsidiaries, the Company shall simultaneously file such notice with the Commission pursuant to a Current Report on Form 8-K. The Holder shall remain entitled to exercise this Warrant during the period commencing on the date of such notice to the effective date of the event triggering such notice except as may otherwise be expressly set forth herein. |
|
iii. |
Voluntary Adjustment by the Company. Subject to the rules and regulations of the Trading Market, the Company may at any time during the term of this Warrant, subject to the prior written consent of the Holder, reduce the then current Exercise Price to any amount and for any period of time deemed appropriate by the board of directors of the Company. |
Section 4. Transfer of Warrant.
a) Transferability. Pursuant to FINRA Rule 5110(e)(1)(A), neither this Warrant nor any Warrant Shares issued upon exercise of this Warrant shall be sold, transferred, assigned, pledged, or hypothecated, or be the subject of any hedging, short sale, derivative, put, or call transaction that would result in the effective economic disposition of the securities by any person for a period of 180 days immediately following the commencement of sales of the offering pursuant to which this Warrant is being issued, except the transfer of any security:
i. by operation of law or by reason of reorganization of the Company;
ii. to any FINRA member firm participating in the offering and the officers or partners thereof, if all securities so transferred remain subject to the lock-up restriction in this Section 4(a) for the remainder of the time period;
iii. if the aggregate amount of securities of the Company held by the Holder or related person do not exceed 1% of the securities being offered;
iv. that is beneficially owned on a pro-rata basis by all equity owners of an investment fund, provided that no participating member manages or otherwise directs investments by the fund, and participating members in the aggregate do not own more than 10% of the equity in the fund; or
v. the exercise or conversion of any security, if all securities received remain subject to the lock-up restriction in this Section 4(a) for the remainder of the time period.
Subject to the foregoing restriction, any applicable securities laws and the conditions set forth in Section 4(d), this Warrant and all rights hereunder (including, without limitation, any registration rights) are transferable, in whole or in part, upon surrender of this Warrant at the principal office of the Company or its designated agent, together with a written assignment of this Warrant substantially in the form attached hereto duly executed by the Holder or its agent or attorney and funds sufficient to pay any transfer taxes payable upon the making of such transfer. Upon such surrender and, if required, such payment, the Company shall execute and deliver a new Warrant or Warrants in the name of the assignee or assignees, as applicable, and in the denomination or denominations specified in such instrument of assignment, and shall issue to the assignor a new Warrant evidencing the portion of this Warrant not so assigned, and this Warrant shall promptly be cancelled. Notwithstanding anything herein to the contrary, the Holder shall not be required to physically surrender this Warrant to the Company unless the Holder has assigned this Warrant in full, in which case, the Holder shall surrender this Warrant to the Company within three (3) Trading Days of the date the Holder delivers an assignment form to the Company assigning this Warrant full. The Warrant, if properly assigned in accordance herewith, may be exercised by a new holder for the purchase of Warrant Shares without having a new Warrant issued.
b) New Warrants. This Warrant may be divided or combined with other Warrants upon presentation hereof at the aforesaid office of the Company, together with a written notice specifying the names and denominations in which new Warrants are to be issued, signed by the Holder or its agent or attorney. Subject to compliance with Section 4(a), as to any transfer which may be involved in such division or combination, the Company shall execute and deliver a new Warrant or Warrants in exchange for the Warrant or Warrants to be divided or combined in accordance with such notice. All Warrants issued on transfers or exchanges shall be dated the Issue Date of this Warrant and shall be identical with this Warrant except as to the number of Warrant Shares issuable pursuant thereto.
c) Warrant Register. The Company shall register this Warrant, upon records to be maintained by the Company for that purpose (the “Warrant Register”), in the name of the record Holder hereof from time to time. The Company may deem and treat the registered Holder of this Warrant as the absolute owner hereof for the purpose of any exercise hereof or any distribution to the Holder, and for all other purposes, absent actual notice to the contrary.
Section 5. Miscellaneous.
a) No Rights as Stockholder Until Exercise; No Settlement in Cash. This Warrant does not entitle the Holder to any voting rights, dividends or other rights as a stockholder of the Company prior to the exercise hereof as set forth in Section 2(d)(i), except as expressly set forth in Section 3. Without limiting the rights of a Holder to receive Warrant Shares on a “cashless exercise” only as permitted in Section 2(c), and to receive the cash payments contemplated pursuant to Sections 2(d)(i) and 2(d)(iv), in no event will the Company be required to net cash settle an exercise of this Warrant.
b) Loss, Theft, Destruction or Mutilation of Warrant. The Company covenants that upon receipt by the Company of evidence reasonably satisfactory to it of the loss, theft, destruction or mutilation of this Warrant or any stock certificate relating to the Warrant Shares, and in case of loss, theft or destruction, of indemnity or security reasonably satisfactory to it (which, in the case of the Warrant, shall not include the posting of any bond), and upon surrender and cancellation of such Warrant or stock certificate, if mutilated, the Company will make and deliver a new Warrant or stock certificate of like tenor and dated as of such cancellation, in lieu of such Warrant or stock certificate.
c) Saturdays, Sundays, Holidays, etc. If the last or appointed day for the taking of any action or the expiration of any right required or granted herein shall not be a Trading Day, then, such action may be taken or such right may be exercised on the next succeeding Trading Day.
d) Authorized Shares. The Company covenants that, at all times during the period the Warrant is outstanding, it will reserve from its authorized and unissued Common Stock a sufficient number of shares to provide for the issuance of the Warrant Shares upon the exercise of any purchase rights under this Warrant. The Company further covenants that its issuance of this Warrant shall constitute full authority to its officers who are charged with the duty of issuing the necessary Warrant Shares upon the exercise of the purchase rights under this Warrant. The Company will take all such reasonable action as may be necessary to assure that such Warrant Shares may be issued as provided herein without violation of any applicable law or regulation, or of any requirements of the Trading Market upon which the Common Stock may be listed. The Company covenants that all Warrant Shares which may be issued upon the exercise of the purchase rights represented by this Warrant will, upon exercise of the purchase rights represented by this Warrant and payment for such Warrant Shares in accordance herewith, be duly authorized, validly issued, fully paid and nonassessable and free from all taxes, liens and charges created by the Company in respect of the issue thereof (other than taxes in respect of any transfer occurring contemporaneously with such issue).
Except and to the extent as waived or consented to by the Holder, the Company shall not by any action, including, without limitation, amending its certificate of incorporation or through any reorganization, transfer of assets, consolidation, merger, dissolution, issue or sale of securities or any other voluntary action, avoid or seek to avoid the observance or performance of any of the terms of this Warrant, but will at all times in good faith assist in the carrying out of all such terms and in the taking of all such actions as may be necessary or appropriate to protect the rights of Holder as set forth in this Warrant against impairment (it being understood that this Warrant shall not in any case prevent the Company from effecting any such amendment, reorganization, transfer, consolidation, merger, dissolution, issuance or sale). Without limiting the generality of the foregoing, the Company will (i) not increase the par value of any Warrant Shares above the amount payable therefor upon such exercise immediately prior to such increase in par value, (ii) take all such action as may be necessary or appropriate in order that the Company may validly and legally issue fully paid and nonassessable Warrant Shares upon the exercise of this Warrant and (iii) use commercially reasonable efforts to obtain all such authorizations, exemptions or consents from any public regulatory body having jurisdiction thereof, as may be, necessary to enable the Company to perform its obligations under this Warrant.
Before taking any action, which would result in an adjustment in the number of Warrant Shares for which this Warrant is exercisable or in the Exercise Price, the Company shall obtain all such authorizations or exemptions thereof, or consents thereto, as may be necessary from any public regulatory body or bodies having jurisdiction thereof.
e) Jurisdiction. All questions concerning the construction, validity, enforcement and interpretation of this Warrant shall be determined in accordance with the provisions of the Underwriting Agreement.
f) Restrictions. The Holder acknowledges that the Warrant Shares acquired upon the exercise of this Warrant, if not registered, and the Holder does not utilize cashless exercise, will have restrictions upon resale imposed by state and federal securities laws.
g) Nonwaiver and Expenses. No course of dealing or any delay or failure to exercise any right hereunder on the part of Holder shall operate as a waiver of such right or otherwise prejudice the Holder’s rights, powers or remedies, notwithstanding the fact that the right to exercise this Warrant terminates on the Termination Date. Without limiting any other provision of this Warrant, if the Company willfully and knowingly fails to comply with any provision of this Warrant, which results in any material damages to the Holder, the Company shall pay to the Holder such amounts as shall be sufficient to cover any costs and expenses including, but not limited to, reasonable attorneys’ fees, including those of appellate proceedings, incurred by the Holder in collecting any amounts due pursuant hereto or in otherwise enforcing any of its rights, powers or remedies hereunder.
h) Notices. Any notice, request or other document required or permitted to be given or delivered to the Holder by the Company shall be delivered in accordance with the notice provisions of the Underwriting Agreement.
i) Limitation of Liability. No provision hereof, in the absence of any affirmative action by the Holder to exercise this Warrant to purchase Warrant Shares, and no enumeration herein of the rights or privileges of the Holder, shall give rise to any liability of the Holder for the purchase price of any Common Stock or as a stockholder of the Company, whether such liability is asserted by the Company or by creditors of the Company.
j) Remedies. The Holder, in addition to being entitled to exercise all rights granted by law, including recovery of damages, will be entitled to specific performance of its rights under this Warrant. The Company agrees that monetary damages would not be adequate compensation for any loss incurred by reason of a breach by it of the provisions of this Warrant and hereby agrees to waive and not to assert the defense in any action for specific performance that a remedy at law would be adequate.
k) Successors and Assigns. Subject to applicable securities laws, this Warrant and the rights and obligations evidenced hereby shall inure to the benefit of and be binding upon the successors and permitted assigns of the Company and the successors and permitted assigns of Holder. The provisions of this Warrant are intended to be for the benefit of any Holder from time to time of this Warrant and shall be enforceable by the Holder or holder of Warrant Shares.
l) Amendment. This Warrant may be modified or amended or the provisions hereof waived with the written consent of the Company, on the one hand, and the Holders of this Warrant, on the other hand.
m) Severability. Wherever possible, each provision of this Warrant shall be interpreted in such manner as to be effective and valid under applicable law, but if any provision of this Warrant shall be prohibited by or invalid under applicable law, such provision shall be ineffective to the extent of such prohibition or invalidity, without invalidating the remainder of such provisions or the remaining provisions of this Warrant.
n) Headings. The headings used in this Warrant are for the convenience of reference only and shall not, for any purpose, be deemed a part of this Warrant.
********************
(Signature Page Follows)
IN WITNESS WHEREOF, the Company has caused this Warrant to be executed by its officer thereunto duly authorized as of the date first above indicated.
|
AUTONOMIX MEDICAL, INC. |
||
|
By: |
||
|
Name: |
||
|
Title: |
||
[SIGNATURE PAGE TO REPRESENTATIVE WARRANT,
AUTONOMIX MEDICAL, INC.]
EXHIBIT A
NOTICE OF EXERCISE
TO: AUTONOMIX MEDICAL, INC.
(1) The undersigned hereby elects to purchase ________ Warrant Shares of the Company pursuant to the terms of the attached Warrant (only if exercised in full), and tenders herewith payment of the exercise price in full, together with all applicable transfer taxes, if any.
(2) Payment shall take the form of (check applicable box):
[ ] in lawful money of the United States; or
[ ] if permitted the cancellation of such number of Warrant Shares as is necessary, in accordance with the formula set forth in subsection 2(c), to exercise this Warrant with respect to the maximum number of Warrant Shares purchasable pursuant to the cashless exercise procedure set forth in subsection 2(c).
(3) Please issue said Warrant Shares in the name of the undersigned or in such other name as is specified below:
_______________________________
The Warrant Shares shall be delivered to the following DWAC Account Number:
_______________________________
_______________________________
_______________________________
[SIGNATURE OF HOLDER]
Name of Investing Entity: ______________________________________________________
Signature of Authorized Signatory of Investing Entity: _________________________________
Name of Authorized Signatory: ___________________________________________________
Title of Authorized Signatory: ____________________________________________________
Date: ________________________________________________________________________
EXHIBIT B
ASSIGNMENT FORM
(To assign the foregoing Warrant, execute this form and supply required information. Do not use this form to exercise the Warrant to purchase shares.)
FOR VALUE RECEIVED, the foregoing Warrant and all rights evidenced thereby are hereby assigned to
|
Name: |
|||
|
(Please Print) |
|||
|
Address: |
|||
|
(Please Print) |
|||
|
Phone Number: |
|||
|
Email Address: |
|||
|
Dated: _______________ __, ______ |
|||
|
Holder’s Signature: |
|||
|
Holder’s Address: |
|||
Exhibit 4.6
WARRANT AGENCY AGREEMENT
WARRANT AGENCY AGREEMENT, dated as of [●], 2024 (“Agreement”), between Autonomix Medical, Inc., a corporation organized under the laws of the State of Delaware (the “Company”), and Equity Stock Transfer LLC (the “Warrant Agent”).
W I T N E S S E T H
WHEREAS, pursuant to a registered offering (the “Offering”) by the Company of units comprised of: (i) shares of the Company’s common stock, par value $0.0001 per share (the “Common Stock”), and/or pre-funded warrants (each, a “Pre-Funded Warrant”), each to purchase one share of Common Stock (a “Pre-Funded Warrant Share”) at a price of $0.001 per share, and (ii) Series A warrants (each, a “Series A Warrant” and collectively with the Pre-Funded Warrants, the “Warrants”) each to purchase one share of Common Stock (a “Series A Warrant Share” and collectively with the Pre-Funded Warrant Shares, the “Warrant Shares”) at a price of $[●] per share; and
WHEREAS, upon the terms and subject to the conditions hereinafter set forth and pursuant to an effective registration statement on Form S-1, as amended (File No. 333-[●]) (the “Registration Statement”), and the terms and conditions of the Warrant Certificates, the Company wishes to issue the Warrants in book entry form entitling the respective holders of the Warrants (the “Holders,” which term shall include a Holder’s transferees, successors and assigns and “Holder” shall include, if the Warrants are held in “street name,” a Participant (as defined below) or a designee appointed by such Participant); and
WHEREAS, the shares of Common Stock and Warrants to be issued in connection with the Offering shall be immediately separable and will be issued separately, but will be purchased together in the Offering; and
WHEREAS, the Company wishes the Warrant Agent to act on behalf of the Company, and the Warrant Agent is willing so to act, in connection with the issuance, registration, transfer, exchange, exercise and replacement of the Warrants and, in the Warrant Agent’s capacity as the Company’s transfer agent, the delivery of the Warrant Shares (as defined below).
NOW, THEREFORE, in consideration of the premises and the mutual agreements herein set forth, the parties hereby agree as follows:
Section 1. Certain Definitions. For purposes of this Agreement, all capitalized terms not herein defined shall have the meanings hereby indicated:
(a) “Affiliate” has the meaning ascribed to it in Rule 12b-2 under the Securities Exchange Act of 1934, as amended (the “Exchange Act”).
(b) “Business Day” means any day other than Saturday, Sunday or other day on which commercial banks in The City of New York are authorized or required by law to remain closed; provided, however, for clarification, commercial banks shall not be deemed to be authorized or required by law to remain closed due to “stay at home”, “shelter-in-place”, “non-essential employee” or any other similar orders or restrictions or the closure of any physical branch locations at the direction of any governmental authority so long as the electronic funds transfer systems (including for wire transfers) of commercial banks in The City of New York generally are open for use by customers on such day.
(c) “Close of Business” on any given date means 5:00 p.m., New York City time, on such date; provided, however, that if such date is not a Business Day it means 5:00 p.m., New York City time, on the next succeeding Business Day.
(d) “Person” means an individual, corporation, association, partnership, limited liability company, joint venture, trust, unincorporated organization, government or political subdivision thereof or governmental agency or other entity.
(e) “Warrant Certificate” means a certificate in substantially the form attached as Exhibit 1 hereto with respect to the Pre-Funded Warrant and in substantially the form attached as Exhibit 2 hereto with respect to the Series A Warrant, representing such number of Warrant Shares as is indicated therein, provided that any reference to the delivery of a Warrant Certificate in this Agreement shall include delivery of a Definitive Certificate or a Global Warrant (each as defined below).
All other capitalized terms used but not otherwise defined herein shall have the meaning ascribed to such terms in the applicable Warrant Certificate.
Section 2. Appointment of Warrant Agent. The Company hereby appoints the Warrant Agent to act as agent for the Company in accordance with the terms and conditions hereof, and the Warrant Agent hereby accepts such appointment.
Section 3. Global Warrants.
(a) The Warrants shall be registered securities and shall be evidenced by a global warrant (the “Global Warrant”), in the form of the Warrant Certificate, which shall be deposited with the Warrant Agent and registered in the name of Cede & Co., a nominee of The Depository Trust Company (the “Depositary”), or as otherwise directed by the Depositary. Ownership of beneficial interests in the Warrants shall be shown on, and the transfer of such ownership shall be effected through, records maintained by (i) the Depositary or its nominee for each Global Warrant or (ii) institutions that have accounts with the Depositary (such institution, with respect to a Warrant in its account, a “Participant”).
(b) If the Depositary subsequently ceases to make its book-entry settlement system available for the Warrants, the Company may instruct the Warrant Agent regarding other arrangements for book-entry settlement. In the event that the Warrants are not eligible for, or it is no longer necessary to have the Warrants available in, book-entry form, the Warrant Agent shall provide written instructions to the Depositary to deliver to the Warrant Agent for cancellation each Global Warrant, and the Company shall instruct the Warrant Agent to deliver to each Holder a Warrant Certificate.
(c) A Holder has the right to elect at any time or from time to time a Warrant Exchange (as defined below) pursuant to a Warrant Certificate Request Notice (as defined below). Upon written notice by a Holder to the Company and the Warrant Agent for the exchange of some or all of such Holder’s interest in the Global Warrant for a separate certificate in the form attached hereto as Exhibit 1 or Exhibit 2, as applicable (such separate certificate, a “Definitive Certificate”) evidencing the same number of Warrants, which request shall be in the form attached hereto as Exhibit 3 (a “Warrant Certificate Request Notice” and the date of delivery of such Warrant Certificate Request Notice by the Holder, the “Warrant Certificate Request Notice Date” and the surrender by the Holder to the Warrant Agent of its beneficial interest in a number of Global Warrants for the same number of Warrants evidenced by a Warrant Certificate, a “Warrant Exchange”), the Company and the Warrant Agent shall promptly effect the Warrant Exchange and the Company shall promptly issue and deliver to the Holder a Definitive Certificate for such number of Warrants in the name set forth in the Warrant Certificate Request Notice. Such Definitive Certificate shall be dated the original issue date of the Warrants, shall be manually executed by an authorized signatory of the Company and shall be in the form attached hereto as Exhibit 1 or Exhibit 2, as applicable. In connection with a Warrant Exchange, the Company agrees to deliver the Definitive Certificate to the Holder within ten (10) Business Days of the Warrant Certificate Request Notice pursuant to the delivery instructions in the Warrant Certificate Request Notice (“Warrant Certificate Delivery Date”). If the Company fails for any reason to deliver to the Holder the Definitive Certificate subject to the Warrant Certificate Request Notice by the Warrant Certificate Delivery Date, the Company shall pay to the Holder, in cash, as liquidated damages and not as a penalty, for each $1,000 of Warrant Shares evidenced by such Definitive Certificate (based on the VWAP (as defined in the Warrants) of the Common Stock on the Warrant Certificate Request Notice Date), $10 per Business Day for each Business Day after such Warrant Certificate Delivery Date until such Definitive Certificate is delivered or, prior to delivery of such Warrant Certificate, the Holder rescinds such Warrant Exchange. The Company covenants and agrees that, upon the date of delivery of the Warrant Certificate Request Notice, the Holder shall be deemed to be the holder of the Definitive Certificate and, notwithstanding anything to the contrary set forth herein, the Definitive Certificate shall be deemed for all purposes to contain all of the terms and conditions of the Warrants evidenced by such Warrant Certificate and the terms of this Agreement, other than Sections 3(c), 3(d) and 9 herein, shall not apply to the Warrants evidenced by the Definitive Certificate. Notwithstanding anything herein to the contrary, the Company shall act as warrant agent with respect to any Definitive Certificate requested and issued pursuant to this section. Notwithstanding anything to the contrary contained in this Agreement, in the event of inconsistency between any provision in this Agreement and any provision in a Definitive Certificate, as it may from time to time be amended, the terms of such Definitive Certificate shall control.
(d) A Holder of a Definitive Certificate (pursuant to a Warrant Exchange or otherwise) has the right to elect at any time or from time to time a Global Warrants Exchange (as defined below) pursuant to a Global Warrants Request Notice (as defined below). Upon written notice by a Holder to the Company for the exchange of some or all of such Holder’s Warrants evidenced by a Definitive Certificate for a beneficial interest in Global Warrants held in book-entry form through the Depositary evidencing the same number of Warrants, which request shall be in the form attached hereto as Exhibit 4 (a “Global Warrants Request Notice” and the date of delivery of such Global Warrants Request Notice by the Holder, the “Global Warrants Request Notice Date” and the surrender upon delivery by the Holder of the Warrants evidenced by Definitive Certificates for the same number of Warrants evidenced by a beneficial interest in Global Warrants held in book-entry form through the Depositary, a “Global Warrants Exchange”), the Company shall promptly effect the Global Warrants Exchange and shall promptly direct the Warrant Agent to issue and deliver to the Holder Global Warrants for such number of Warrants in the Global Warrants Request Notice, which beneficial interest in such Global Warrants shall be delivered by the Depositary’s Deposit or Withdrawal at Custodian system to the Holder pursuant to the instructions in the Global Warrants Request Notice. In connection with a Global Warrants Exchange, the Company shall direct the Warrant Agent to deliver the beneficial interest in such Global Warrants to the Holder within ten (10) Business Days of the Global Warrants Request Notice pursuant to the delivery instructions in the Global Warrant Request Notice (“Global Warrants Delivery Date”). If the Company fails for any reason to deliver to the Holder Global Warrants subject to the Global Warrants Request Notice by the Global Warrants Delivery Date, the Company shall pay to the Holder, in cash, as liquidated damages and not as a penalty, for each $1,000 of Warrant Shares evidenced by such Global Warrants (based on the VWAP (as defined in the Warrants) of the Common Stock on the Global Warrants Request Notice Date), $10 per Business Day for each Business Day after such Global Warrants Delivery Date until such Global Warrants are delivered or, prior to delivery of such Global Warrants, the Holder rescinds such Global Warrants Exchange. The Company covenants and agrees that, upon the date of delivery of the Global Warrants Request Notice, the Holder shall be deemed to be the beneficial holder of such Global Warrants.
Section 4. Form of Warrant Certificates. The Warrant Certificate, together with the form of election to purchase Common Stock (“Notice of Exercise”) and the form of assignment to be printed on the reverse thereof, shall be in the form of Exhibit 1 or Exhibit 2, as applicable, hereto.
Section 5. Countersignature and Registration. The Global Warrant shall be executed on behalf of the Company by its Chief Executive Officer or Chief Financial Officer. The Global Warrant shall be countersigned by the Warrant Agent and shall not be valid for any purpose unless so countersigned. In case any officer of the Company who shall have signed any of the Global Warrant shall cease to be such officer of the Company before countersignature by the Warrant Agent and issuance and delivery by the Company, such Global Warrant, nevertheless, may be countersigned by the Warrant Agent, issued and delivered with the same force and effect as though the person who signed such Global Warrant had not ceased to be such officer of the Company; and any Global Warrant may be signed on behalf of the Company by any person who, at the actual date of the execution of such Global Warrant, shall be a proper officer of the Company to sign such Global Warrant, although at the date of the execution of this Warrant Agreement any such person was not such an officer.
The Warrant Agent will keep or cause to be kept, at one of its offices, or at the office of one of its agents, books for registration and transfer of the Global Warrants issued hereunder. Such books shall show the names and addresses of the respective Holders of the Global Warrant, the number of warrants evidenced on the face of each of such Global Warrant and the date of each of such Global Warrant. The Warrant Agent will create a special account for the issuance of Global Warrants.
The Company will keep or cause to be kept at one of its offices, books for the registration and transfer of any Definitive Certificates issued hereunder and the Warrant Agent shall not have any obligation to keep books and records with respect to any Definitive Warrants. Such Company books shall show the names and addresses of the respective Holders of the Definitive Certificates, the number of warrants evidenced on the face of each such Definitive Certificate and the date of each such Definitive Certificate.
Section 6. Transfer, Split Up, Combination and Exchange of Warrant Certificates; Mutilated, Destroyed, Lost or Stolen Warrant Certificates. With respect to the Global Warrant, subject to the provisions of the Warrant Certificate and the last sentence of this first paragraph of Section 6 and subject to applicable law, rules or regulations, or any “stop transfer” instructions the Company may give to the Warrant Agent, at any time after the closing date of the Offering, and at or prior to the Close of Business on the Termination Date (as such term is defined in the Warrant Certificate), any Global Warrant or Global Warrants may be transferred, split up, combined or exchanged for another Global Warrant or Global Warrants, entitling the Holder to purchase a like number of shares of Common Stock as the Global Warrant or Global Warrants surrendered then entitled such Holder to purchase. Any Holder desiring to transfer, split up, combine or exchange any Global Warrant shall make such request in writing delivered to the Warrant Agent, and shall surrender the Global Warrant to be transferred, split up, combined or exchanged at the principal office of the Warrant Agent. Any requested transfer of Warrants, whether in book-entry form or certificate form, shall be accompanied by reasonable evidence of authority of the party making such request that may be required by the Warrant Agent. Thereupon the Warrant Agent shall, subject to the last sentence of this first paragraph of Section 6, countersign and deliver to the Person entitled thereto a Global Warrant or Global Warrants, as the case may be, as so requested. The Company may require payment from the Holder of a sum sufficient to cover any tax or governmental charge that may be imposed in connection with any transfer, split up, combination or exchange of Global Warrants. The Company shall compensate the Warrant Agent per the fee schedule mutually agreed upon by the parties hereto and provided separately on the date hereof.
Upon receipt by the Warrant Agent of evidence reasonably satisfactory to it of the loss, theft, destruction or mutilation of a Warrant Certificate, which evidence shall include an affidavit of loss, or in the case of mutilated certificates, the certificate or portion thereof remaining, and, in case of loss, theft or destruction, of indemnity in customary form and amount (but, with respect to any Definitive Certificates, shall not include the posting of any bond by the Holder unless required by the Warrant Agent), and satisfaction of any other reasonable requirements established by Section 8-405 of the Uniform Commercial Code as in effect in the State of Delaware, and reimbursement to the Company and the Warrant Agent of all reasonable expenses incidental thereto, and upon surrender to the Warrant Agent and cancellation of the Warrant Certificate if mutilated, the Company will make and deliver a new Warrant Certificate of like tenor to the Warrant Agent for delivery to the Holder in lieu of the Warrant Certificate so lost, stolen, destroyed or mutilated.
Section 7. Exercise of Warrants; Exercise Price; Termination Date.
(a) The Warrants shall be exercisable commencing on the Issue Date. The Warrants shall cease to be exercisable and shall terminate and become void as set forth in the Warrant Certificate. Subject to the foregoing and to Section 7(b) below, the Holder of a Warrant may exercise the Warrant in whole or in part pursuant to the procedures set forth in Section 2 of the Warrant Certificate. Subject to Section 7(b) below, payment of the Exercise Price may be made, at the option of the Holder, by wire transfer or by certified or official bank check in United States dollars, to the Warrant Agent at the principal office of the Warrant Agent or to the office of one of its agents as may be designated by the Warrant Agent from time to time. In the case of the Holder of a Global Warrant, the Holder shall deliver the executed Notice of Exercise and the payment of the Exercise Price as described herein. Notwithstanding any other provision in this Agreement, a holder whose interest in a Global Warrant is a beneficial interest in a Global Warrant held in book-entry form through the Depositary (or another established clearing corporation performing similar functions), shall effect exercises by delivering to the Depositary (or such other clearing corporation, as applicable) the appropriate instruction form for exercise, complying with the procedures to effect exercise that are required by the Depositary (or such other clearing corporation, as applicable). Delivery of the Warrant Shares shall be made by the Warrant Share Delivery Date. The Company acknowledges that the bank accounts maintained by the Warrant Agent in connection with the services provided under this Agreement will be in its name and that the Warrant Agent may receive investment earnings in connection with the investment at Warrant Agent risk and for its benefit of funds held in those accounts from time to time. Neither the Company nor the Holders will receive interest on any deposits or Exercise Price. No ink-original Notice of Exercise shall be required, nor shall any medallion guarantee (or other type of guarantee or notarization) of any Notice of Exercise be required. The Company hereby acknowledges and agrees that, with respect to a Holder whose interest in a Global Warrant is a beneficial interest in a Global Warrant held in book-entry form through the Depositary (or another established clearing corporation performing similar functions), upon delivery of irrevocable instructions to such Holder’s Participant to exercise such warrants, that solely for purposes of Regulation SHO that such Holder shall be deemed to have exercised such warrants.
(b) Upon receipt of a Notice of Exercise for a Cashless Exercise at a time when Cashless Exercise is available under the Warrants, the Company will promptly calculate and transmit to the Warrant Agent the number of Warrant Shares issuable in connection with such Cashless Exercise and deliver a copy of the Notice of Exercise to the Warrant Agent, which shall issue such number of Warrant Shares in connection with such Cashless Exercise.
(c) Upon the exercise of the Warrant Certificate pursuant to the terms of Section 2 of the Warrant Certificate, the Warrant Agent shall cause the Warrant Shares underlying such Warrant Certificate or Global Warrant to be delivered to or upon the order of the Holder of such Warrant Certificate or Global Warrant, registered in such name or names as may be designated by such Holder, no later than the Warrant Share Delivery Date (as such term is defined in the Warrant Certificate). If the Company is then a participant in the DWAC system of the Depositary and either (A) there is an effective registration statement permitting the issuance of the Warrant Shares to or resale of the Warrant Shares by Holder or (B) the Warrant is being exercised via Cashless Exercise, then the certificates for Warrant Shares shall be transmitted by the Warrant Agent to the Holder by crediting the account of the Holder’s broker with the Depositary through its DWAC system. For the avoidance of doubt, if the Company becomes obligated to pay any amounts to any Holders pursuant to Section 2(d)(i) or 2(d)(iv) of the Warrant Certificate, such obligation shall be solely that of the Company and not that of the Warrant Agent. Notwithstanding anything else to the contrary in this Agreement, except in the case of a Cashless Exercise, if any Holder fails to duly deliver payment to the Warrant Agent of an amount equal to the aggregate Exercise Price of the Warrant Shares to be purchased upon exercise of such Holder’s Warrant as set forth in Section 7(a) hereof by the Warrant Share Delivery Date, the Warrant Agent will not obligated to deliver such Warrant Shares (via DWAC or otherwise) until following receipt of such payment, and the applicable Warrant Share Delivery Date shall be deemed extended by one day for each day (or part thereof) until such payment is delivered to the Warrant Agent.
(d) The Warrant Agent shall deposit all funds received by it in payment of the Exercise Price for all Warrants in the account of the Company maintained with the Warrant Agent for such purpose (or to such other account as directed by the Company in writing) and shall advise the Company via email at the end of each day on which notices of exercise are received or funds for the exercise of any Warrant are received of the amount so deposited to its account.
Section 8. Cancellation and Destruction of Warrant Certificates. All Warrant Certificates surrendered for the purpose of exercise, transfer, split up, combination or exchange shall, if surrendered to the Company or to any of its agents, be delivered to the Warrant Agent for cancellation or in canceled form, or, if surrendered to the Warrant Agent, shall be canceled by it, and no Warrant Certificate shall be issued in lieu thereof except as expressly permitted by any of the provisions of this Agreement. The Company shall deliver to the Warrant Agent for cancellation and retirement, and the Warrant Agent shall so cancel and retire, any other Warrant Certificate purchased or acquired by the Company otherwise than upon the exercise thereof. The Warrant Agent shall deliver all canceled Warrant Certificates to the Company, or shall, at the written request of the Company, destroy such canceled Warrant Certificates, and in such case shall deliver a certificate of destruction thereof to the Company, subject to any applicable law, rule or regulation requiring the Warrant Agent to retain such canceled certificates.
Section 9. Certain Representations; Reservation and Availability of Shares of Common Stock or Cash.
(a) This Agreement has been duly authorized, executed and delivered by the Company and, assuming due authorization, execution and delivery hereof by the Warrant Agent, constitutes a valid and legally binding obligation of the Company enforceable against the Company in accordance with its terms, and the Warrants have been duly authorized, executed and issued by the Company and, assuming due authentication thereof by the Warrant Agent pursuant hereto and payment therefor by the Holders as provided in the Registration Statement, constitute valid and legally binding obligations of the Company enforceable against the Company in accordance with their terms and entitled to the benefits hereof; in each case except as enforceability may be limited by bankruptcy, insolvency, reorganization, moratorium and other similar laws relating to or affecting creditors’ rights generally or by general equitable principles (regardless of whether such enforceability is considered in a proceeding in equity or at law).
(b) The Company covenants and agrees that it will cause to be reserved and kept available out of its authorized and unissued shares of Common Stock or its authorized and issued shares of Common Stock held in its treasury, free from preemptive rights, the number of shares of Common Stock that will be sufficient to permit the exercise in full of all outstanding Warrants.
(c) The Warrant Agent will create a special account for the issuance of Common Stock upon the exercise of Warrants.
(d) The Company further covenants and agrees that it will pay when due and payable any and all federal and state transfer taxes and charges which may be payable in respect of the original issuance or delivery of the Warrant Certificates or certificates evidencing Common Stock upon exercise of the Warrants. The Company shall not, however, be required to pay any tax or governmental charge which may be payable in respect of any transfer involved in the transfer or delivery of Warrant Certificates or the issuance or delivery of certificates for Common Stock in a name other than that of the Holder of the Warrant Certificate evidencing Warrants surrendered for exercise or to issue or deliver any certificate for shares of Common Stock upon the exercise of any Warrants until any such tax or governmental charge shall have been paid (any such tax or governmental charge being payable by the Holder of such Warrant Certificate at the time of surrender) or until it has been established to the Company’s reasonable satisfaction that no such tax or governmental charge is due.
(e) The Company agrees that all shares of Common Stock issued upon the proper exercise of a Warrant in conformity with this Agreement shall be validly issued, fully paid and nonassessable.
Section 10. Common Stock Record Date. Each Person in whose name any certificate for shares of Common Stock is issued (or to whose broker’s account is credited shares of Common Stock through the DWAC system) upon the exercise of Warrants shall for all purposes be deemed to have become the holder of record for the Common Stock represented thereby on, and such certificate shall be dated, the date on which submission of the Notice of Exercise was made, provided that the Warrant Certificate evidencing such Warrant is duly surrendered (but only if required herein) and payment of the Exercise Price (and any applicable transfer taxes) is received on or prior to the Warrant Share Delivery Date; provided, however, that if the date of submission of the Notice of Exercise is a date upon which the Common Stock transfer books of the Company are closed, such Person shall be deemed to have become the record holder of such shares on, and such certificate shall be dated, the next succeeding day on which the Common Stock transfer books of the Company are open.
Section 11. Adjustment of Exercise Price, Number of Shares of Common Stock or Number of the Company Warrants. The Exercise Price, the number of shares covered by each Warrant and the number of Warrants outstanding are subject to adjustment from time to time as provided in Section 3 of the Warrant Certificate. In the event that at any time, as a result of an adjustment made pursuant to Section 3 of the Warrant Certificate, the Holder of any Warrant thereafter exercised shall become entitled to receive any shares of capital stock of the Company other than shares of Common Stock, thereafter the number of such other shares so receivable upon exercise of any Warrant shall be subject to adjustment from time to time in a manner and on terms as nearly equivalent as practicable to the provisions with respect to the shares contained in Section 3 of the Warrant Certificate and the provisions of Sections 7, 11 and 12 of this Agreement with respect to the shares of Common Stock shall apply on like terms to any such other shares. All Warrants originally issued by the Company subsequent to any adjustment made to the Exercise Price pursuant to the Warrant Certificate shall evidence the right to purchase, at the adjusted Exercise Price, the number of shares of Common Stock purchasable from time to time hereunder upon exercise of the Warrants, all subject to further adjustment as provided herein.
Section 12. Certification of Adjusted Exercise Price or Number of Shares of Common Stock. Whenever the Exercise Price or the number of shares of Common Stock issuable upon the exercise of each Warrant is adjusted as provided in Section 11 or 13, the Company shall (a) promptly prepare a certificate setting forth the Exercise Price of each Warrant as so adjusted, and a brief statement of the facts accounting for such adjustment, (b) promptly file with the Warrant Agent and with each transfer agent for the Common Stock a copy of such certificate and (c) instruct the Warrant Agent to send a brief summary thereof to each Holder of a Warrant Certificate.
Section 13. Fractional Shares of Common Stock.
(a) The Company shall not issue fractions of Warrants or distribute Warrant Certificates which evidence fractional Warrants. Whenever any fractional Warrant would otherwise be required to be issued or distributed, the actual issuance or distribution shall reflect a rounding of such fraction to the nearest whole Warrant (rounded down).
(b) The Company shall not issue fractions of shares of Common Stock upon exercise of Warrants or distribute stock certificates which evidence fractional shares of Common Stock. Whenever any fraction of a share of Common Stock would otherwise be required to be issued or distributed, the actual issuance or distribution in respect thereof shall be made in accordance with Section 2(d)(v) of the Warrant Certificate.
Section 14. Conditions of the Warrant Agent’s Obligations. The Warrant Agent accepts its obligations herein set forth upon the terms and conditions hereof, including the following to all of which the Company agrees and to all of which the rights hereunder of the Holders from time to time of the Warrant Certificates shall be subject:
(a) Compensation and Indemnification. The Company agrees promptly to pay the Warrant Agent the compensation detailed on Exhibit 5 hereto for all services rendered by the Warrant Agent and to reimburse the Warrant Agent for reasonable out-of-pocket expenses (including reasonable counsel fees) incurred without gross negligence or willful misconduct finally adjudicated to have been directly caused by the Warrant Agent in connection with the services rendered hereunder by the Warrant Agent. The Warrant Agent shall be liable hereunder only for its own gross negligence, willful misconduct or bad faith. The Company agrees to indemnify the Warrant Agent and save it harmless against any and all liabilities, including judgments, costs and reasonable counsel fees, for anything done or omitted by the Warrant Agent in the execution of this Agreement, except as a result of the Warrant Agent’s gross negligence, willful misconduct or bad faith. The Warrant Agent shall be under no obligation to institute or defend any action, suit, or legal proceeding in connection herewith or to take any other action likely to involve the Warrant Agent in expense, unless first indemnified to the Warrant Agent’s satisfaction. The indemnities provided by this paragraph shall survive the resignation or discharge of the Warrant Agent or the termination of this Agreement. Anything in this Agreement to the contrary notwithstanding, in no event shall the Warrant Agent be liable under or in connection with the Agreement for indirect, special, incidental, punitive or consequential losses or damages of any kind whatsoever, including but not limited to lost profits, whether or not foreseeable, even if the Warrant Agent has been advised of the possibility thereof and regardless of the form of action in which such damages are sought, and the Warrant Agent’s aggregate liability to the Company, or any of the Company’s representatives or agents, under this Section 14(a) or under any other term or provision of this Agreement, whether in contract, tort, or otherwise, is expressly limited to, and shall not exceed in any circumstances, one (1) year’s fees received by the Warrant Agent as fees and charges under this Agreement, but not including reimbursable expenses previously reimbursed to the Warrant Agent by the Company hereunder.
(b) Agent for the Company. In acting under this Warrant Agreement and in connection with the Warrant Certificates, the Warrant Agent is acting solely as agent of the Company and does not assume any obligations or relationship of agency or trust for or with any of the Holders of Warrant Certificates or beneficial owners of Warrants.
(c) Counsel. The Warrant Agent may consult with counsel satisfactory to it, which may include counsel for the Company, and the written advice of such counsel shall be full and complete authorization and protection in respect of any action taken, suffered or omitted by it hereunder in good faith and in accordance with the advice of such counsel.
(d) Documents. The Warrant Agent shall be protected and shall incur no liability for or in respect of any action taken or omitted by it in reliance upon any Warrant Certificate, notice, direction, consent, certificate, affidavit, statement or other paper or document reasonably believed by it to be genuine and to have been presented or signed by the proper parties.
(e) Certain Transactions. The Warrant Agent, and its officers, directors and employees, may become the owner of, or acquire any interest in, Warrants, with the same rights that it or they would have if it were not the Warrant Agent hereunder, and, to the extent permitted by applicable law, it or they may engage or be interested in any financial or other transaction with the Company and may act on, or as depositary, trustee or agent for, any committee or body of Holders of Warrant Securities or other obligations of the Company as freely as if it were not the Warrant Agent hereunder. Nothing in this Warrant Agreement shall be deemed to prevent the Warrant Agent from acting as trustee under any indenture to which the Company is a party.
(f) No Liability for Interest. Unless otherwise agreed with the Company, the Warrant Agent shall have no liability for interest on any monies at any time received by it pursuant to any of the provisions of this Agreement or of the Warrant Certificates.
(g) No Liability for Invalidity. The Warrant Agent shall have no liability with respect to any invalidity of this Agreement or the Warrant Certificates (except as to the Warrant Agent’s countersignature thereon).
(h) No Responsibility for Representations. The Warrant Agent shall not be responsible for any of the recitals or representations herein or in the Warrant Certificate (except as to the Warrant Agent’s countersignature thereon), all of which are made solely by the Company.
(i) No Implied Obligations. The Warrant Agent shall be obligated to perform only such duties as are herein and in the Warrant Certificates specifically set forth and no implied duties or obligations shall be read into this Agreement or the Warrant Certificates against the Warrant Agent. The Warrant Agent shall not be under any obligation to take any action hereunder which may tend to involve it in any expense or liability, the payment of which within a reasonable time is not, in its reasonable opinion, assured to it. The Warrant Agent shall not be accountable or under any duty or responsibility for the use by the Company of any of the Warrant Certificates authenticated by the Warrant Agent and delivered by it to the Company pursuant to this Agreement or for the application by the Company of the proceeds of the Warrant Certificate. The Warrant Agent shall have no duty or responsibility in case of any default by the Company in the performance of its covenants or agreements contained herein or in the Warrant Certificates or in the case of the receipt of any written demand from a Holder of a Warrant Certificate with respect to such default, including, without limiting the generality of the foregoing, any duty or responsibility to initiate or attempt to initiate any proceedings at law.
Section 15. Purchase or Consolidation or Change of Name of Warrant Agent. Any corporation into which the Warrant Agent or any successor Warrant Agent may be merged or with which it may be consolidated, or any corporation resulting from any merger or consolidation to which the Warrant Agent or any successor Warrant Agent shall be party, or any corporation succeeding to the corporate trust business of the Warrant Agent or any successor Warrant Agent, shall be the successor to the Warrant Agent under this Agreement without the execution or filing of any paper or any further act on the part of any of the parties hereto, provided that such corporation would be eligible for appointment as a successor Warrant Agent under the provisions of Section 17. In case at the time such successor Warrant Agent shall succeed to the agency created by this Agreement any of the Warrant Certificates shall have been countersigned but not delivered, any such successor Warrant Agent may adopt the countersignature of the predecessor Warrant Agent and deliver such Warrant Certificates so countersigned; and in case at that time any of the Warrant Certificates shall not have been countersigned, any successor Warrant Agent may countersign such Warrant Certificates either in the name of the predecessor Warrant Agent or in the name of the successor Warrant Agent; and in all such cases such Warrant Certificates shall have the full force provided in the Warrant Certificates and in this Agreement.
In case at any time the name of the Warrant Agent shall be changed and at such time any of the Warrant Certificates shall have been countersigned but not delivered, the Warrant Agent may adopt the countersignature under its prior name and deliver such Warrant Certificates so countersigned; and in case at that time any of the Warrant Certificates shall not have been countersigned, the Warrant Agent may countersign such Warrant Certificates either in its prior name or in its changed name; and in all such cases such Warrant Certificates shall have the full force provided in the Warrant Certificates and in this Agreement.
Section 16. Duties of Warrant Agent. The Warrant Agent undertakes the duties and obligations imposed by this Agreement upon the following terms and conditions, by all of which the Company, by its acceptance hereof, shall be bound:
(a) The Warrant Agent may consult with legal counsel reasonably acceptable to the Company (who may be legal counsel for the Company), and the opinion of such counsel shall be full and complete authorization and protection to the Warrant Agent as to any action taken or omitted by it in good faith and in accordance with such opinion.
(b) Whenever in the performance of its duties under this Agreement the Warrant Agent shall deem it necessary or desirable that any fact or matter be proved or established by the Company prior to taking or suffering any action hereunder, such fact or matter (unless other evidence in respect thereof be herein specifically prescribed) may be deemed to be conclusively proved and established by a certificate signed by the Chief Executive Officer or Chief Financial Officer or suffered in good faith by it under the provisions of this Agreement in reliance upon such certificate.
(c) Subject to the limitation set forth in Section 14, the Warrant Agent shall be liable hereunder only for its own gross negligence or willful misconduct, or for a breach by it of this Agreement.
(d) The Warrant Agent shall not be liable for or by reason of any of the statements of fact or recitals contained in this Agreement or in the Warrant Certificate (except its countersignature thereof) by the Company or be required to verify the same, but all such statements and recitals are and shall be deemed to have been made by the Company only.
(e) The Warrant Agent shall not be under any responsibility in respect of the validity of this Agreement or the execution and delivery hereof (except the due execution hereof by the Warrant Agent) or in respect of the validity or execution of any Warrant Certificate (except its countersignature thereof); nor shall it be responsible for any breach by the Company of any covenant or condition contained in this Agreement or in any Warrant Certificate; nor shall it be responsible for the adjustment of the Exercise Price or the making of any change in the number of shares of Common Stock required under the provisions of Section 11 or 13 or responsible for the manner, method or amount of any such change or the ascertaining of the existence of facts that would require any such adjustment or change (except with respect to the exercise of Warrants evidenced by the Warrant Certificates after actual notice of any adjustment of the Exercise Price); nor shall it by any act hereunder be deemed to make any representation or warranty as to the authorization or reservation of any shares of Common Stock to be issued pursuant to this Agreement or any Warrant Certificate or as to whether any shares of Common Stock will, when issued, be duly authorized, validly issued, fully paid and nonassessable.
(f) Each party hereto agrees that it will perform, execute, acknowledge and deliver or cause to be performed, executed, acknowledged and delivered all such further and other acts, instruments and assurances as may reasonably be required by the other party hereto for the carrying out or performing by any party of the provisions of this Agreement.
(g) The Warrant Agent is hereby authorized to accept instructions with respect to the performance of its duties hereunder from the Chief Executive Officer or Chief Financial Officer of the Company, and to apply to such officers for advice or instructions in connection with its duties, and it shall not be liable and shall be indemnified and held harmless for any action taken or suffered to be taken by it in good faith in accordance with instructions of any such officer, provided Warrant Agent carries out such instructions without gross negligence or willful misconduct.
(h) The Warrant Agent and any shareholder, director, officer or employee of the Warrant Agent may buy, sell or deal in any of the Warrants or other securities of the Company or become pecuniarily interested in any transaction in which the Company may be interested, or contract with or lend money to the Company or otherwise act as fully and freely as though it were not Warrant Agent under this Agreement. Nothing herein shall preclude the Warrant Agent from acting in any other capacity for the Company or for any other legal entity.
(i) The Warrant Agent may execute and exercise any of the rights or powers hereby vested in it or perform any duty hereunder either itself or by or through its attorney or agents, and the Warrant Agent shall not be answerable or accountable for any act, default, neglect or misconduct of any such attorney or agents or for any loss to the Company resulting from any such act, default, neglect or misconduct, provided reasonable care was exercised in the selection and continued employment thereof.
Section 17. Change of Warrant Agent. The Warrant Agent may resign and be discharged from its duties under this Agreement upon 30 days’ notice in writing sent to the Company and to each transfer agent of the Common Stock, and to the Holders of the Warrant Certificates. The Company may remove the Warrant Agent or any successor Warrant Agent upon 30 days’ notice in writing, sent to the Warrant Agent or successor Warrant Agent, as the case may be, and to each transfer agent of the Common Stock, and to the Holders of the Warrant Certificates. If the Warrant Agent shall resign or be removed or shall otherwise become incapable of acting, the Company shall appoint a successor to the Warrant Agent. If the Company shall fail to make such appointment within a period of 30 days after such removal or after it has been notified in writing of such resignation or incapacity by the resigning or incapacitated Warrant Agent or by the Holder of a Warrant Certificate (who shall, with such notice, submit his Warrant Certificate for inspection by the Company), then the Holder of any Warrant Certificate may apply to any court of competent jurisdiction for the appointment of a new Warrant Agent, provided that, for purposes of this Agreement, the Company shall be deemed to be the Warrant Agent until a new warrant agent is appointed. Any successor Warrant Agent, whether appointed by the Company or by such a court, shall be a corporation organized and doing business under the laws of the United States or of a state thereof, in good standing, which is authorized under such laws to exercise corporate trust powers and is subject to supervision or examination by federal or state authority and which has at the time of its appointment as Warrant Agent a combined capital and surplus of at least $50,000,000. After appointment, the successor Warrant Agent shall be vested with the same powers, rights, duties and responsibilities as if it had been originally named as Warrant Agent without further act or deed; but the predecessor Warrant Agent shall deliver and transfer to the successor Warrant Agent any property at the time held by it hereunder, and execute and deliver any further assurance, conveyance, act or deed necessary for the purpose. Not later than the effective date of any such appointment, the Company shall file notice thereof in writing with the predecessor Warrant Agent and each transfer agent of the Common Stock, and mail a notice thereof in writing to the Holders of the Warrant Certificates. However, failure to give any notice provided for in this Section 17, or any defect therein, shall not affect the legality or validity of the resignation or removal of the Warrant Agent or the appointment of the successor Warrant Agent, as the case may be.
Section 18. Issuance of New Warrant Certificates. Notwithstanding any of the provisions of this Agreement or of the Warrants to the contrary, the Company may, at its option, issue new Warrant Certificates evidencing Warrants in such form as may be approved by its Board of Directors to reflect any adjustment or change in the Exercise Price per share and the number or kind or class of shares of stock or other securities or property purchasable under the several Warrant Certificates made in accordance with the provisions of this Agreement.
Section 19. Notices. Notices or demands authorized by this Agreement to be given or made (i) by the Warrant Agent or by the Holder of any Warrant Certificate to or on the Company, (ii) subject to the provisions of Section 17, by the Company or by the Holder of any Warrant Certificate to or on the Warrant Agent or (iii) by the Company or the Warrant Agent to the Holder of any Warrant Certificate shall be deemed given (a) on the date delivered, if delivered personally, (b) on the first Business Day following the deposit thereof with Federal Express or another recognized overnight courier, if sent by Federal Express or another recognized overnight courier, (c) on the fourth Business Day following the mailing thereof with postage prepaid, if mailed by registered or certified mail (return receipt requested), and (d) the time of transmission, if such notice or communication is delivered via facsimile or email attachment at or prior to 5:30 p.m. (New York City time) on a Business Day and (e) the next Business Day after the time of transmission, if such notice or communication is delivered via facsimile or email attachment on a day that is not a Business Day or later than 5:30 p.m. (New York City time) on any Business Day, in each case to the parties at the following addresses (or at such other address for a party as shall be specified by like notice):
|
(a) |
If to the Company, to: |
Autonomix Medical, Inc.
21 Waterway Avenue, Suite 300
The Woodlands, Texas 77380
Attention: Chief Financial Officer
|
(b) |
If to the Warrant Agent, to: |
Equity Stock Transfer LLC
[●]
Attention: _________
For any notice delivered by email to be deemed given or made, such notice must be followed by notice sent by overnight courier service to be delivered on the next business day following such email, unless the recipient of such email has acknowledged via return email receipt of such email.
(c) If to the Holder of any Warrant Certificate to the address of such Holder as shown on the registry books of the Company. Any notice required to be delivered by the Company to the Holder of any Warrant may be given by the Warrant Agent on behalf of the Company. Notwithstanding any other provision of this Agreement, where this Agreement provides for notice of any event to a Holder of any Warrant, such notice shall be sufficiently given if given to the Depositary (or its designee) pursuant to the procedures of the Depositary or its designee.
Section 20. Supplements and Amendments.
(a) The Company and the Warrant Agent may from time to time supplement or amend this Agreement without the approval of any Holders of Global Warrants in order to (i) add to the covenants and agreements of the Company for the benefit of the Holders of the Global Warrants, (ii) to surrender any rights or power reserved to or conferred upon the Company in this Agreement, (iii) to cure any ambiguity, (iv) to correct or supplement any provision contained herein which may be defective or inconsistent with any other provision herein, or (v) to make any other provisions with regard to matters or questions arising hereunder which the Company and Warrant Agent may deem necessary or desirable, provided that such addition, correction or surrender shall not adversely affect the interests of the Holders of the Global Warrants or Warrant Certificates in any material respect.
(b) In addition to the foregoing, with the consent of Holders of Warrants entitled, upon exercise thereof, to receive not less than a majority of the shares of Common Stock issuable thereunder, the Company and the Warrant Agent may modify this Agreement for the purpose of adding any provisions to or changing in any manner or eliminating any of the provisions of this Warrant Agreement or modifying in any manner the rights of the Holders of the Global Warrants; provided, however, that no modification of the terms (including but not limited to the adjustments described in Section 11) upon which the Warrants are exercisable or the rights of Holders of Warrants to receive liquidated damages or other payments in cash from the Company or reducing the percentage required for consent to modification of this Agreement may be made without the consent of the Holder of each outstanding Warrant Certificate affected thereby; provided further, however, that no amendment hereunder shall affect any terms of any Warrant Certificate issued in a Warrant Exchange. As a condition precedent to the Warrant Agent’s execution of any amendment, the Company shall deliver to the Warrant Agent a certificate from a duly authorized officer of the Company that states that the proposed amendment complies with the terms of this Section 20.
Section 21. Successors. All covenants and provisions of this Agreement by or for the benefit of the Company or the Warrant Agent shall bind and inure to the benefit of their respective successors and assigns hereunder.
Section 22. Benefits of this Agreement. Nothing in this Agreement shall be construed to give any Person other than the Company, the Holders of Warrant Certificates and the Warrant Agent any legal or equitable right, remedy or claim under this Agreement. This Agreement shall be for the sole and exclusive benefit of the Company, the Warrant Agent and the Holders of the Warrant Certificates. Notwithstanding anything to the contrary contained herein, to the extent any provision of a Warrant Certificate conflicts with any provision of this Agreement, the provisions of the Warrant Certificate shall govern and be controlling.
Section 23. Applicable Law. The validity, interpretation and performance of this Agreement and each Warrant Certificate and Global Warrant shall be governed in all respects by the laws of the State of New York, without giving effect to conflicts of law principles that would result in the application of the substantive laws of another jurisdiction. The Company hereby agrees that any action, proceeding or claim against it arising out of or relating in any way to this Agreement shall be brought and enforced in the courts of the State of New York or the United States District Court for the Southern District of New York, and irrevocably submits to such jurisdiction, which jurisdiction shall be exclusive. The Company hereby waives any objection to such exclusive jurisdiction and that such courts represent an inconvenient forum. Any such process or summons to be served upon the Company may be served by transmitting a copy thereof by registered or certified mail, return receipt requested, postage prepaid, addressed to it at the address set forth in Section 19 hereof. Such mailing shall be deemed personal service and shall be legal and binding upon the Company in any action, proceeding or claim.
Section 24. Counterparts. This Agreement may be executed in any number of counterparts and each of such counterparts shall for all purposes be deemed to be an original, and all such counterparts shall together constitute but one and the same instrument.
Section 25. Captions. The captions of the sections of this Agreement have been inserted for convenience only and shall not control or affect the meaning or construction of any of the provisions hereof.
Section 26. Information. The Company agrees to promptly provide to the Holders of the Warrants any information it provides to the holders of the Common Stock, except to the extent any such information is publicly available on the EDGAR system (or any successor thereof) of the Securities and Exchange Commission.
[signature page to follow]
IN WITNESS WHEREOF, the parties hereto have caused this Agreement to be duly executed as of the day and year first above written.
|
|
AUTONOMIX MEDICAL, INC. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
By: |
|
|
|
|
|
Name: Steve Bond Title: Chief Financial Officer |
|
|
|
|
|
|
| EQUITY STOCK TRANSFER LLC | |||
| By: | |||
|
[Name: [Title: |
|||
Exhibit 1
Form of Pre-Funded Warrant Certificate
Exhibit 2
Form of Series A Warrant Certificate
Exhibit 3
Form of Warrant Certificate Request Notice
WARRANT CERTIFICATE REQUEST NOTICE
To: Equity Stock Transfer LLC, as Warrant Agent for Autonomix Medical, Inc. (the “Company”)
The undersigned Holder of Common Stock Purchase Warrants (“Warrants”) in the form of Global Warrants issued by the Company hereby elects to receive a Warrant Certificate evidencing the Warrants held by the Holder as specified below:
|
1. |
Name of Holder of Warrants in form of Global Warrants: _____________________________ |
|
2. |
Name of Holder in Warrant Certificate (if different from name of Holder of Warrants in form of Global Warrants): ________________________________ |
|
3. |
Number of Warrants in name of Holder in form of Global Warrants: ___________________ |
|
4. |
Number of Warrants for which Warrant Certificate shall be issued: __________________ |
|
5. |
Number of Warrants in name of Holder in form of Global Warrants after issuance of Warrant Certificate, if any: ___________ |
|
6. |
Warrant Certificate shall be delivered to the following address: |
______________________________
______________________________
______________________________
______________________________
The undersigned hereby acknowledges and agrees that, in connection with this Warrant Exchange and the issuance of the Warrant Certificate, the Holder is deemed to have surrendered the number of Warrants in form of Global Warrants in the name of the Holder equal to the number of Warrants evidenced by the Warrant Certificate.
[SIGNATURE OF HOLDER]
Name of Investing Entity: ____________________________________________________
Signature of Authorized Signatory of Investing Entity: ______________________________
Name of Authorized Signatory: ________________________________________________
Title of Authorized Signatory: _________________________________________________
Date: _______________________________________________________________
Exhibit 4
Form of Global Warrant Request Notice
GLOBAL WARRANT REQUEST NOTICE
To: Equity Stock Transfer LLC, as Warrant Agent for Autonomix Medical, Inc. (the “Company”)
The undersigned Holder of Common Stock Purchase Warrants (“Warrants”) in the form of Warrants Certificates issued by the Company hereby elects to receive a Global Warrant evidencing the Warrants held by the Holder as specified below:
|
1. |
Name of Holder of Warrants in form of Warrant Certificates: _____________________________ |
|
2. |
Name of Holder in Global Warrant (if different from name of Holder of Warrants in form of Warrant Certificates): ________________________________ |
|
3. |
Number of Warrants in name of Holder in form of Warrant Certificates: ___________________ |
|
4. |
Number of Warrants for which Global Warrant shall be issued: __________________ |
|
5. |
Number of Warrants in name of Holder in form of Warrant Certificates after issuance of Global Warrant, if any: ___________ |
|
6. |
Global Warrant shall be delivered to the following address: |
______________________________
______________________________
______________________________
______________________________
The undersigned hereby acknowledges and agrees that, in connection with this Global Warrant Exchange and the issuance of the Global Warrant, the Holder is deemed to have surrendered the number of Warrants in form of Warrant Certificates in the name of the Holder equal to the number of Warrants evidenced by the Global Warrant.
[SIGNATURE OF HOLDER]
Name of Investing Entity: ____________________________________________________
Signature of Authorized Signatory of Investing Entity: ______________________________
Name of Authorized Signatory: ________________________________________________
Title of Authorized Signatory: _________________________________________________
Date: _______________________________________________________________
Exhibit 5
Warrant Agent Fee Schedule
Exhibit 5.1
 |
 |
November 1, 2024
Autonomix Medical, Inc.
21 Waterway Avenue, Suite 300
The Woodlands, Texas 77380
|
Re: |
Registration Statement on Form S-1 |
Ladies and Gentlemen:
We have acted as counsel to Autonomix Medical, Inc., a Delaware corporation (the “Company”), in connection with the Registration Statement on Form S-1 (as amended, the “Registration Statement”), filed by the Company on November 1, 2024, with the Securities and Exchange Commission (the “Commission”) under the Securities Act of 1933, as amended (the “Securities Act”), for the proposed offering (the “Offering”) of (1)$11.5 million of (a) common stock units (the “Common Stock Units”) (including any sold as a result of the exercise of the underwriters’ over-allotment option), each Common Stock Unit consisting of: (i) one share of the Company’s common stock, par value $0.001 per share (“Common Stock,” such Common Stock when issued as part of Units (defined below), “Unit Shares”), and (ii) one Series A warrant to purchase one share of Common Stock (the “Series A Warrants”), and/or (b) pre-funded units (the “PFW Units” and together with the Common Stock Units, the “Units”) (including any sold as a result of the exercise of the underwriters’ over-allotment option), with each PFW Unit consisting of (i): one pre-funded warrant to purchase one share of Common Stock (the “Pre-Funded Warrants”), and (ii) one Series A Warrant; and (2) underwriter warrants to purchase a number of shares of Common Stock equal to 6% of the aggregate number of Common Stock Units and PFW Units (including any sold as a result of the exercise of the underwriters’ over-allotment option) (the “Representative Warrants”), which will have a term of five years and an exercise price equal to 155% of the public offering price. The Common Stock, Unit Shares, Pre-Funded Warrants, Series A Warrants, and Representative Warrants are referred to herein collectively as the “Securities.” The Securities are being sold to the several underwriters named in, and pursuant to, an underwriting agreement among the Company and such underwriters (“Underwriting Agreement”).
In connection with our opinion, we have examined the Registration Statement, including the exhibits thereto, the forms of Pre-Funded Warrant, Series A Warrant, and Representative Warrant, and such other documents, corporate records and instruments, and have examined such laws and regulations, as we have deemed necessary for the purposes of this opinion. In making our examination, we have assumed the genuineness of all signatures, the authenticity of all documents submitted to us as originals, the conformity with the originals of all documents submitted to us as copies and the legal capacity of all natural persons. As to matters of fact material to our opinions in this letter, we have relied on certificates and statements from officers and other employees of the Company, public officials and other appropriate persons.
Smart In
Your World®
 |
Autonomix Medical, Inc. November 1, 2024 Page 2 |
Based on the foregoing and subject to the qualifications set forth below, we are of the opinion that:
|
1. |
The Securities have been duly authorized for issuance by all necessary corporate action by the Company. |
|
2. |
The Unit Shares, when issued by the Company against payment therefor, as described in the Registration Statement, will be validly issued, fully paid, and non-assessable. |
|
3. |
Provided that the Pre-Funded Warrants, the Series A Warrants, and Representative Warrants have been duly executed and delivered by the Company and duly delivered to the purchasers or underwriters, such Pre-Funded Warrants, Series A Warrants, and Representative Warrants, when issued by the Company against payment therefor, as contemplated in the Registration Statement, will be valid and binding obligations of the Company. |
|
4. |
The shares of Common Stock issuable upon exercise of the Pre-Funded Warrants, the Series A Warrants, and the Representative Warrants, upon payment to the Company of the required consideration, and when issued and sold by the Company and paid for in accordance with the terms of the Pre-Funded Warrants, the Series A Warrants, and Representative Warrants, as applicable, and as described in the Registration Statement, will be validly issued, fully paid, and non-assessable. |
The opinions set forth above are subject to the following qualifications:
|
A. |
The opinion expressed herein with respect to the legality, validity, binding nature and enforceability of the Pre-Funded Warrants, Series A Warrants, and Representative Warrants is subject to (i) applicable laws relating to bankruptcy, insolvency, reorganization, moratorium, fraudulent transfer or other similar laws affecting creditors’ rights generally, whether now or hereafter in effect and (ii) general principles of equity, including, without limitation, concepts of materiality, laches, reasonableness, good faith and fair dealing and the principles regarding when injunctive or other equitable remedies will be available (regardless of whether considered in a proceeding at law or in equity). |
|
B. |
The foregoing opinions are limited to the laws of the State of New York and the General Corporation Law of Delaware, and we express no opinion as to the laws of any other jurisdiction. |
The opinions expressed in this opinion letter are as of the date of this opinion letter only and as to laws covered hereby only as they are in effect on that date, and we assume no obligation to update or supplement such opinion to reflect any facts or circumstances that may come to our attention after that date or any changes in law that may occur or become effective after that date. The opinions herein are limited to the matters expressly set forth in this opinion letter, and no opinion or representation is given or may be inferred beyond the opinions expressly set forth in this opinion letter.
 |
Autonomix Medical, Inc. November 1, 2024 Page 3 |
We hereby consent to the filing of this opinion with the Commission as an exhibit to the Registration Statement and to the use of this firm’s name under the caption “Legal Matters” in the Registration Statement. In giving this consent, we do not thereby admit that we are within the category of persons whose consent is required under Section 7 of the Securities Act or the rules and regulations of the Commission promulgated thereunder.
|
Very truly yours,
/s/ ArentFox Schiff, LLP |
Exhibit 23.1
Consent of Independent Registered Public Accounting Firm
We consent to the inclusion in this Registration Statement on Form S-1 of Autonomix Medical, Inc. for the registration of common stock and warrants of our reports dated May 31, 2024, with respect to the financial statements of Autonomix Medical, Inc. included in the Annual Report on Form 10-K for the year ended March 31, 2024. Our report contains an explanatory paragraph describing conditions that raise substantial doubt about Autonomix Medical, Inc.'s ability to continue as a going concern as described in Note 1 to the financial statements. We also consent to the reference to our firm under the caption "Experts" in the Registration Statement.
/s/ Forvis Mazars, LLP
Atlanta, Georgia
November 1, 2024
EXHIBIT 107
Calculation of Filing Fee Table
Form S-1
(Form Type)
Autonomix Medical, Inc.
(Exact Name of Registrant as Specified in its Charter)
Table 1: Newly Registered Securities
|
Security Type |
Security Class Title |
Fee Calculation Rule |
Amount Registered |
Proposed Maximum Offering Price Per Unit |
Maximum Aggregate Offering Price (1), (2),(3) |
Fee Rate |
Amount of Registration Fee |
|||||||||||||||||||||
|
Fees to be paid |
Equity |
Common Stock Units, each unit consisting of (i) one share of common stock, par value $0.001 per share and (ii) one warrant to purchase one share of common stock (“Series A Warrant”)(3) |
457 |
(o) |
-- |
-- |
$ |
11,500,000 |
0.00015310 |
$ |
1,760.65 |
|||||||||||||||||
|
Equity |
Pre-Funded Warrant Units, each unit consisting of (i) one pre-funded warrant to purchase one share of common stock (“Pre-Funded Warrant”), and (ii) one Series A Warrant (3) |
457 |
(o) |
-- |
-- |
(a) |
-- |
-- |
||||||||||||||||||||
|
Equity |
Common Stock included in Common Stock Units (4) |
-- |
-- |
-- |
Included above. |
-- |
-- |
|||||||||||||||||||||
|
Equity |
Pre-Funded Warrants included in the Pre-Funded Warrant Units |
-- |
-- |
-- |
$ |
-- |
(5) |
-- |
-- |
|||||||||||||||||||
|
Equity |
Common Stock Underlying Series A Warrants |
457 |
(o) |
-- |
-- |
$ |
13,225,000 |
0.00015310 |
$ |
2,024.748 |
||||||||||||||||||
|
Equity |
Common Stock Underlying Pre-Funded Warrants (4) |
457 |
(o) |
-- |
-- |
Included above. |
-- |
-- |
||||||||||||||||||||
|
Equity |
Representative Warrants (6) |
457 |
(g) |
-- |
-- |
$ |
-- |
(4) |
-- |
-- |
||||||||||||||||||
|
Equity |
Common Stock Underlying Representative Warrants (6) |
457 |
(o) |
-- |
-- |
$ |
1,069,500 |
0.00015310 |
$ |
163.741 |
||||||||||||||||||
|
Total Offering Amounts |
$ |
25,794,500 |
0.00015310 |
$ |
3,949.14 |
|||||||||||||||||||||||
|
Total Fees Previously Paid |
-- |
-- |
$ |
-- |
||||||||||||||||||||||||
|
Net Fee Due |
$ |
3,949.14 |
||||||||||||||||||||||||||
(a) The proposed maximum offering price of the Common Stock Units proposed to be sold in the offering will be reduced on a dollar-for-dollar basis based on the offering price of any Pre-Funded Warrant Units offered and sold in the offering, and as such the proposed aggregate maximum offering price of the Common Stock Units together with the Pre-Funded Warrant Units (including the common stock issuable upon exercise of the Pre-Funded Warrants), if any, is $11,500,000. The registrant may issue Pre-Funded Warrants to purchase shares of common stock in the offering. The purchase price of Pre-Funded Warrant Units will equal the price per unit at which Common Stock Units are being sold to the public in this offering, minus the exercise price for the Pre-Funded Warrants.
|
(1) |
Estimated solely for the purpose of calculating the registration fee pursuant to Rule 457(o) under the Securities Act of 1933, as amended (the “Securities Act”). |
|
|
|
|
(2) |
Pursuant to Rule 416 under the Securities Act of 1933, as amended, this registration statement also covers such an indeterminate amount of shares of common stock as may become issuable to prevent dilution resulting from stock splits, stock dividends and similar events. |
|
|
|
|
(3) |
Includes Units that may be purchased by the underwriters pursuant to their option to purchase additional Units to cover over-allotments. |
|
|
|
|
(4) |
No separate fee is required pursuant to Rule 457(g) under the Securities Act. |
|
|
|
|
(5) |
Consistent with the response to Question 240.06 of the Securities Act Rules Compliance and Disclosure Interpretations, the registration fee with respect to the Series A Warrants included in the units and Pre-Funded Warrant Units, as well as the Pre-Funded Warrants included in the Pre-Funded Units, has been allocated to the shares of common stock underlying such warrants, and those shares of common stock are included in the registration fee as calculated herein. |
|
|
|
|
(6) |
We have agreed to grant to the underwriter a warrant covering a number of shares of common stock equal to 6% of the aggregate number of Common Stock Units and Pre-Funded Warrant Units (including any sold as a result of the exercise of the underwriters’ over-allotment option) (the “Representative Warrant”). The Representative Warrants will be exercisable at any time and from time to time, in whole or in part, during the four- and one-half year period commencing 180 days following the commencement of sales of the securities issued in this offering. The Representative Warrant will be exercisable at a price equal to 155% of the public offering price. We have registered the Representative Warrant and the shares underlying the Representative Warrant in this offering. Accordingly, as estimated solely for the purpose of calculating the registration fee pursuant to Rule 457(o), the proposed maximum aggregate offering price of the common stock underlying the Representative Warrant is $1,069,500, which is equal to 155% of $690,000 (6% of $11,500,000, which is the proposed maximum aggregate offering price of the Common Stock Units and Pre-Funded Warrant Units. |
1 Year Autonomix Medical Chart |
1 Month Autonomix Medical Chart |

It looks like you are not logged in. Click the button below to log in and keep track of your recent history.
Support: +44 (0) 203 8794 460 | support@advfn.com
By accessing the services available at ADVFN you are agreeing to be bound by ADVFN's Terms & Conditions