
We could not find any results for:
Make sure your spelling is correct or try broadening your search.
| Share Name | Share Symbol | Market | Type |
|---|---|---|---|
| Aeolus Pharmaceuticals Inc (CE) | USOTC:AOLS | OTCMarkets | Common Stock |
| Price Change | % Change | Share Price | Bid Price | Offer Price | High Price | Low Price | Open Price | Shares Traded | Last Trade | |
|---|---|---|---|---|---|---|---|---|---|---|
| 0.00 | 0.00% | 0.000001 | 0.00 | 00:00:00 |
|
Delaware
(State or other jurisdiction of incorporation or organization) |
56-1953785
(I.R.S. Employer Identification No.) |
|
26361 Crown Valley Parkway, Suite 150
Mission Viejo, California (Address of principal executive offices) |
92691
(Zip Code) |
|
Registrant's telephone number, including area code: 949-481-9825
|
|
|
Securities registered pursuant to Section 12(b) of the Act: None
|
|
|
Securities registered pursuant to Section 12(g) of the Act:
Common Stock, $.01 par value per share (Title of class) |
|
| Page | |
|
PART I
|
1 |
|
Item 1. Business.
|
1 |
|
Item 1A. Risk Factors.
|
39 |
|
Item 1B. Unresolved Staff Comments.
|
55 |
|
Item 2. Properties.
|
55 |
|
Item 3. Legal Proceedings.
|
55 |
|
Item 4. Mine Safety Disclosures.
|
55 |
|
PART II
|
55 |
|
Item 5. Market for Registrant's Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities.
|
55 |
|
Item 6. Selected Financial Data.
|
56 |
|
Item 7. Management's Discussion and Analysis of Financial Condition and Results of Operations.
|
57 |
|
Item 7A. Quantitative and Qualitative Disclosures About Market Risk.
|
65 |
|
Item 8. Financial Statements and Supplementary Data.
|
66 |
|
Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure.
|
85 |
|
Item 9A. Controls and Procedures.
|
86 |
|
Item 9B. Other Information.
|
87 |
|
PART III
|
87 |
|
Item 10. Directors, Executive Officers and Corporate Governance.
|
88 |
|
Item 11. Executive Compensation.
|
88 |
|
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters.
|
88 |
|
Item 13. Certain Relationships and Related Transactions, and Director Independence.
|
90 |
|
Item 14. Principal Accounting Fees and Services.
|
90 |
|
PART IV
|
90 |
|
Item 15. Exhibits and Financial Statement Schedules.
|
90 |
|
·
|
our need for, and our ability to obtain, additional funds;
|
|
·
|
our ability to obtain grants to develop our drug candidates;
|
|
·
|
uncertainties regarding the effectiveness of proceeds deployment and our actual future use of proceeds from our 2015 Securities Placement described below;
|
|
·
|
uncertainties relating to non-clinical studies, clinical trials and regulatory reviews and approvals;
|
|
·
|
uncertainties relating to our pre-clinical studies and trials and regulatory reviews and approvals;
|
|
·
|
uncertainties regarding our ability to successfully advance our Phase I study for Lung-Acute Radiation Syndrome (or Lung ARS) and other commercialization studies and projects involving AEOL 10150, in light of the clinical hold placed on our Lung ARS study by the Food & Drug Administration on September 22, 2014;
|
|
·
|
uncertainties regarding whether our compounds could inhibit formation of fibrosis in the lungs.
|
|
·
|
uncertainties concerning whether we can position our compounds for a pre-Emergency Use Authorization application or we can obtain procurements from the Biomedical Advanced Research and Development Authority following any such application;
|
|
·
|
our dependence on a limited number of therapeutic compounds;
|
|
·
|
the early stage of the drug candidates we are developing;
|
|
·
|
the acceptance of any future products by physicians and patients;
|
|
·
|
competition with and dependence on collaborative partners;
|
|
·
|
loss of key consultants, management or scientific personnel;
|
|
·
|
our ability to obtain adequate intellectual property protection and to enforce these rights; and
|
|
·
|
our ability to avoid infringement of the intellectual property rights of others.
|
|
·
|
Retain the catalytic mechanism and high antioxidant efficiency of the natural enzymes, and
|
|
·
|
Create and develop stable and small molecule antioxidants without the limitations of SOD so that they:
|
|
o
|
Have broader antioxidant activity,
|
|
o
|
Have better tissue penetration,
|
|
o
|
Have a longer life in the body, and
|
|
o
|
Are not proteins, which are more difficult and expensive to manufacture.
|
|
AEOL 10150 Overview
|
|
|
Product Type
|
√ Catalytic antioxidants
|
|
(manganoporphyrin)
|
|
|
Administration Route
|
√ Subcutaneous administration; self-injection possible
√ Oral formulation developed
|
|
Indications in Development
|
√ Radiation Oncology
√ Pulmonary Fibrosis
|
|
√ Pulmonary ARS/DEARE
|
|
|
√ Sulfur Mustard; Chlorine Gas; Nerve Gas
|
|
|
Technical Readiness Level (TRL)
|
√ TRL 7/8 for Pulmonary Effects
|
|
of ARS/DEARE
|
|
|
Regulatory Status
|
√ Active IND (IND-67741)
|
|
Phase I (3 studies, 50 patients)
|
|
|
√ IND on Clinical Hold (IND-
|
|
|
Indication
|
Funding Source
|
Amount of Grant/Contract
|
Research Partners
|
|
Lung-ARS
|
BARDA
|
Up to $118.4 million
|
University of Maryland
|
|
Chlorine Gas
|
NIH CounterACT
|
$20.3 million
|
National Jewish Health
|
|
Mustard Gas
|
NIH CounterACT
|
Part of the NIH-CounterACT grant above
|
National Jewish Health
University of Colorado
|
|
Nerve Agents
|
NIH CounterACT
|
$5 million
|
University of Colorado
|
|
Treatment
|
Survival
|
Number of Animals
|
|
Radiation Only
|
10%
|
20
|
|
Radiation + 5 mg/kg AEOL 10150
|
16%
|
19
|
|
Radiation + 10 mg/kg AEOL 10150
|
16%
|
19
|
|
Radiation + 25 mg/kg AEOL 10150
|
40%
|
20
|
|
Radiation + 40 mg/kg AEOL 10150
|
30%
|
20
|
|
- Increased mean and median overall survival time
|
|
|
- Increased mean and median survival time in subjects that did not survive to 180 days
|
|
|
- Increased time to onset of increased respiratory rate, a clinical measure of lung injury
|
|
|
- Decreased mortality in subjects with elevated respiratory rate
|
|
|
- Decreased wet lung weight in all animals, suggesting less parenchymal damage and edema
|
|
|
- Increased Sp02, a measure of compensated lung function
|
|
|
- Diminished radiographic evidence of pneumonitis and fibrosis during the later stages of the study (days 90 -180)
|
|
1.
|
Exposure of the whole thorax to 11.5 Gy resulted in radiation-induced lung injury in all NHPs in the study and proved 100% fatal in the control animals, despite supportive care including dexamethasone. 11.5 Gy is, therefore, equal to or greater than the LD100/180dose for the WTLI model.
|
|
2.
|
10150, as administered in this pilot study (daily for 28 days at a dose of 5mg/kg subcutaneously), demonstrated potential efficacy in mitigating against fatal radiation-induced lung injury. Treatment with the drug resulted in 28.6% survival following exposure to a radiation dose that proved to be 100% fatal in the untreated control group.
|
|
3.
|
Serial CT scans demonstrated less quantitative radiographic injury (pneumonitis, fibrosis, effusions) in the 10150-treated cohort, suggesting that the drug reduces the severity of the radiographically detectable lung injury.
|
|
4.
|
Dexamethasone administration yielded a transient benefit on both clinical and radiographic evidence of pneumonitis. The 10150 treated cohort required 1/3 less dexamethasone support due to reduced pulmonary injury in the 10150 treated group, resulting in less frequent clinical "triggers" (respiratory rate≥80) to treat with dexamethasone.
|
|
5.
|
The results of this pilot study are encouraging and suggest that treatment with 10150 results in reduced clinical, radiographic and anatomic evidence of radiation-induced lung injury, which also results in improved survival. 10150 merits further study as a post-exposure MCM against radiation-induced lung injury.
|
|
·
|
Demonstrated survival increase in animal studies of lung ARS when administered 24 hours after exposure,
|
|
·
|
Demonstrated reduction in lung fibrosis in animal studies when administered up to 24 hours post exposure,
|
|
·
|
Demonstrated histological improvement in lung tissue post-radiation exposure,
|
|
·
|
Addresses an unmet medical need as an MCM to Lung-ARS,
|
|
·
|
Established safety profile in both clinical and pre-clinical studies,
|
|
·
|
Subcutaneous self-administration possible by exposed individuals during emergency,
|
|
·
|
Rapid administration, allowing large numbers of patients to be treated quickly,
|
|
·
|
Original formulation stable for up to 4½ years at 0–8°C and 1 year at room temperature,
|
|
·
|
New formulation stability tested in bulk drug for 2 years at room temperature (25°C) and refrigerated conditions (2-8°C); stability testing in bulk drug will continue to three years,
|
|
·
|
New formulation stability tested in final drug product to 18 months under room temperature (25°C), accelerated conditions (40°C) and refrigerated conditions (2-8°C); stability testing in final drug product will continue to 5 years,
|
|
·
|
Requires no non-standard storage conditions (i.e., not photosensitive),
|
|
·
|
Currently in development as an adjunct to radiation therapy and lung fibrosis; if approved will provide a pre-existing distribution and stockpile resource at oncology centers in the event of a radiological emergency,
|
|
·
|
Demonstrated advantage when used in combination with Neupogen®,
|
|
·
|
Demonstrated potential as both a therapeutic and prophylactic,
|
|
·
|
Demonstrated efficacy against sulfur mustard gas, phosgene gas, chlorine gas and nerve agent exposures,
|
|
·
|
Potential dual use as an adjunct treatment for cancer patients receiving radiation therapy and treatment of pulmonary fibrosis, subject to separate FDA approvals for these indications.
|
|
·
|
Knowledge of the mechanism of radiation-induced damage to the lung and its amelioration by the candidate drug.
|
|
·
|
Pharmacokinetic and pharmacodynamic analysis to provide information on relevant dose and administration schedule.
|
|
·
|
Direct correlation of key study parameters (e.g., survival or major morbidity) with the desired clinical benefit in humans.
|
|
·
|
Collection of efficacy data in two species relevant to the human radiation response and its treatment unless otherwise justified under GLP-compliant conditions.
|
|
·
|
A Phase I safety trial using the same product and formulation as used in the pivotal trial(s) is required.
|

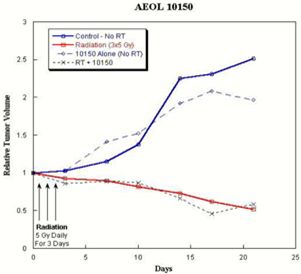
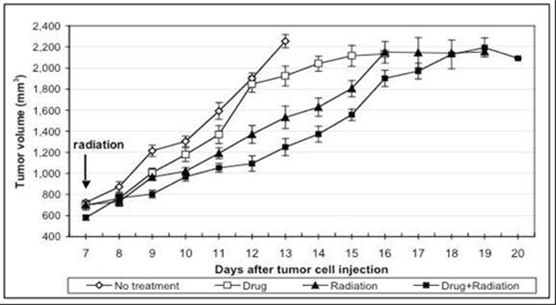

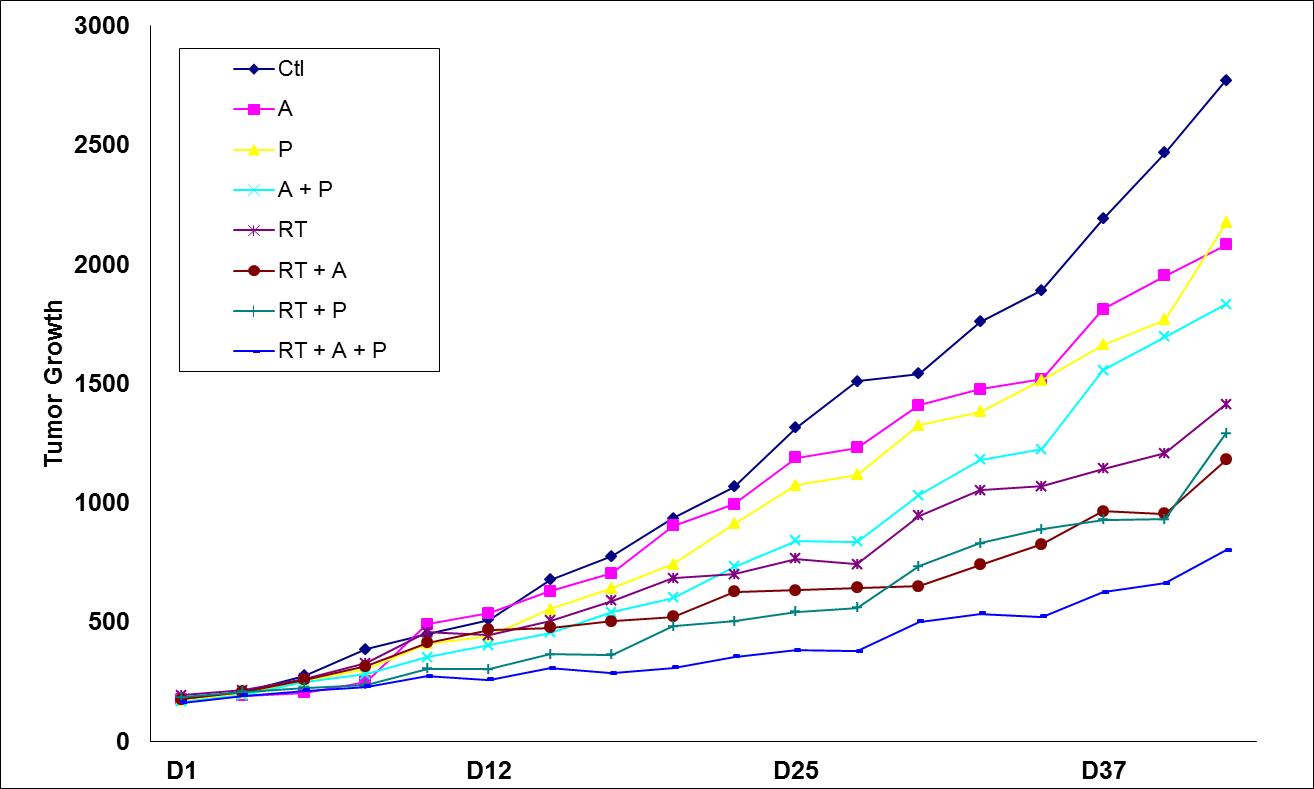
|
·
|
Increases in Cmax and AUC (0-8) appear to correlate with increases in dose, but the correlation is not strong.
|
|
·
|
The mean Cmax for the 40 mg cohort was 1,735 ng/mL; 2,315 ng/mL for the 60 mg cohort and 1,653 ng/mL for the 2 mg/kg cohort.
|
|
·
|
There were probable linear correlations between both Cmax and AUC(0-8) and dose based on body weight.
|
|
·
|
The terminal half-life (a measurement of the time period for which a compound stays in the body) as determined from Day 7 data was approximately 8 to 9 hours.
|
|
·
|
Steady-state occurs within three days of multiple dosing. There was no evidence for a third longer half-life that would be associated with long term accumulation. Thus, compound accumulation is not expected beyond the third day with multiple dosing.
|
|
·
|
From 48 hours to the end of the infusion, the plasma concentrations of 10150 during the infusion showed little variability, indicating a smoother delivery of the drug than with twice-daily injections.
|
|
•
|
Increased overall survival from 25% in the untreated control group to 50%
|
|
•
|
Increased mean and median overall survival time
|
|
•
|
Increased mean and median survival time in subjects that did not survive to 180 days
|
|
•
|
Increased time to onset of increased respiratory rate, a clinical measure of lung injury
|
|
•
|
Decreased mortality in subjects with elevated respiratory rate
|
|
•
|
Decreased wet lung weight in all animals, suggesting less parenchymal damage and edema
|
|
•
|
Increased Sp02, a measure of compensated lung function
|
|
•
|
Diminished radiographic evidence of pneumonitis and fibrosis during the later stages of the study (days 90 -180)
|
|
·
|
Utilize a selective detoxification pathway that preserves host defense and limits inflammation
|
|
·
|
Create and develop stable, small molecules so that they:
|
|
o
|
Maintain pathogen killing with less off target damage,
|
|
o
|
Provide faster resolution of inflammation,
|
|
o
|
Decrease irreversible injury and oxidative stress, and
|
|
·
|
U.S. Civilian: The U.S. civilian market includes funds to protect the U.S. population from biological agents and is largely funded by the Project BioShield Act of 2004 ("Project BioShield"). Project BioShield is the U.S. government's largest biodefense initiative. Project BioShield was extended through the Pandemic All Hazards and Preparedness Reauthorization Act of 2013, which authorized BARDA to administer a Special Reserve Fund of $2.8 billion for MCM procurement.
|
|
·
|
U.S. Military: The DoD is responsible for the development and procurements of countermeasures for the military segment, which focuses on providing protection for military personnel and civilians who are on active duty.
|
|
·
|
Non-U.S. Markets: Non-U.S. markets address protection against biowarfare agents for both civilians and military personnel in foreign countries. We anticipate that foreign countries will want to procure biodefense products as they are developed and validated by procurements by the U.S. government.
|
|
·
|
the agent for which the countermeasure is designed can cause serious or life-threatening disease;
|
|
·
|
the product may reasonably be believed to be effective in detecting, diagnosing, treating or preventing the disease;
|
|
·
|
the known and potential benefits of the product outweigh its known and potential risks; and
|
|
·
|
there is no adequate alternative to the product that is approved and available.
|
|
·
|
completion of preclinical studies;
|
|
·
|
the submission to the FDA of a request for authorization to conduct clinical trials on an IND, which must become effective before clinical trials may commence;
|
|
·
|
adequate and well-controlled Phase I clinical trials, which typically involves normal, healthy volunteers. The tests study a drug candidate's safety profile, including the safe dosage range. The studies also determine how a drug is absorbed, distributed, metabolized and excreted as well as the duration of its action;
|
|
·
|
adequate and well-controlled Phase II clinical trials which typically involve treating patients with the targeted disease with the drug candidate to assess a drug's effectiveness;
|
|
·
|
adequate and well-controlled Phase III clinical trials involving a larger population of patients with the targeted disease are treated with the drug candidate to confirm efficacy of the drug candidate in the treatment of the targeted indication and to identify adverse events;
|
|
·
|
submission to the FDA of an NDA; and
|
|
·
|
review and approval of the NDA by the FDA before the product may be shipped or sold commercially.
|
|
Name
|
Age
|
Position(s)
|
||
|
David Cavalier
|
46
|
Chairman of the Board, Chief Financial Officer
|
||
|
John L. McManus
|
51
|
President and Chief Executive Officer
|
|
·
|
developing our existing drug candidates and developing and testing new drug candidates;
|
|
·
|
protecting our intellectual property;
|
|
·
|
establishing our competitive position;
|
|
·
|
achieving third-party collaborations;
|
|
·
|
receiving regulatory approvals;
|
|
·
|
manufacturing and marketing products; and
|
|
·
|
receiving government funding and identifying new government funding opportunities.
|
|
·
|
any or all of these proposed products or procedures are found to be unsafe or ineffective or otherwise fail to receive necessary regulatory approvals;
|
|
·
|
the proposed products or procedures are not economical to market or do not achieve broad market acceptance;
|
|
·
|
third parties hold proprietary rights that preclude us from marketing the proposed products or procedures; and
|
|
·
|
third parties market a superior or equivalent product.
|
|
·
|
difficulty in securing research laboratories to conduct research activities;
|
|
·
|
difficulty in securing centers to conduct trials;
|
|
·
|
difficulty in enrolling patients in conformity with required protocols or projected timelines;
|
|
·
|
unexpected adverse reactions by patients in trials;
|
|
·
|
difficulty in obtaining clinical supplies of the product;
|
|
·
|
changes in the FDA's or other regulatory body's requirements for our testing during the course of that testing;
|
|
·
|
inability to generate statistically significant data confirming the efficacy of the product being tested;
|
|
·
|
modification of the drug during testing; and
|
|
·
|
reallocation of our limited financial and other resources to other clinical programs.
|
|
·
|
succeed in developing competitive products sooner than us or our strategic partners or licensees;
|
|
·
|
obtain FDA and other regulatory approvals (including EUA approvals) for their products before approval of any of our products;
|
|
·
|
obtain patents that block or otherwise inhibit the development and commercialization of our drug candidates;
|
|
·
|
develop products that are safer or more effective than our products;
|
|
·
|
devote greater resources to marketing or selling their products;
|
|
·
|
introduce or adapt more quickly to new technologies or scientific advances;
|
|
·
|
introduce products that render our products obsolete;
|
|
·
|
withstand price competition more successfully than us or our strategic partners or licensees;
|
|
·
|
negotiate third-party strategic alliances or licensing arrangements more effectively; or
|
|
·
|
take advantage of other opportunities more readily.
|
|
·
|
the receipt of regulatory approvals for the indications that we are studying;
|
|
·
|
the establishment and demonstration in the medical community of the safety, clinical efficacy and cost-effectiveness of our products and their potential advantages over existing therapeutic products;
|
|
·
|
marketing and distribution support;
|
|
·
|
the introduction, market penetration and pricing strategies of competing and future products; and
|
|
·
|
coverage and reimbursement policies of governmental and other third-party payors such as insurance companies, health maintenance organizations and other plan administrators.
|
|
·
|
termination of contracts;
|
|
·
|
forfeiture of profits;
|
|
·
|
suspension of payments;
|
|
·
|
fines; and
|
|
·
|
suspension or prohibition from conducting business with the U.S. government.
|
|
·
|
the Federal Acquisition Regulations, and agency-specific regulations supplemental to the Federal Acquisition Regulations, which comprehensively regulate the procurement, formation, administration and performance of government contracts;
|
|
·
|
the business ethics and public integrity obligations, which govern conflicts of interest and the hiring of former government employees, restrict the granting of gratuities and funding of lobbying activities and incorporate other requirements such as the Anti-Kickback Act and Foreign Corrupt Practices Act;
|
|
·
|
export and import control laws and regulations; and
|
|
·
|
laws, regulations and executive orders restricting the use and dissemination of information classified for national security purposes and the exportation of certain products and technical data.
|
|
·
|
terminate existing contracts, in whole or in part, for any reason or no reason;
|
|
·
|
unilaterally reduce or modify contracts or subcontracts, including equitable price adjustments;
|
|
·
|
cancel multi-year contracts and related orders if funds for contract performance for any subsequent year become unavailable;
|
|
·
|
decline to exercise an option to renew a contract;
|
|
·
|
exercise an option to purchase only the minimum amount specified in a contract;
|
|
·
|
decline to exercise an option to purchase the maximum amount specified in a contract;
|
|
·
|
claim rights to products, including intellectual property, developed under the contract;
|
|
·
|
take actions that result in a longer development timeline than expected;
|
|
·
|
audit and object to the contractor's contract-related costs and fees, including allocated indirect costs;
|
|
·
|
direct the course of a development program in a manner not chosen by the government contractor;
|
|
·
|
suspend or debar the contractor from doing business with the government or a specific government agency;
|
|
·
|
pursue criminal or civil remedies under the False Claims Act and False Statements Act; and
|
|
·
|
control or prohibit the export of products.
|
|
High
|
Low
|
||
|
Fiscal Year Ended September 30, 2014
|
|||
|
October 1, 2013 through December 31, 2013
|
$0.29
|
$0.23
|
|
|
January 1, 2014 through March 31, 2014
|
$0.33
|
$0.23
|
|
|
April 1, 2014 through June 30, 2014
|
$0.36
|
$0.24
|
|
|
July 1, 2014 through September 30, 2014
|
$0.51
|
$0.23
|
|
|
Fiscal Year Ended September 30, 2015
|
|||
|
October 1, 2014 through December 31, 2014
|
$0.29
|
$0.20
|
|
|
January 1, 2015 through March 31, 2015
|
$0.35
|
$0.21
|
|
|
April 1, 2015 through June 30, 2015
|
$0.41
|
$0.28
|
|
|
July 1, 2015 through September 30, 2015
|
$0.32
|
$0.22
|
|
Year Ended September 30,
|
||||||||||||||||||||
|
2015
|
2014
|
2013
|
2012
|
2011
|
||||||||||||||||
|
(in thousands, except per share data)
|
||||||||||||||||||||
|
Revenue:
|
||||||||||||||||||||
|
Grant income and contract revenue
|
$
|
3,111
|
$
|
9,631
|
$
|
3,928
|
$
|
7,293
|
$
|
4,821
|
||||||||||
|
Costs and expenses:
|
||||||||||||||||||||
|
Research and development
|
3,509
|
6,966
|
3,360
|
6,468
|
5,055
|
|||||||||||||||
|
General and administrative
|
2,228
|
2,745
|
3,266
|
3,196
|
3,668
|
|||||||||||||||
|
Total costs and expenses
|
5,737
|
9,711
|
6,626
|
9,664
|
8,723
|
|||||||||||||||
|
Loss from operations
|
(2,626
|
)
|
(80
|
)
|
(2,698
|
)
|
(2,371
|
)
|
(3,902
|
)
|
||||||||||
|
Other income (expenses), net
|
—
|
—
|
(510
|
)
|
4,069
|
4,222
|
||||||||||||||
|
Interest income (expense), net
|
(2
|
)
|
—
|
—
|
—
|
(21
|
)
|
|||||||||||||
|
Net income (loss)
|
(2,628
|
)
|
(80
|
)
|
(3,208
|
)
|
1,698
|
299
|
||||||||||||
|
Preferred stock dividend and accretion
|
—
|
—
|
—
|
—
|
—
|
|||||||||||||||
|
Net income (loss) attributable to common stockholders
|
$
|
(2,628
|
)
|
$
|
(80
|
)
|
$
|
(3,208
|
)
|
$
|
856
|
$
|
149
|
|||||||
|
Basic net income (loss) per share attributable to common stockholders
|
$
|
(0.02
|
)
|
$
|
0.00
|
$
|
(0.03
|
)
|
$
|
0.01
|
$
|
0.00
|
||||||||
|
Diluted net income (loss) per share attributable to common stockholders
|
$
|
(0.02
|
)
|
$
|
0.00
|
$
|
(0.03
|
)
|
$
|
(0.03
|
)
|
$
|
(0.04
|
)
|
||||||
|
Weighted average common shares outstanding:
|
||||||||||||||||||||
|
Basic
|
135,883
|
134,667
|
106,554
|
61,593
|
59,474
|
|||||||||||||||
|
Diluted
|
135,883
|
134,667
|
106,554
|
71,041
|
85,862
|
|||||||||||||||
|
September 30,
|
||||||||||||||||||||
|
2015
|
2014
|
2013
|
2012
|
2011
|
||||||||||||||||
|
(in thousands)
|
||||||||||||||||||||
|
Cash and cash equivalents and marketable securities
|
94
|
1,517
|
869
|
281
|
518
|
|||||||||||||||
|
Working capital (deficiency)
|
(876
|
)
|
1,553
|
674
|
(1,048
|
)
|
114
|
|||||||||||||
|
Total assets
|
1,777
|
3,580
|
1,966
|
1,256
|
2,290
|
|||||||||||||||
|
Long-term portion of capital lease obligations and notes payable
|
—
|
—
|
—
|
—
|
—
|
|||||||||||||||
|
Total liabilities
|
2,621
|
1,995
|
1,261
|
21,591
|
25,549
|
|||||||||||||||
|
Total stockholders' equity (deficit)
|
845
|
1,585
|
705
|
(20,335
|
)
|
(23,259
|
)
|
|||||||||||||
|
·
|
as a MCM for the pulmonary and delayed effects of acute radiation exposure under contract with the U.S Government
|
|
·
|
as a MCM for exposure to chemical gas and nerve gas exposure with grant funding from the U.S. Government
|
|
·
|
as a treatment for idiopathic pulmonary fibrosis
|
|
·
|
as a treatment for the side effects of radiation therapy for solid tumors in oncology
|
|
·
|
development AEOL 1114B ("11114") as a treatment for Parkinson's disease
|
|
·
|
development of AEOL 20415 ("20415") as a treatment for infectious disease
|
|
Payments due by period
|
||||||||||||||||||||
|
Contractual Obligations
|
Total
|
Less
than 1
Year
|
1-3
Years
|
3-5
Years
|
More
than 5
Years
|
|||||||||||||||
|
Short and long-term debt
|
$
|
1,000
|
$
|
1,000
|
$
|
—
|
$
|
—
|
$
|
—
|
||||||||||
|
Capital lease obligations
|
—
|
—
|
—
|
—
|
—
|
|||||||||||||||
|
Operating leases
|
9
|
9
|
—
|
—
|
—
|
|||||||||||||||
|
Purchase obligations
|
—
|
—
|
—
|
—
|
—
|
|||||||||||||||
|
Total
|
$
|
1,009
|
$
|
1,009
|
$
|
—
|
$
|
—
|
$
|
—
|
||||||||||
|
Page
|
|
|
Report of Independent Registered Public Accounting Firm
|
67
|
|
Consolidated Balance Sheets – As of September 30, 2015 and 2014
|
68
|
|
Consolidated Statements of Operations – For the fiscal years ended September 30, 2015 and 2014
|
69
|
|
Consolidated Statements of Stockholders' Equity (Deficit) – For the fiscal years ended September 30, 2015 and 2014
|
70
|
|
Consolidated Statements of Cash Flows – For the fiscal years ended September 30, 2015 and 2014
|
71
|
|
Notes to Consolidated Financial Statements
|
72
|
|
|
September 30,
|
|||||||
|
|
2015
|
2014
|
||||||
|
ASSETS
|
||||||||
|
Current assets:
|
||||||||
|
Cash and cash equivalents
|
$
|
94
|
$
|
1,517
|
||||
|
Accounts receivable
|
1,585
|
1,559
|
||||||
|
Deferred subcontractor cost
|
21
|
426
|
||||||
|
Prepaid expenses and other current assets
|
45
|
46
|
||||||
|
Total current assets
|
1,745
|
3,548
|
||||||
|
Investment in CPEC LLC
|
32
|
32
|
||||||
|
Total assets
|
$
|
1,777
|
$
|
3,580
|
||||
|
LIABILITIES AND STOCKHOLDERS' (DEFICIT) EQUITY
|
||||||||
|
Current liabilities:
|
||||||||
|
Accounts payable and accrued expenses
|
$
|
1,598
|
$
|
1,552
|
||||
|
Deferred revenue
|
22
|
443
|
||||||
|
Note payable to shareholders, net of debt discount of $273
|
727
|
—
|
||||||
|
Note payable to shareholders redemption liability
|
275
|
—
|
||||||
|
Total current liabilities
|
2,622
|
1,995
|
||||||
|
Total liabilities
|
2,622
|
1,995
|
||||||
|
Commitments and Contingencies (Notes E and K)
|
||||||||
|
Stockholders' equity:
|
||||||||
|
Preferred stock, $0.01 par value per share, 10,000,000 shares authorized:
|
||||||||
|
Series A nonredeemable convertible preferred stock, 1,250,000 shares authorized as of September 30, 2015 and 2014, respectively; no shares issued and outstanding as of September 30, 2015 and 2014, respectively
|
—
|
—
|
||||||
|
Series B nonredeemable convertible preferred stock, 1,600,000 and 1,600,000 shares authorized as of September 30, 2015 and 2014, respectively; 526,080 and 526,080 shares issued and outstanding as of September 30, 2015 and 2014, respectively
|
5
|
5
|
||||||
|
Common stock, $0.01 par value per share, 200,000,000 shares authorized; 135,930,068 and 135,850,068 shares issued and outstanding at September 30, 2015 and 2014, respectively
|
1,359
|
1,359
|
||||||
|
Additional paid-in capital
|
184,421
|
184,223
|
||||||
|
Accumulated deficit
|
(186,630
|
)
|
(184,002
|
)
|
||||
|
Total stockholders' (deficit) equity
|
(845
|
)
|
1,585
|
|||||
|
Total liabilities and stockholders' (deficit) equity
|
$
|
1,777
|
$
|
3,580
|
||||
|
Fiscal Year Ended September 30,
|
||||||||
|
2015
|
2014
|
|||||||
|
Revenue:
|
||||||||
|
Contract revenue
|
$
|
3,111
|
$
|
9,631
|
||||
|
Costs and expenses:
|
||||||||
|
Research and development
|
3,509
|
6,966
|
||||||
|
General and administrative
|
2,228
|
2,745
|
||||||
|
Total costs and expenses
|
5,737
|
9,711
|
||||||
|
Loss from operations
|
(2,626
|
)
|
(80
|
)
|
||||
|
Interest expense
|
2
|
—
|
||||||
|
Net loss
|
$
|
(2,628
|
)
|
$
|
(80
|
)
|
||
|
Net loss attributable to common stockholders – basic
|
$
|
(2,628
|
)
|
$
|
(80
|
)
|
||
|
Net loss attributable to common stockholders – diluted
|
$
|
(2,628
|
)
|
$
|
(80
|
)
|
||
|
Basic net loss per common share
|
$
|
(0.02
|
)
|
$
|
0.00
|
|||
|
Diluted net loss per common share
|
$
|
(0.02
|
)
|
$
|
0.00
|
|||
|
Weighted average common shares outstanding:
|
||||||||
|
Basic
|
135,883
|
134,667
|
||||||
|
Diluted
|
135,883
|
134,667
|
||||||
|
Series B Preferred Stock
|
Common Stock
|
Additional
|
Accumulated
|
Total Stockholders'
|
||||||||||||||||||||||||
|
Shares
|
Par Value
|
Shares
|
Par Value
|
Paid-in Capital
|
Deficit
|
Equity (Deficit)
|
||||||||||||||||||||||
|
Balance at September 30, 2013
|
526,080
|
$
|
5
|
134,550,068
|
$
|
1,346
|
$
|
183,276
|
$
|
(183,922
|
)
|
$
|
705
|
|||||||||||||||
|
Exercise of warrants
|
—
|
—
|
1,300,000
|
13
|
312
|
—
|
325
|
|||||||||||||||||||||
|
Issuance of warrants to consultants
|
—
|
—
|
—
|
—
|
9
|
—
|
9
|
|||||||||||||||||||||
|
Stock-based compensation
|
—
|
—
|
—
|
—
|
626
|
—
|
626
|
|||||||||||||||||||||
|
Net loss for the fiscal year ended September 30, 2014
|
—
|
—
|
—
|
—
|
—
|
(80
|
)
|
(80
|
)
|
|||||||||||||||||||
|
Balance at September 30, 2014
|
526,080
|
5
|
135,850,068
|
1,359
|
184,223
|
(184,002
|
)
|
1,585
|
||||||||||||||||||||
|
Exercise of warrants
|
—
|
—
|
80,000
|
—
|
20
|
—
|
20
|
|||||||||||||||||||||
|
Issuance of warrants to consultants
|
—
|
—
|
—
|
—
|
14
|
—
|
14
|
|||||||||||||||||||||
|
Stock-based compensation
|
—
|
—
|
—
|
—
|
164
|
—
|
164
|
|||||||||||||||||||||
|
Net loss for the fiscal year end September 30, 2015
|
—
|
—
|
—
|
—
|
—
|
(2,628
|
)
|
(2,628
|
)
|
|||||||||||||||||||
|
Balance at September 30, 2015
|
526,080
|
$
|
5
|
135,930,068
|
$
|
1,359
|
$
|
184,421
|
$
|
(186,630
|
)
|
$
|
(845
|
)
|
||||||||||||||
|
Fiscal Year Ended September 30,
|
||||||||
|
2015
|
2014
|
|||||||
|
Cash flows from operating activities:
|
||||||||
|
Net loss
|
$
|
(2,628
|
)
|
$
|
(80
|
)
|
||
|
Adjustments to reconcile net loss to net cash (used in) provided by operating activities:
|
||||||||
|
Accrued interest
|
2
|
—
|
||||||
|
Noncash compensation
|
178
|
635
|
||||||
|
Change in assets and liabilities:
|
||||||||
|
Accounts receivable
|
(26
|
)
|
(1,189
|
)
|
||||
|
Deferred subcontractor cost
|
405
|
230
|
||||||
|
Prepaid expenses and other current assets
|
1
|
(7
|
)
|
|||||
|
Accounts payable and accrued expenses
|
46
|
973
|
||||||
|
Deferred revenue
|
(421
|
)
|
(239
|
)
|
||||
|
Net cash (used in) provided by operating activities
|
(2,443
|
)
|
323
|
|||||
|
|
||||||||
|
Cash flows from financing activities:
|
||||||||
|
Proceeds from exercise of common stock warrants
|
20
|
325
|
||||||
|
Proceeds from issuance of note payable to shareholders
|
1,000
|
—
|
||||||
|
Net cash provided by financing activities
|
1,020
|
325
|
||||||
|
|
||||||||
|
Net (decrease) increase in cash and cash equivalents
|
(1,423
|
)
|
648
|
|||||
|
|
||||||||
|
Cash and cash equivalents at beginning of year
|
1,517
|
869
|
||||||
|
Cash and cash equivalents at end of year
|
$
|
94
|
$
|
1,517
|
||||
|
|
||||||||
|
Supplemental disclosure of non-cash financing activities:
|
||||||||
|
Note payable to shareholders redemption liability
|
$
|
275
|
$
|
—
|
||||
|
·
|
Level 1: Observable inputs such as quoted prices (unadjusted) in active markets for identical assets or liabilities.
|
|
·
|
Level 2: Inputs other than quoted prices that are observable for the asset or liability, either directly or indirectly. These include quoted prices for similar assets or liabilities in active markets and quoted prices for identical or similar assets or liabilities in markets that are not active.
|
|
·
|
Level 3: Unobservable inputs that reflect the reporting entity's own assumptions.
|
|
Fair value at September 30, 2015
|
|||||||
|
Level 1
|
Level 2
|
Level 3
|
|||||
|
$
|
275
|
$
|
—
|
$
|
—
|
||
|
Fair value measurement of redemption liability
|
|||
|
Balance at September 30, 2014
|
$
|
—
|
|
|
Issuance of convertible promissory notes with redemption feature
|
275
|
||
|
Change in fair value of redemption liability
|
—
|
||
|
Balance at September 30, 2015
|
$
|
275
|
|
|
In thousands, except per share data
|
||||||||
|
Fiscal Year Ended September 30,
|
||||||||
|
2015
|
2014
|
|||||||
|
Numerator:
|
||||||||
|
Net income (loss)
|
$
|
(2,628
|
)
|
$
|
(80
|
)
|
||
|
Net income attributable to participating securities
|
—
|
—
|
||||||
|
Net income (loss) attributable to common stockholders – basic
|
$
|
(2,628
|
)
|
$
|
(80
|
)
|
||
|
Net income (loss)
|
$
|
(2,628
|
)
|
$
|
(80
|
)
|
||
|
Less gain (loss) on warrant liability for participating common warrants
|
—
|
—
|
||||||
|
Net income (loss) attributable to common stockholders – diluted
|
$
|
(2,628
|
)
|
$
|
(80
|
)
|
||
|
Denominator:
|
||||||||
|
Weighted-average shares used in computing net income (loss) per share attributable to common stockholders – basic
|
135,883
|
134,667
|
||||||
|
Effect of potentially dilutive securities:
|
||||||||
|
Common stock warrants
|
—
|
—
|
||||||
|
Convertible preferred warrants
|
—
|
—
|
||||||
|
Convertible preferred stock
|
—
|
—
|
||||||
|
Common stock options
|
—
|
—
|
||||||
|
Non-participating common stock warrants
|
—
|
—
|
||||||
|
Weighted-average shares used in computing net income (loss) per share attributable to common stockholders – diluted
|
135,883
|
134,667
|
||||||
|
Basic net income (loss) per common share
|
$
|
(0.02
|
)
|
$
|
0.00
|
|||
|
Diluted net income (loss) per common share
|
$
|
(0.02
|
)
|
$
|
0.00
|
|||
|
Number of Shares
|
Exercise Price
|
Expiration Date
|
|||||
|
50,000
|
$
|
0.50
|
May 2016
|
||||
|
50,000
|
$
|
0.50
|
July 2016
|
||||
|
50,000
|
$
|
1.00
|
July 2016
|
||||
|
50,000
|
$
|
1.50
|
July 2016
|
||||
|
50,000
|
$
|
2.00
|
July 2016
|
||||
|
50,000
|
$
|
2.50
|
July 2016
|
||||
|
1,337,627
|
$
|
0.40
|
March 2017
|
||||
|
325,000
|
$
|
0.40
|
April 2017
|
||||
|
300,000
|
$
|
0.258
|
June 2017
|
||||
|
50,000
|
$
|
0.26
|
June 2017
|
||||
|
140,000
|
$
|
0.35
|
October 2017
|
||||
|
12,205,000
|
$
|
0.25
|
February 2018
|
||||
|
1,242,000
|
$
|
0.25
|
March 2018
|
||||
|
50,000
|
$
|
0.49
|
January 2020
|
||||
|
15,949,627
|
|||||||
|
Number of Shares
|
Exercise Price
|
Expiration Date
|
|||||
|
50,000
|
$
|
0.38
|
April 2015
|
||||
|
50,000
|
$
|
0.50
|
May 2016
|
||||
|
50,000
|
$
|
0.50
|
July 2016
|
||||
|
50,000
|
$
|
1.00
|
July 2016
|
||||
|
50,000
|
$
|
1.50
|
July 2016
|
||||
|
50,000
|
$
|
2.00
|
July 2016
|
||||
|
50,000
|
$
|
2.50
|
July 2016
|
||||
|
1,337,627
|
$
|
0.40
|
March 2017
|
||||
|
325,000
|
$
|
0.40
|
April 2017
|
||||
|
300,000
|
$
|
0.258
|
June 2017
|
||||
|
50,000
|
$
|
0.26
|
June 2017
|
||||
|
140,000
|
$
|
0.35
|
October 2017
|
||||
|
12,285,000
|
$
|
0.25
|
February 2018
|
||||
|
1,242,000
|
$
|
0.25
|
March 2018
|
||||
|
16,029,627
|
|||||||
|
Weighted Average
|
|||||||||||||
|
Remaining
|
Aggregate
|
||||||||||||
|
Number
|
Exercise
|
Contractual
|
Intrinsic
|
||||||||||
|
of Shares
|
Price
|
Term (in years)
|
Value
|
||||||||||
|
Outstanding at 9/30/2013
|
18,775,664
|
$
|
0.29
|
4.1 years
|
$
|
693,340
|
|||||||
|
Granted
|
50,000
|
$
|
0.26
|
$
|
-
|
||||||||
|
Exercised
|
(1,300,000
|
)
|
$
|
0.25
|
$
|
318,000
|
|||||||
|
Cancelled
|
(600,000
|
)
|
$
|
0.86
|
$
|
-
|
|||||||
|
Forfeited
|
-
|
$
|
-
|
$
|
-
|
||||||||
|
Outstanding at 9/30/2014
|
16,925,664
|
$
|
0.27
|
3.1 years
|
$
|
215,048
|
|||||||
|
Granted
|
50,000
|
$
|
0.49
|
$
|
-
|
||||||||
|
Exercised
|
(80,000
|
)
|
$
|
0.25
|
$
|
10,975
|
|||||||
|
Cancelled
|
(50,000
|
)
|
$
|
0.38
|
$
|
-
|
|||||||
|
Forfeited
|
-
|
$
|
-
|
$
|
-
|
||||||||
|
Outstanding at 9/30/2015
|
16,845,664
|
$
|
0.27
|
2.2 years
|
$
|
206,626
|
|||||||
|
Exercisable at 9/30/2015
|
16,845,664
|
$
|
0.27
|
2.2 years
|
$
|
206,626
|
|||||||
|
Weighted Average
|
|||||||||||||
|
Remaining
|
Aggregate
|
||||||||||||
|
Number
|
Exercise
|
Contractual
|
Intrinsic
|
||||||||||
|
of Shares
|
Price
|
Term (in years)
|
Value
|
||||||||||
|
Outstanding at 9/30/2013
|
11,214,898
|
$
|
0.52
|
6.7 years
|
$
|
2
|
|||||||
|
Granted
|
775,000
|
$
|
0.26
|
$
|
-
|
||||||||
|
Exercised
|
-
|
$
|
-
|
$
|
-
|
||||||||
|
Cancelled
|
(393,307
|
)
|
$
|
2.64
|
$
|
-
|
|||||||
|
Forfeited
|
-
|
$
|
-
|
$
|
-
|
||||||||
|
Outstanding at 9/30/2014
|
11,596,591
|
$
|
0.43
|
6.1 years
|
$
|
1
|
|||||||
|
Granted
|
775,000
|
$
|
0.27
|
$
|
-
|
||||||||
|
Exercised
|
-
|
$
|
-
|
$
|
-
|
||||||||
|
Cancelled
|
(207,000
|
)
|
$
|
0.90
|
$
|
-
|
|||||||
|
Forfeited
|
-
|
$
|
-
|
$
|
-
|
||||||||
|
Outstanding at 9/30/2015
|
12,164,591
|
$
|
0.42
|
5.4 years
|
$
|
1
|
|||||||
|
Exercisable at 9/30/2015
|
12,002,089
|
$
|
0.42
|
5.4 years
|
$
|
1
|
|||||||
|
Weighted Average
|
||||||||
|
Number
|
Grant-Date
|
|||||||
|
of Shares
|
Fair Value
|
|||||||
|
Unvested at September 30, 2013
|
1,482,286
|
$
|
0.37
|
|||||
|
Granted
|
775,000
|
$
|
0.22
|
|||||
|
Vested
|
(2,088,534
|
)
|
$
|
0.33
|
||||
|
Forfeited
|
-
|
$
|
-
|
|||||
|
Unvested at September 30, 2014
|
168,752
|
$
|
0.22
|
|||||
|
Granted
|
775,000
|
$
|
0.24
|
|||||
|
Vested
|
(781,250
|
)
|
$
|
0.24
|
||||
|
Forfeited
|
-
|
$
|
-
|
|||||
|
Unvested at September 30, 2015
|
162,502
|
$
|
0.23
|
|||||
|
Options Outstanding
|
Options Exercisable
|
|||||||||||||||||||||||||
|
Weighted
|
Weighted
|
|||||||||||||||||||||||||
|
Number
|
Weighted
|
Average
|
Number
|
Weighted
|
Average
|
|||||||||||||||||||||
|
Range of
|
Outstanding at
|
Average
|
Remaining
|
Exercisable at
|
Average
|
Remaining
|
||||||||||||||||||||
|
Exercise
|
September 30,
|
Exercise
|
Contractual
|
September 30,
|
Exercise
|
Contractual
|
||||||||||||||||||||
|
Prices
|
2015
|
Price
|
Life (in years)
|
2015
|
Price
|
Life (in years)
|
||||||||||||||||||||
|
$
|
0.23-$0.30
|
3,087,500
|
$
|
0.28
|
6.37
|
2,924,998
|
$
|
0.28
|
6.20
|
|||||||||||||||||
|
$
|
0.31-$0.40
|
6,551,500
|
$
|
0.39
|
5.83
|
6,551,500
|
$
|
0.39
|
5.83
|
|||||||||||||||||
|
$
|
0.41-$0.50
|
502,000
|
$
|
0.45
|
6.25
|
502,000
|
$
|
0.45
|
6.25
|
|||||||||||||||||
|
$
|
0.51-$0.60
|
982,250
|
$
|
0.59
|
3.71
|
982,250
|
$
|
0.59
|
3.71
|
|||||||||||||||||
|
$
|
0.61-$0.70
|
60,000
|
$
|
0.68
|
1.03
|
60,000
|
$
|
0.68
|
1.03
|
|||||||||||||||||
|
$
|
0.71-$0.80
|
366,750
|
$
|
0.75
|
1.75
|
366,750
|
$
|
0.75
|
1.75
|
|||||||||||||||||
|
$
|
0.81-$0.90
|
570,591
|
$
|
0.87
|
1.26
|
570,591
|
$
|
0.87
|
1.26
|
|||||||||||||||||
|
$
|
0.91-$1.00
|
14,000
|
$
|
0.95
|
0.25
|
14,000
|
$
|
0.95
|
0.25
|
|||||||||||||||||
|
$
|
1.01-$1.19
|
30,000
|
$
|
1.10
|
0.22
|
30,000
|
$
|
1.10
|
0.22
|
|||||||||||||||||
|
12,164,591
|
$
|
0.42
|
5.43
|
12,002,089
|
$
|
0.42
|
5.38
|
|||||||||||||||||||
|
For the fiscal year ended September 30,
|
||||||||
|
2015
|
2014
|
|||||||
|
Research and Development Expenses
|
$
|
—
|
$
|
11
|
||||
|
General and Administrative Expenses
|
178
|
624
|
||||||
|
Total Stock-based Compensation Expense
|
$
|
178
|
$
|
635
|
||||
|
For the fiscal year ended September 30,
|
||||||||
|
2015
|
2014
|
|||||||
|
Dividend yield
|
0
|
%
|
0
|
%
|
||||
|
Unvested forfeiture rate
|
5.35
|
%
|
0
|
%
|
||||
|
Expected volatility
|
137
|
%
|
122
|
%
|
||||
|
Risk-free interest rate
|
1.70
|
%
|
1.54
|
%
|
||||
|
Expected option life after shares are vested
|
5.27 years
|
5.27 years
|
||||||
|
2015
|
2014
|
|||||||
|
Accrued payroll related liabilities
|
$
|
1,036
|
$
|
1,103
|
||||
|
Depreciation and amortization
|
586
|
704
|
||||||
|
Total deferred tax assets
|
1,622
|
1,807
|
||||||
|
State Taxes
|
(113
|
) |
(126
|
) | ||||
|
Total deferred tax liabilities
|
(113
|
) |
(126
|
) | ||||
|
Net deferred tax assets
|
1,509 | 1,681 | ||||||
|
Valuation allowance for deferred assets
|
(1,509
|
)
|
(1,681
|
)
|
||||
|
Net deferred tax asset
|
$
|
—
|
$
|
—
|
||||
|
2015
|
2014
|
|||||||
|
Effective income tax rate
|
0
|
%
|
0
|
%
|
||||
|
United States Federal income tax at statutory rate
|
$
|
(891
|
)
|
$
|
(25
|
)
|
||
|
State income taxes (net of federal benefit)
|
1
|
1
|
||||||
|
NQSO forfeiture
|
234
|
—
|
||||||
|
Prior year deferred true up
|
39
|
(280
|
)
|
|||||
|
Change in valuation reserves
|
(153
|
)
|
96
|
|||||
|
FIN 48
|
883
|
354
|
||||||
|
Other
|
(111
|
)
|
(144
|
)
|
||||
|
Provision for income taxes
|
$
|
2
|
$
|
2
|
||||
|
Amount
|
||||
|
(in thousands)
|
||||
|
Unrecognized tax benefits at October 1,
|
$
|
4,859
|
||
|
Additions for tax positions related to current year
|
226
|
|||
|
Additions/reductions for tax positions taken in prior years
|
||||
|
Settlements
|
—
|
|||
|
Lapse of Limitations
|
—
|
|||
|
Unrecognized tax benefits at September 30, 2015
|
$
|
5,085
|
||
|
Current – Federal
|
$
|
—
|
||
|
Current – State
|
2
|
|||
|
2
|
||||
|
Deferred - Federal
|
134
|
|||
|
Deferred – State
|
38
|
|||
|
172
|
||||
|
Less: Valuation Allowance
|
(172
|
)
|
||
|
—
|
||||
|
Total current and deferred
|
$
|
2
|
|
First
Quarter
|
Second
Quarter
|
Third
Quarter
|
Fourth
Quarter
|
Total
Year
|
||||||||||||||||
|
(in thousands, except per share amounts)
|
||||||||||||||||||||
|
Fiscal 2015
|
||||||||||||||||||||
|
Total revenue
|
$
|
925
|
$
|
1,189
|
$
|
63
|
$
|
934
|
$
|
3,111
|
||||||||||
|
Net income (loss)
|
$
|
(698
|
)
|
$
|
(712
|
)
|
$
|
(784
|
)
|
$
|
(434
|
)
|
$
|
(2,628
|
)
|
|||||
|
Net income available to stockholders – Basic
|
$
|
(698
|
)
|
$
|
(712
|
)
|
$
|
(784
|
)
|
$
|
(434
|
)
|
$
|
(2,628
|
)
|
|||||
|
Net income available to stockholders – Diluted
|
$
|
(698
|
)
|
$
|
(712
|
)
|
$
|
(784
|
)
|
$
|
(434
|
)
|
$
|
(2,628
|
)
|
|||||
|
Basic net income (loss) per common share attributable to common stockholders
|
$
|
(0.01
|
)
|
$
|
(0.01
|
)
|
$
|
(0.01
|
)
|
$
|
0.00
|
$
|
(0.02
|
)
|
||||||
|
Diluted net income (loss) per common share attributable to common stockholders
|
$
|
(0.01
|
)
|
$
|
(0.01
|
)
|
$
|
(0.01
|
)
|
$
|
0.00
|
$
|
(0.02
|
)
|
||||||
|
Fiscal 2014
|
||||||||||||||||||||
|
Total revenue
|
$
|
793
|
$
|
1,438
|
$
|
4,983
|
$
|
2,417
|
$
|
9,631
|
||||||||||
|
Net income (loss)
|
$
|
(695
|
)
|
$
|
(437
|
)
|
$
|
1,576
|
$
|
(524
|
)
|
$
|
(80
|
)
|
||||||
|
Net income available to stockholders – Basic
|
$
|
(695
|
)
|
$
|
(437
|
)
|
$
|
1,576
|
$
|
(524
|
)
|
$
|
(80
|
)
|
||||||
|
Net income available to stockholders – Diluted
|
$
|
(695
|
)
|
$
|
(437
|
)
|
$
|
1,576
|
$
|
(524
|
)
|
$
|
(80
|
)
|
||||||
|
Basic net income (loss) per common share attributable to common stockholders
|
$
|
(0.01
|
)
|
$
|
0.00
|
$
|
0.01
|
$
|
0.00
|
$
|
0.00
|
|||||||||
|
Diluted net income (loss) per common share attributable to common stockholders
|
$
|
(0.01
|
)
|
$
|
0.00
|
$
|
0.01
|
$
|
0.00
|
$
|
0.00
|
|||||||||
|
·
|
pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of our assets;
|
|
·
|
provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that our receipts and expenditures are being made only in accordance with authorizations of our management and directors; and
|
|
·
|
provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of our assets that could have a material effect on our financial statements.
|
|
Plan category
|
(a)Number of securities to be issued upon exercise of outstanding options, warrants and rights
|
(b)Weighted-average exercise price of outstanding options, warrants and rights
|
(c)Number of securities remaining available for future issuance under equity compensation plans (excluding securities reflected in column (a))
|
|||||||||
|
Equity compensation plans approved by our stockholders:
|
||||||||||||
|
2004 Stock Option Plan
|
7,749,091
|
$
|
0.47
|
2,250,909
|
||||||||
|
Equity compensation plans and securities not approved by our stockholders:
|
||||||||||||
|
Warrants to purchase Common Stock Issued to National Securities
|
50,000
|
$
|
0.38
|
Not applicable
|
||||||||
|
Warrants to Purchase Common Stock Issued to Dan Delmonico
|
50,000
|
$
|
0.49
|
Not applicable
|
||||||||
|
Warrants to Purchase Common Stock Issued to Michael Kruger
|
50,000
|
$
|
0.50
|
Not applicable
|
||||||||
|
Warrants to Purchase Common Stock Issued to Noble International Investments, Inc.
|
300,000
|
$
|
1.00
|
Not applicable
|
||||||||
|
Warrants to Purchase Common Stock Issued to CEOcast, Inc.
|
250,000
|
$
|
0.83
|
Not applicable
|
||||||||
|
Warrants to Purchase Common Stock Issued to Market Pathways
|
250,000
|
$
|
1.50
|
Not applicable
|
||||||||
|
Warrants to Purchase Common Stock Issued to Roberts Mitani, LLC
|
300,000
|
$
|
0.258
|
Not applicable
|
||||||||
|
Warrants to Purchase Common Stock Issued to Columbia Capital Securities, Inc.
|
35,000
|
$
|
0.37
|
Not applicable
|
||||||||
|
Warrants to Purchase Common Stock Issued to Monarch Bay Associates, LLC
|
35,000
|
$
|
0.37
|
Not applicable
|
||||||||
|
Total – Common Stock
|
10,793,661
|
2,250,909
|
||||||||||
|
Warrants to purchase Series B Preferred Stock
|
896,037
|
$
|
0.01
|
Not applicable
|
||||||||
|
Total – Series B Preferred Stock
|
896,037
|
177,883
|
||||||||||
|
Incorporated by Reference To
|
|||||
|
Exhibit
|
Registrant's
|
Date Filed
|
Exhibit
|
Filed
|
|
|
Number
|
Description of Document
|
Form
|
with the SEC |
Number
|
Herewith
|
|
2.1
|
Agreement and Plan of Merger and Reorganization dated September 16, 2003 between Incara, Inc. and Incara Pharmaceuticals Corporation
|
S-4
|
9/19/2003
|
2.1
|
|
|
3.1
|
Amended and Restated Certificate of Incorporation
|
10-K
|
12/31/2012
|
3.1
|
|
| 3.2 | Certificate of Designation for Series C Preferred | 8-K | 12/15/15 | 10.1 | |
| 3.3 | Bylaws | 8-K | 10/27/15 | 3.1 | |
|
4.1
|
Form of Common Stock Certificate
|
10-Q
|
8/11/2004
|
4.1
|
|
|
4.2
|
Form of Series B Preferred Stock Certificate
|
S-4
|
9/19/2003
|
4.8
|
|
|
4.3
|
Form of Warrant to Purchase Common Stock dated June 5, 2006.
|
8-K
|
6/6/2006
|
10.3
|
|
|
4.4
|
Registration Rights Agreement dated May 22, 2007 by and among the Company and each of the Purchasers whose names appear on the Schedule attached thereto.
|
8-K
|
5/23/2007
|
4.1
|
|
|
4.5
|
Registration Rights Agreement dated October 6, 2009 by and among the Company and the investors whose names appear on the signature pages thereof.
|
8-K
|
10/6/2009
|
4.1
|
|
|
4.6
|
Form of Warrant to Purchase Common Stock dated May 22, 2007.
|
8-K
|
5/23/2007
|
10.2
|
|
|
4.7
|
Form of Warrant to Purchase Common Stock
|
8-K
|
10/6/2009
|
10.2
|
|
|
4.8
|
Registration Rights Agreement dated September 16, 2003 among Incara Pharmaceuticals Corporation, Incara, Inc. and Goodnow Capital, L.L.C.
|
S-4
|
9/19/2003
|
10.101
|
|
|
4.9
|
Registration Rights Agreement dated August 11, 2010 by and among Aeolus Pharmaceuticals, Inc. and the investors listed therein
|
8-K
|
8/12/2010
|
4.1
|
|
|
4.1
|
Registration Rights Agreement dated March 4, 2013 by and among Aeolus Pharmaceuticals, Inc. and the investors listed therein
|
8-K
|
3/6/2013
|
10.2
|
|
|
4.11
|
Form of Warrant to Purchase Common Stock dated March 4, 2013.
|
8-K
|
3/6/2013
|
10.3
|
|
|
4.12
|
Warrant Repricing, Exercise and Lockup Agreement dated February 19, 2013 by and among the Company, Xmark JV Investment Partners, LLC and affiliates
|
8-K
|
2/19/2013
|
10.4
|
|
| 4.13 | Form of Series C Preferred Stock Certificate | X | |||
| 4.14 | Form of Warrant | 8-K | 12/15/15 | 10.2 | |
|
10.1*
|
License Agreement between Duke University and Aeolus Pharmaceuticals, Inc., dated July 21, 1995
|
S-1
|
12/8/1995
|
10.4
|
|
|
10.2
|
Amended and Restated Limited Liability Company Agreement of CPEC LLC dated July 15, 1999, among CPEC LLC, Intercardia, Inc. and Interneuron Pharmaceuticals, Inc.
|
8-K
|
7/23/1999
|
10.42
|
|
|
10.3
|
Assignment, Assumption and License Agreement dated July 15, 1999, between CPEC LLC and Intercardia, Inc.
|
8-K
|
7/23/1999
|
10.43
|
|
|
Incorporated by Reference To
|
|||||
|
Exhibit
|
Registrant's
|
Date Filed
|
Exhibit
|
Filed
|
|
|
Number
|
Description of Document
|
Form
|
with the SEC |
Number
|
Herewith
|
|
10.4*
|
License Agreement dated January 19, 2001 between Incara Pharmaceuticals Corporation and Incara Development, Ltd.
|
10-Q
|
2/13/2001
|
10.59
|
|
|
10.5*
|
License Agreement dated January 19, 2001 between Elan Corporation, plc, Elan Pharma International Ltd. and Incara Development, Ltd.
|
10-Q
|
2/13/2001
|
10.6
|
|
|
10.6
|
Registration Rights Agreement dated December 21, 2000 among Incara Pharmaceuticals Corporation, Elan International Services, Ltd. and Elan Pharma International Ltd.
|
10-Q
|
2/13/2001
|
10.62
|
|
|
10.7
|
Agreement and Amendment, effective as of January 22, 2001, by and among Incara Pharmaceuticals Corporation, Elan International Services, Ltd. and Elan Pharma International Limited
|
10-Q
|
5/14/2001
|
10.64
|
|
|
10.8
|
Second Agreement and Amendment, effective as of January 22, 2001, by and among Incara Pharmaceuticals Corporation, Elan International Services, Ltd. and Elan Pharma International Limited
|
10-Q
|
5/14/2001
|
10.65
|
|
|
10.9
|
Third Agreement and Amendment, effective as of January 22, 2001, by and among Incara Pharmaceuticals Corporation, Elan International Services, Ltd. and Elan Pharma International Limited
|
8-K
|
6/1/2001
|
10.66
|
|
|
10.1
|
Agreement and Fourth Amendment, effective February 13, 2002, by and among Incara Pharmaceuticals Corporation, Elan International Services, Ltd., Elan Pharma International Limited and Elan Pharmaceutical Investments III, Ltd.
|
10-Q
|
2/14/2002
|
10.75
|
|
|
10.11*
|
License Agreement dated June 25, 1998 between Duke University and Aeolus Pharmaceuticals, Inc.
|
10-Q
|
5/15/2002
|
10.82
|
|
|
10.12*
|
License Agreement dated May 7, 2002 between Duke University and Aeolus Pharmaceuticals, Inc.
|
10-Q
|
5/15/2002
|
10.83
|
|
|
10.13*
|
License Agreement dated November 17, 2000 between National Jewish Medical and Research Center and Aeolus Pharmaceuticals, Inc.
|
10-Q
|
2/13/2001
|
10.56
|
|
|
10.14
|
Exclusive License Agreement, dated January 15, 2009, by and between the Company and National Jewish Health
|
10-Q
|
5/16/2011
|
10.7
|
|
|
10.15*
|
Securities Purchase Agreement dated as of May 15, 2002, among Incara Pharmaceuticals Corporation, Aeolus Pharmaceuticals, Inc., Elan Pharma International Limited and Elan International Services, Ltd.
|
8-K/A
|
7/3/2002
|
10.84
|
|
|
10.16*
|
Development and Option Agreement dated May 15, 2002, among Elan Pharma International Limited, Incara Pharmaceuticals Corporation and Aeolus Pharmaceuticals, Inc.
|
8-K/A
|
7/3/2002
|
10.85
|
|
|
10.17
|
Amended and Restated Registration Rights Agreement dated as of May 15, 2002, among Incara Pharmaceuticals Corporation, Elan International Services, Ltd. and Elan Pharma International Limited
|
8-K/A
|
7/3/2002
|
10.86
|
|
|
Incorporated by Reference To
|
|||||
|
Exhibit
|
Registrant's
|
Date Filed
|
Exhibit
|
Filed
|
|
|
Number
|
Description of Document
|
Form
|
with the SEC |
Number
|
Herewith
|
|
10.18
|
Amendment No. 1 to License Agreement dated May 14, 2002, between Aeolus Pharmaceuticals, Inc. and Duke University (amending License Agreement dated July 21, 1995)
|
8-K/A
|
7/3/2002
|
10.87
|
|
|
10.19
|
Amendment No. 1 to License Agreement dated May 14, 2002, between Aeolus Pharmaceuticals, Inc. and Duke University (amending License Agreement dated June 25, 1998)
|
8-K/A
|
7/3/2002
|
10.88
|
|
|
10.2
|
Amendment No. 1 to License Agreement dated May 14, 2002, between Aeolus Pharmaceuticals, Inc. and National Jewish Medical and Research Center (amending License Agreement dated November 17, 2000)
|
8-K/A
|
7/3/2002
|
10.89
|
|
|
10.21*
|
Subaward Agreement, dated March 16, 2011, by and between the Company and the Office of Research and Development of the University of Maryland, Baltimore
|
10-Q
|
5/16/2011
|
10.4
|
|
|
10.22
|
Letter dated May 17, 2004 from Elan International Services, Limited and Elan Pharma International Limited to Incara Pharmaceuticals Corporation
|
10-Q
|
8/11/2004
|
10.106
|
|
|
10.23+
|
Aeolus Pharmaceuticals, Inc. 1994 Stock Option Plan, as amended
|
10-Q
|
8/11/2004
|
10.109
|
|
|
10.24+
|
Aeolus Pharmaceuticals, Inc. Amended and Restated 2004 Stock Incentive Plan
|
14-C
|
11/16/2012
|
D
|
|
|
10.25+
|
Amended and Restated Employment Agreement dated July 30, 2010 between Aeolus Pharmaceuticals, Inc. and John L. McManus
|
8-K
|
8/2/2010
|
10.4
|
|
|
10.26+
|
Letter Agreement dated July 10, 2006 between Aeolus Pharmaceuticals, Inc. and McManus & Company, Inc.
|
8-K
|
7/10/2006
|
10.2
|
|
|
10.27+
|
Form of Indemnity Agreement
|
10-K
|
12/27/2011
|
10.27
|
|
|
10.28
|
Terms of Outside Director Compensation
|
10-K
|
12/17/2004
|
10.114
|
|
|
10.29+
|
Form of Incentive Stock Option Agreement
|
10-Q
|
2/8/2005
|
10.115
|
|
|
10.30+
|
Form of Nonqualified Stock Option Agreement
|
10-Q
|
2/8/2005
|
10.116
|
|
|
10.31
|
Subscription Agreement dated June 5, 2006 by and between the Company and the investors whose names appear on the signature pages thereof.
|
8-K
|
6/6/2006
|
10.1
|
|
|
10.32
|
Board Observer Letter dated June 5, 2006 by and among the Company and Efficacy Biotech Master Fund Ltd.
|
8-K
|
6/6/2006
|
10.6
|
|
|
10.33+
|
Consulting Agreement, dated December 1, 2010, between Aeolus Pharmaceuticals, Inc. and Brian J. Day
|
8-K
|
12/3/2010
|
10.1
|
|
|
10.34*
|
Sponsored Research Agreement (Non-Clinical), dated April 12, 2011, by and between the Company and Duke University
|
10-Q
|
5/16/2011
|
10.5
|
|
|
10.35
|
Securities Purchase Agreement dated August 11, 2010 by and among Aeolus Pharmaceuticals, Inc. and the investors listed therein
|
8-K
|
8/12/2010
|
10.1
|
|
|
10.36
|
Form of Warrant pursuant to Securities Purchase Agreement dated August 11, 2010 by and among Aeolus Pharmaceuticals, Inc. and the investors listed therein
|
8-K
|
8/12/2010
|
10.2
|
|
|
Incorporated by Reference To
|
|||||
|
Exhibit
|
Registrant's
|
Date Filed
|
Exhibit
|
Filed
|
|
|
Number
|
Description of Document
|
Form
|
with the SEC |
Number
|
Herewith
|
|
10.37
|
Convertible Promissory Note dated February 7, 2007 issued by Aeolus Pharmaceuticals, Inc. to Elan Pharma International Ltd.
|
S-1
|
6/4/2007
|
10.43
|
|
|
10.38
|
Amendment No. 1 To Convertible Promissory Note dated February 7, 2009 by and between Aeolus Pharmaceuticals, Inc. and Elan Pharma International Limited
|
8-K
|
3/16/2009
|
10.1
|
|
|
10.39+
|
Form of Restricted Share Award Agreement
|
S-8 POS
|
3/31/2008
|
99.2
|
|
|
10.4
|
Securities Purchase and Exchange Agreement dated October 6, 2009 by and among the Company and the investors whose names appear on the signature pages thereof
|
8-K
|
10/6/2009
|
10.1
|
|
|
10.41
|
Amendment Agreement to the Securities Purchase and Exchange Agreement, dated December 24, 2009, by and among the Company and the investors whose names appear on the signature pages thereof
|
8-K
|
12/28/2009
|
10.1
|
|
|
10.42+
|
Intentionally Omitted
|
8-K
|
2/16/2011
|
10.1
|
|
|
10.43*
|
Contract No. HHSO100201100007C, dated February 11, 2011, by and between the Company and the U.S. Department of Health and Human Services Biomedical Advanced Research and Development Authority
|
10-Q
|
5/16/2011
|
10.1
|
|
|
10.44*
|
Research and Manufacturing Agreement, dated February 18, 2011 (the "JMPS Agreement"), by and between the Company and Johnson Matthey Pharmaceutical Materials, Inc. (d/b/a Johnson Matthey Pharma Services).
|
10-Q
|
5/16/2011
|
10.2
|
|
|
10.45*
|
Appendix 2 to the JMPS Agreement, dated February 18, 2011
|
10-Q
|
8/14/2012
|
10.4
|
|
|
10.46*
|
Appendix 3 to the JMPS Agreement, dated April 30, 2012
|
10-Q
|
8/14/2012
|
10.5
|
|
|
10.47*
|
Appendix 4 to the JMPS Agreement, dated April 30, 2012
|
10-Q
|
8/14/2012
|
10.6
|
|
|
10.48*
|
Appendix 5 to the JMPS Agreement, dated April 30, 2012
|
10-Q
|
8/14/2012
|
10.7
|
|
|
10.49*
|
Appendix 6 to the JMPS Agreement, dated April 30, 2012
|
10-Q
|
8/14/2012
|
10.8
|
|
|
10.50*
|
General Management Consulting Assignment, dated February 23, 2011, by and between the Company and Booz Allen Hamilton Inc.
|
10-Q
|
5/16/2011
|
10.3
|
|
|
10.51
|
Form of Securities Purchase Agreement by and among the Company and the investors whose names appear on the signature pages thereof
|
8-K
|
4/5/2012
|
10.1
|
|
|
10.52
|
Form of Registration Rights Agreement by and among the Company and the investors party thereto
|
8-K
|
4/5/2012
|
10.2
|
|
|
10.53
|
Form of Warrant issued to investors in March and April 2012
|
8-K
|
4/5/2012
|
10.3
|
|
|
10.54
|
Amended and Restated Employment Agreement by and between the Company and John L. McManus
|
8-K
|
3/5/2013
|
10.1
|
|
| 10.55 | Form of Registration Rights Agreement | 8-K | 12/15/15 | 10.2 | |
|
List of Subsidiaries
|
X
|
||||
|
Consent of Grant Thornton LLP, Independent Registered Public Accounting Firm
|
X
|
||||
|
Incorporated by Reference To
|
|||||
|
Exhibit
|
Registrant's
|
Date Filed
|
Exhibit
|
Filed
|
|
|
Number
|
Description of Document |
Form
|
with the SEC |
Number
|
Herewith
|
|
Certification of the Principal Executive Officer pursuant to Rule 13a-14(a) and 15d-14(a)
|
X
|
||||
|
Certification of the Principal Financial and Accounting Officer pursuant to Rule 13a-14(a) and 15d-14(a)
|
X
|
||||
|
Certification by the Principal Executive Officer and Principal Financial and Accounting Officer pursuant to 18 U.S.C. 1350 as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002
|
X
|
||||
|
101.INS†
|
XBRL Instance Document
|
X
|
|||
|
101.SCH†
|
XBRL Taxonomy Extension Schema Document
|
X
|
|||
|
101.CAL†
|
XBRL Taxonomy Extension Calculation Linkbase Document
|
X
|
|||
|
101.DEF†
|
XBRL Taxonomy Extension Definition Linkbase Document
|
X
|
|||
|
101.LAB†
|
XBRL Taxonomy Extension Label Linkbase Document
|
X
|
|||
|
101.PRE†
|
XBRL Taxonomy Extension Presentation Linkbase Document
|
X
|
|||
|
SIGNATURE
|
TITLE(S)
|
DATE
|
||
| /s/ John L. McManus |
President and Chief Executive Officer
|
December 18, 2015
|
||
|
John L. McManus
|
(Principal Executive Officer)
|
|||
| /s/ David C. Cavalier |
Chief Financial Officer and Secretary
|
December 18, 2015
|
||
|
David C. Cavalier
|
(Principal Financial and Accounting Officer)
|
|||
| /s/ David C. Cavalier |
Chairman of the Board of Directors
|
December 18, 2015
|
||
|
David C. Cavalier
|
||||
| /s/ John M. Clerici |
Director
|
December 18, 2015
|
||
|
John M. Clerici
|
||||
|
/s/ John M. Farah, Jr., Ph.D.
|
Director
|
December 18, 2015
|
||
|
John M. Farah, Jr., Ph.D.
|
||||
|
Director
|
|
|||
|
Mitchell D. Kaye
|
||||
| /s/ Amit Kumar, Ph.D. |
Director
|
December 18, 2015
|
||
|
Amit Kumar, Ph.D.
|
||||
| /s/ Chris A. Rallis |
Director
|
December 18, 2015
|
||
|
Chris A. Rallis
|
||||
| /s/ Jeffrey Scott |
Director
|
December 18, 2015
|
||
|
Jeffrey Scott
|


| 1. | I have reviewed this Annual Report on Form 10-K of Aeolus Pharmaceuticals, Inc.; |
| 2. | Based on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report; |
| 3. | Based on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of operations and cash flows of the registrant as of, and for, the periods presented in this report; |
| 4. | The registrant's other certifying officer and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) and internal control over financial reporting (as defined in Exchange Act Rules 13a-15(f) and 15d-15(f)) for the registrant and have: |
| (a) | Designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the registrant, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this report is being prepared; |
| (b) | Designed such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles; |
| (c) | Evaluated the effectiveness of the registrant's disclosure controls and procedures and presented in this report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this report based on such evaluation; and |
| (d) | Disclosed in this report any change in the registrant's internal control over financial reporting that occurred during the registrant's most recent fiscal quarter (the registrant's fourth fiscal quarter in the case of an annual report) that has materially affected, or is reasonably likely to materially affect, the registrant's internal control over financial reporting; and |
| 5. | The registrant's other certifying officer and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the registrant's auditors and the audit committee of the registrant's board of directors (or persons performing the equivalent functions): |
| (a) | All significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the registrant's ability to record, process, summarize and report financial information; and |
| (b) | Any fraud, whether or not material, that involves management or other employees who have a significant role in the registrant's internal control over financial reporting. |
| 1. | I have reviewed this Annual Report on Form 10-K of Aeolus Pharmaceuticals, Inc.; |
| 2. | Based on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report; |
| 3. | Based on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of operations and cash flows of the registrant as of, and for, the periods presented in this report; |
| 4. | The registrant's other certifying officer and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) and internal control over financial reporting (as defined in Exchange Act Rules 13a-15(f) and 15d-15(f)) for the registrant and have: |
| (a) | Designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the registrant, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this report is being prepared; |
| (b) | Designed such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles; |
| (c) | Evaluated the effectiveness of the registrant's disclosure controls and procedures and presented in this report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this report based on such evaluation; and |
| (d) | Disclosed in this report any change in the registrant's internal control over financial reporting that occurred during the registrant's most recent fiscal quarter (the registrant's fourth fiscal quarter in the case of an annual report) that has materially affected, or is reasonably likely to materially affect, the registrant's internal control over financial reporting; and |
| 5. | The registrant's other certifying officer and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the registrant's auditors and the audit committee of the registrant's board of directors (or persons performing the equivalent functions): |
| (a) | All significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the registrant's ability to record, process, summarize and report financial information; and |
| (b) | Any fraud, whether or not material, that involves management or other employees who have a significant role in the registrant's internal control over financial reporting. |
| 1. | The Report fully complies with the requirements of Section 13(a) or 15(d) of the Securities Exchange Act of 1934, as amended; and |
| 2. | The information contained in the Report fairly presents, in all material respects, the financial condition and results of operations of the Company for the period covered by the Report. |
Document and Entity Information - USD ($) |
12 Months Ended | ||
|---|---|---|---|
Sep. 30, 2015 |
Dec. 18, 2015 |
Mar. 31, 2015 |
|
| Document And Entity Information | |||
| Entity Registrant Name | AEOLUS PHARMACEUTICALS, INC. | ||
| Entity Central Index Key | 0001261734 | ||
| Document Type | 10-K | ||
| Document Period End Date | Sep. 30, 2015 | ||
| Amendment Flag | false | ||
| Current Fiscal Year End Date | --09-30 | ||
| Is Entity a Well-known Seasoned Issuer? | No | ||
| Is Entity a Voluntary Filer? | No | ||
| Is Entity's Reporting Status Current? | Yes | ||
| Entity Filer Category | Smaller Reporting Company | ||
| Entity Common Stock, Shares Outstanding | 151,559,745 | ||
| Public Float | $ 13,758,209 | ||
| Document Fiscal Period Focus | FY | ||
| Document Fiscal Year Focus | 2015 |
CONDENSED CONSOLIDATED BALANCE SHEETS (Unaudited) (Parenthetical) - USD ($) $ in Thousands |
Sep. 30, 2015 |
Sep. 30, 2014 |
|---|---|---|
| Stockholder's deficit: | ||
| Note payable discount | $ 273,000 | $ 0 |
| Preferred stock par value | $ 0.01 | $ 0.01 |
| Preferred stock, authorized shares | 10,000,000 | 10,000,000 |
| Series A nonredeemable convertible preferred stock, authorized shares | 1,250,000 | 1,250,000 |
| Series A nonredeemable convertible preferred stock, issued shares | 0 | 0 |
| Series A nonredeemable convertible preferred stock, outstanding shares | 0 | 0 |
| Series B nonredeemable convertible preferred stock, authorized shares | 1,600,000 | 1,600,000 |
| Series B nonredeemable convertible preferred stock, issued shares | 526,080 | 526,080 |
| Series B nonredeemable convertible preferred stock, outstanding shares | 526,080 | 526,080 |
| Common stock, par value | $ 0.01 | $ 0.01 |
| Common stock, Authorized | 200,000,000 | 200,000,000 |
| Common stock, Issued | 135,930,068 | 135,850,068 |
| Common stock, outstanding | 135,930,068 | 135,850,068 |
CONDENSED CONSOLIDATED STATEMENTS OF OPERATIONS (Unaudited) - USD ($) $ in Thousands |
12 Months Ended | |
|---|---|---|
Sep. 30, 2015 |
Sep. 30, 2014 |
|
| Revenue: | ||
| Contract revenue | $ 3,111 | $ 9,631 |
| Costs and expenses: | ||
| Research and development | 3,509 | 6,966 |
| General and administrative | 2,228 | 2,745 |
| Total costs and expenses | 5,737 | 9,711 |
| Loss from operations | (2,626) | (80) |
| Interest expense | 2 | 0 |
| Net loss | (2,628) | (80) |
| Net income (loss) per weighted share attributable to common stockholders - basic | (2,628) | (80) |
| Net income (loss) per weighted share attributable to common stockholders - diluted | $ (2,628) | $ (80) |
| Basic net loss per common share | $ (0.02) | $ 0.00 |
| Diluted net loss per common share | $ (0.02) | $ 0.00 |
| Weighted average common shares outstanding: | ||
| Basic | 135,883 | 134,667 |
| Diluted | 135,883 | 134,667 |
CONSOLIDATED STATEMENTS OF STOCKHOLDERS EQUITY (DEFICIT) - USD ($) $ in Thousands |
Series B Preferred Stock |
Common Stock [Member] |
Additional Paid-In Capital |
Accumulated Deficit |
Total |
|---|---|---|---|---|---|
| Beginning Balance - Shares at Sep. 30, 2013 | 526,080 | 134,550,068 | |||
| Beginning Balance - Amount at Sep. 30, 2013 | $ 5 | $ 1,346 | $ 183,276 | $ (183,922) | $ 705 |
| Exercise of warrants, Shares | 1,300,000 | ||||
| Exercise of warrants, Amount | $ 13 | 312 | 325 | ||
| Stock-based compensation | 626 | 626 | |||
| Issuance of warrants to consultants | 9 | 9 | |||
| Net income (loss) | $ (80) | (80) | |||
| Ending Balance, Shares at Sep. 30, 2014 | 526,080 | 135,850,068 | |||
| Ending Balance, Amount at Sep. 30, 2014 | $ 5 | $ 1,359 | 184,223 | (184,002) | 1,585 |
| Exercise of warrants, Shares | 80,000 | ||||
| Exercise of warrants, Amount | 20 | 20 | |||
| Stock-based compensation | 164 | 164 | |||
| Issuance of warrants to consultants | 14 | 14 | |||
| Net income (loss) | (2,628) | (2,628) | |||
| Ending Balance, Shares at Sep. 30, 2015 | 526,080 | 135,930,068 | |||
| Ending Balance, Amount at Sep. 30, 2015 | $ 5 | $ 1,359 | $ 184,421 | $ (186,630) | $ (845) |
A. Organization, Business and Summary of Significant Accounting Policies |
12 Months Ended |
|---|---|
Sep. 30, 2015 | |
| Notes to Financial Statements | |
| A. Organization, Business and Summary of Significant Accounting Policies | Organization The accompanying audited consolidated financial statements include the accounts of Aeolus Pharmaceuticals, Inc. and its wholly-owned subsidiary, Aeolus Sciences, Inc. (collectively "we," "us," "Company" or "Aeolus"). All significant intercompany accounts and transactions have been eliminated in consolidation. Aeolus is a Delaware corporation. The Company's primary operations are located in Mission Viejo, California.
Business Aeolus is developing a new class of broad-spectrum, catalytic antioxidant compounds based on technology discovered at Duke University and National Jewish Health. The Company's lead compound, 10150, is a metalloporphyrin specifically designed to neutralize reactive oxygen and nitrogen species. The Company is developing 10150 as a medical countermeasure against the pulmonary effects of radiation exposure under a contract ("BARDA Contract") valued at up to $118.4 million with the Biomedical Advanced Research and Development Authority ("BARDA"), a division of the Department of Health and Human Services ("HHS"). Additionally, Aeolus receives development support from the National Institutes of Health ("NIH") for development of the compound as a medical countermeasure against radiation and chemical exposure. |
B. Summary of Significant Accounting Policies |
12 Months Ended | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Sep. 30, 2015 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Accounting Policies [Abstract] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| B. Summary of Significant Accounting Policies |
Basis of Presentation The consolidated financial statements include the accounts of Aeolus and its wholly owned subsidiary. All significant intercompany accounts and transactions have been eliminated. The Company uses the equity method to account for its 35% ownership interest in CPEC, which is further discussed in Note D. Use of Estimates The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosures of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Such estimates include revenue recognition, allowance for doubtful accounts and stock-based compensation. Actual results could differ from those estimates. Cash and Cash Equivalents The Company invests available cash in short-term bank deposits. Cash and cash equivalents include investments with maturities of three months or less at the date of purchase. The carrying value of cash and cash equivalents approximate their fair market value at September 30, 2015 and 2014 due to their short-term nature. Also, the Company maintains cash balances with financial institutions in excess of federally insured limits. The Company does not anticipate any losses with such cash balances. Significant customers and accounts receivable For the year ended September 30, 2015, the Company's only customer was BARDA. For the year ended September 30, 2015, revenues from BARDA comprised 100% of total revenues. As of September 30, 2015, the Company's receivable balances were comprised 100% from this customer. Unbilled accounts receivable, included in accounts receivable, totaling $589,000 as of September 30, 2015 relate to work that has been performed, though invoicing has not yet occurred. All of the unbilled receivables are expected to be billed and collected within the next year. Accounts receivable are stated at invoice amounts and consist primarily of amounts due from HHS as well as amounts due under reimbursement contracts with other government entities and non-government and philanthropic organizations. If necessary, the Company records a provision for doubtful receivables to allow for any amounts which may be unrecoverable. This provision is based upon an analysis of the Company's prior collection experience, customer creditworthiness and current economic trends. As of September 30, 2015 and 2014, an allowance for doubtful accounts was not recorded as the collection history from the Company's customers indicated that collection was probable. Concentrations of credit risk Financial instruments that potentially subject the Company to concentrations of credit risk consist primarily of cash and cash equivalents and accounts receivable. The Company places its cash and cash equivalents with high quality financial institutions. Management believes that the financial risks associated with its cash and cash equivalents and investments are minimal. Because accounts receivable consist entirely of amounts due from the U.S. federal government agencies, management deems there to be minimal credit risk.
Revenue Recognition Aeolus recognizes revenue in accordance with the authoritative guidance for revenue recognition. Revenue is recognized when all of the following criteria are met: (i) persuasive evidence of an arrangement exists, (ii) delivery (or passage of title) has occurred or services have been rendered, (iii) the seller's price to the buyer is fixed or determinable, and (iv) collectability is reasonably assured.
The BARDA Contract is classified as a "cost-plus-fixed-fee" contract. Aeolus recognizes government contract revenue in accordance with the authoritative guidance for revenue recognition including the authoritative guidance specific to federal government contracts. Reimbursable costs under the contract primarily include direct labor, subcontract costs, materials, equipment, travel, and indirect costs.
Aeolus estimates subcontractor costs, materials and related revenue for some vendors when invoices are not received timely. We receive regular updates from our subcontractors regarding estimated completion of individual projects. Management evaluates the status of each project with respect to budgeted work completed, actual work completed, and cost of actual work completed. We are required to provide BARDA with monthly reports in addition to our bi-weekly conference calls with BARDA regarding the progress of each project.
In addition, we receive a fixed fee on consultant labor and subcontract labor under the BARDA Contract, which is unconditionally earned as allowable costs are incurred and is not contingent on success factors. Reimbursable costs under this BARDA Contract, including the fixed fee, are generally recognized as revenue in the period the reimbursable costs are incurred and become billable.
Fair Value of Financial Instruments
The carrying amounts of our short-term financial instruments, which include cash and cash equivalents, accounts receivable, accounts payable, accrued liabilities, debt, and redemption liability, approximate their fair values due to their short maturities.
Fair Value Measurements
The Company adopted Accounting Standards Codification ("ASC") Topic 820, Fair Value Measurements and Disclosures, for financial and non-financial assets and liabilities.
ASC 820 discusses valuation techniques, such as the market approach (comparable market prices), the income approach (present value of future income or cash flow) and the cost approach (cost to replace the service capacity of an asset or replacement cost). The Company utilizes the market approach. The statement utilizes a fair value hierarchy that prioritizes the inputs to valuation techniques used to measure fair value into three broad levels. The following is a brief description of those three levels:
The redemption liability, discussed further at Note F. Debt, is measured at fair market value on a recurring basis as of September 30, 2015 and is summarized below (in thousands):
The following table summarizes, as of September 30, 2015, the redemption liability activity subject to Level 1 inputs which are measured on a recurring basis:
Research and Development Research and development costs are expensed in the period incurred. Leases The Company leases office space and office equipment under month to month operating lease agreements. For the years ended September 30, 2015 and 2014, total rent expense was approximately $42,000 and $42,000, respectively. Income Taxes The Company recognizes liabilities or assets for the deferred tax consequences of temporary differences between the tax bases of assets or liabilities and their reported amounts in the financial statements. These temporary differences will result in taxable or deductible amounts in future years when the reported amounts of the assets or liabilities are recovered or settled. A valuation allowance is established when management determines that it is more likely than not that all or a portion of a deferred tax asset will not be realized. Management evaluates the Company's ability to realize its net deferred tax assets on a quarterly basis and valuation allowances are provided, as necessary. During this evaluation, management reviews its forecasts of income in conjunction with other positive and negative evidence surrounding the Company's ability to realize its deferred tax assets to determine if a valuation allowance is required. Adjustments to the valuation allowance will increase or decrease the Company's income tax provision or benefit. Management also applies the relevant guidance to determine the amount of income tax expense or benefit to be allocated among continuing operations, discontinued operations, and items charged or credited directly to stockholders' equity (deficit). A tax position must meet a minimum probability threshold before a financial statement benefit is recognized. The minimum threshold is a tax position that is more likely than not to be sustained upon examination by the applicable taxing authority, including resolution of any related appeals or litigation process, based on the technical merits of the position. The Company recognizes interest and penalties related to uncertain tax positions in income tax expense. Net Income (Loss) Per Common Share The Company computes basic net income (loss) per weighted average share attributable to common stockholders using the weighted average number of shares of common stock outstanding during the period. The Company computes diluted net income (loss) per weighted average share attributable to common stockholders using the weighted average number of shares of common and dilutive potential common shares outstanding during the period. Potential common shares outstanding consist of stock options, warrants and convertible preferred stock using the treasury stock method and are excluded if their effect is anti-dilutive. Diluted weighted average common shares did not include any incremental shares for the fiscal year ended September 30, 2015 and 2014. Diluted weighted average common shares excluded incremental shares from common and preferred shares and warrants of approximately 29,536,000 and 29,048,000 for the fiscal year 2015 and 2014, respectively, due to their anti-dilutive effect.
Accounting for Stock-Based Compensation The Company recognizes stock-based compensation expense in the statement of operations based upon the fair value of the equity award amortized over the vesting period. Segment Reporting The Company currently operates in one segment. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
C. Liquidity |
12 Months Ended |
|---|---|
Sep. 30, 2015 | |
| Notes to Financial Statements | |
| C. Liquidity |
The Company completed a financing in December 2015, which is discussed further at Note H. Subsequent event. The primary use of our cash from operations is to further develop our various drug compounds and depending on the results of that development we may need to raise additional capital in the future. |
D. Investments |
12 Months Ended |
|---|---|
Sep. 30, 2015 | |
| Equity Method Investments and Joint Ventures [Abstract] | |
| D. Investments | Investment in CPEC LLC The Company uses the equity method to account for its 35% ownership interest in CPEC. CPEC had $91,000 of net assets at each of September 30, 2015 and 2014. Aeolus' 35% share of CPEC's net assets, which is approximately $32,000, is included in other assets and the Company has no operations or activities unrelated to the out licensing of bucindolol. The other 65% is owned by Endo Pharmaceuticals. |
E. Commitments |
12 Months Ended |
|---|---|
Sep. 30, 2015 | |
| Notes to Financial Statements | |
| E. Commitments | The Company acquires assets still in development and enters into license and research and development arrangements with third parties that often require milestone and royalty payments to the third party contingent upon the occurrence of certain future events linked to the success of the asset in development. Milestone payments may be required, contingent upon the successful achievement of an important point in the development life-cycle of the pharmaceutical product (e.g., approval of the product for marketing by a regulatory agency). If required by the arrangement, the Company may also be required to make royalty payments based upon a percentage of the net sales of the pharmaceutical product in the event that regulatory approval for marketing is obtained. Because of the contingent nature of these payments, they are not included in the table of contractual obligations. No milestones have been met, nor have any payments been made, as of September 30, 2015.
We are also obligated to pay patent filing, prosecution, maintenance and defense costs, if any, for the intellectual property the Company has licensed from National Jewish Health ("NJH"), National Jewish Medical and Research Center (the "NJMRC") and Duke University.
These arrangements may be material individually, and in the unlikely event that milestones for multiple products covered by these arrangements were reached in the same period, the aggregate charge to expense could be material to the results of operations in any one period. In addition, these arrangements often give Aeolus the discretion to unilaterally terminate development of the product, which would allow Aeolus to avoid making the contingent payments; however, Aeolus is unlikely to cease development if the compound successfully achieves clinical testing objectives. |
F. Debt |
12 Months Ended |
|---|---|
Sep. 30, 2015 | |
| F. Debt | |
| F. Debt |
Convertible Promissory Notes September 29, 2015, the Company received funding in exchange for issuance of convertible promissory notes (the "Notes") from Biotechnology Value Fund, L.P. and other affiliates of BVF Partners, L.P. The Notes have an aggregate principal balance of $1,000,000, accrue interest at a rate of 6% per annum and have a scheduled maturity date of September 28, 2016 (the "Maturity Date"). The outstanding principal and accrued interest on the Notes shall automatically convert into Company equity securities issued in a Qualified Financing (as defined below) at a conversion rate carrying a 15% discount to the lowest price per share (or share equivalent) issued in a Qualified Financing (an "Automatic Conversion"). If, prior to the Maturity Date, the Company enters into an agreement pertaining to a Corporate Transaction (as defined below) and the Note has not been previously converted pursuant to an Automatic Conversion, then, the outstanding principal balance and unpaid accrued interest of the Note shall automatically convert in whole into the right of the holder to receive, in lieu of any other payment and in cancellation of the Note, an amount in cash upon closing of the Corporate Transaction equal to two times the outstanding principal amount of the Note. For purposes of the foregoing: "Qualified Financing" means a bona fide new money equity securities financing on or before the Maturity Date with total proceeds to the Company of not less than four million dollars; and "Corporate Transaction" means a sale, lease or other disposition of all or substantially all of the Company's assets or a consolidation or merger of the Company with or into any other corporation or other entity or person, or any other corporate reorganization, in which the stockholders of the Company immediately prior to such consolidation, merger or reorganization own less than fifty percent (50%) of the voting power of the surviving entity immediately after such consolidation, merger or reorganization. The $1,000,000 principal balance of the Notes is recorded in the financial statements at face value and is net with a discount of $273,000 as a result of separating the fair value of the Qualified Financing redemption discount ("Redemption Feature") of 15% on the price per share in the Notes. The Redemption Feature qualifies as a derivative and is subject to fair value treatment. The Redemption Feature is amortized over the expected life of the derivative, and the amortization expense is presented net with the interest expense from the Notes, yielding an effective interest rate that is different than the 6% stated in the Notes. On December 11, 2015, following the completion of a Qualified Financing described above, the principal and accrued interest amounts under the Notes were converted into 5,414,402 shares of the Company's common stock and warrants to purchase an additional 5,414,402 shares of the Company's common stock at an exercise price per share of $0.22 subject to adjustment. As a result, the Notes were no longer outstanding as of that date. |
G. Stockholders' Equity (Deficit) |
12 Months Ended | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Sep. 30, 2015 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Notes to Financial Statements | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| G. Stockholders' Equity (Deficit) |
Basis of Presentation Preferred Stock The Certificate of Incorporation of the Company authorizes the issuance of up to 10,000,000 shares of Preferred Stock, at a par value of $0.01 per share, of which 1,250,000 shares are designated Series A Convertible Preferred Stock and 1,600,000 shares are designated Series B Convertible Preferred Stock. The Board of Directors has the authority to issue Preferred Stock in one or more series, to fix the designation and number of shares of each such series, and to determine or change the designation, relative rights, preferences, and limitations of any series of Preferred Stock, without any further vote or action by the stockholders of the Company. As of September 30, 2015 and 2014, 526,080 shares of Series B Stock were outstanding, respectively. There are no shares of Series A Convertible Preferred Stock issued or outstanding. With respect to dividend rights and rights upon liquidation, winding up and dissolution, the Series B Stock ranks pari passu with the common stock. Subject to any rights of senior stock, holders of Series B Stock are entitled to receive dividends or distributions as, when and if declared by the Board of Directors. In the event the Board of Directors declares a dividend or distribution with respect to the outstanding common stock, the holders of Series B Stock are entitled to receive the amount of dividends per share in the same form payable on the common stock based on the largest number of shares of common stock issuable upon conversion of the outstanding Series B Stock. In the event of a liquidation, winding up or dissolution of the Company, subject to any rights of senior stock, the holders of Series B Stock are entitled to receive, pari passu with the holders of the common stock, the assets of the Company based on the largest number of shares of common stock issuable upon conversion of the outstanding Series B Stock. Each share of Series B Stock is convertible into one share of common stock. The Series B Stock can be converted into common stock at any time upon the election of the holders of the Series B Stock except to the extent such conversion would result in the holders of Series B Stock owning in the aggregate more than 9.99% of the outstanding common stock. The Series B Stock is not entitled to vote on any matter submitted to the vote of holders of the common stock except that the Company must obtain the approval of a majority of the outstanding shares of Series B Stock to either amend the Company's Certificate of Incorporation in a manner that would adversely affect the Series B Stock (including by creating an additional class or series of stock with rights that are senior or pari passu to the Series B Stock) or change the rights of the holders of the Series B Stock in any other respect. On December 10, 2015, the Company entered into securities purchase agreements with certain accredited investors to sell and issue 4,500 preferred stock units issued to existing investors, Biotechnology Value Fund, L.P. and other affiliates of BVF Partners, L.P., for an aggregate purchase price of $4.5 million, resulting in aggregate gross proceeds to the Company of approximately $6.75 million. The preferred units collectively consist of (i) 4,500 shares of Series C Convertible Preferred Stock of the Company that are collectively convertible into an aggregate of 20,454,546 common shares and (ii) warrants to purchase an aggregate of 20,454,546 Common Shares, in each case subject to adjustment. The warrants have an initial exercise price of $0.22 per share. The warrants may not be exercised until after 90 days following the date of issuance. The Series C Stock and warrants contain provisions restricting the conversion or exercise of such securities in circumstances where such event would result in the holder and its affiliates to beneficially own in excess of 9.99% of the Company's outstanding common stock.
Common Stock On December 10, 2015, the Company entered into securities purchase agreements with certain accredited investors to sell and issue (i) an aggregate of 10,215,275 common units issued at a purchase price of $0.22 per unit. Each common unit consists of one share of the Company's common stock and a five year warrant to purchase one share of the Company's common stock, subject to adjustment. The warrants may not be exercised until after 90 days following the date of issuance. The warrants contain provisions restricting the conversion or exercise of such securities in circumstances where such event would result in the holder and its affiliates to beneficially own in excess of 9.99% of the Company's outstanding common stock.
On September 29, 2015, the Company received funding in the form of convertible promissory notes (the "BVF Notes") from Biotechnology Value Fund, L.P. and certain other affiliates of BVF Partners, L.P. The BVF Notes have an aggregate principal balance of $1,000,000, accrue interest at a rate of 6% per annum and have a scheduled maturity date of September 28, 2016. The outstanding principal and accrued interest on the BVF Notes will automatically convert into Company equity securities, provided a Qualified Financing of not less than $4MM occurs.
On December 11, 2015, following the completion of a Qualified Financing described above, the principal and accrued interest amounts under the BVF Notes were converted into 5,414,402 shares of the Company's common stock and warrants to purchase an additional 5,414,402 shares of the Company's common stock at an exercise price per share of $0.22 subject to adjustment. As a result, the BVF Notes were not longer outstanding as of that date.
Dividends The Company has never paid a cash dividend on its common stock and does not anticipate paying cash dividends on its common stock in the foreseeable future. If we pay a cash dividend on our common stock, we also must pay the same dividend on an as converted basis on our Series B preferred stock. Warrants As of September 30, 2015, warrants to purchase an aggregate of 15,949,627 shares of common stock were outstanding. Details of the warrants for common stock outstanding at September 30, 2015 were as follows:
As of September 30, 2015, one warrant to purchase an aggregate of 896,037 shares of preferred stock was outstanding. As of September 30, 2014, warrants to purchase an aggregate of 16,029,627 shares of common stock were outstanding. Details of the warrants for common stock outstanding at September 30, 2014 were as follows:
As of September 30, 2014, one warrant to purchase an aggregate of 896,037 shares of preferred stock was outstanding. Below is a summary of warrant activity for the last two fiscal years ended September 30:
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
H. Subsequent Event |
12 Months Ended |
|---|---|
Sep. 30, 2015 | |
| H. Subsequent Event | |
| H. Subsequent Event |
On December 10, 2015, the Company entered into securities purchase agreements with certain accredited investors to sell and issue (i) an aggregate of 10,215,275 common units issued at a purchase price of $0.22 per unit, and (ii) 4,500 preferred stock units issued to existing investors, Biotechnology Value Fund, L.P. and other affiliates of BVF Partners, L.P., for an aggregate purchase price of $4.5 million, resulting in aggregate gross proceeds to the Company of approximately $6.75 million
Each common unit consists of one share of the Company's common stock and a five year warrant to purchase one share of the Company's common stock, subject to adjustment. The preferred units collectively consist of (i) 4,500 shares of Series C Convertible Preferred Stock of the Company that are collectively convertible into an aggregate of 20,454,546 common shares and (ii) warrants to purchase an aggregate of 20,454,546 Common Shares, in each case subject to adjustment. The warrants have an initial exercise price of $0.22 per share. The warrants may not be exercised until after 90 days following the date of issuance. The Series C Stock and warrants contain provisions restricting the conversion or exercise of such securities in circumstances where such event would result in the holder and its affiliates to beneficially own in excess of 9.99% of the Company's outstanding common stock.
On September 29, 2015, the Company received funding in the form of convertible promissory notes (the "BVF Notes") from Biotechnology Value Fund, L.P. and certain other affiliates of BVF Partners, L.P. The BVF Notes have an aggregate principal balance of $1,000,000, accrue interest at a rate of 6% per annum and have a scheduled maturity date of September 28, 2016. The outstanding principal and accrued interest on the BVF Notes will automatically convert into Company equity securities, provided a Qualified Financing of not less than $4 million occurs.
On December 11, 2015, following the completion of a Qualified Financing described above, the principal and accrued interest amounts under the BVF Notes were converted into 5,414,402 shares of the Company's common stock and warrants to purchase an additional 5,414,402 shares of the Company's common stock at an exercise price per share of $0.22 subject to adjustment. As a result, the BVF Notes were not longer outstanding as of that date. |
I. Stock-Based Compensation |
12 Months Ended | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Sep. 30, 2015 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Notes to Financial Statements | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| I. Stock-Based Compensation | As an integral component of a management and employee retention program designed to motivate, retain and provide incentive to the Company's management, employees and key consultants, the Board of Directors approved the 2004 Stock Incentive Plan (the "2004 Plan") and reserved 25,000,000 shares of common stock for issuance under the 2004 Plan. As of September 30, 2015, 12,835,409 shares were available to be granted under the 2004 Plan. The exercise price of the incentive stock options ("ISOs") granted under the 2004 Plan must not be less than the fair market value of the common stock as determined on the date of the grant. The options may have a term up to 10 years. Options typically vest immediately or up to one year following the date of the grant. Below is a summary of stock option activity for the last two fiscal years ended September 30:
Stock options granted to consultants during fiscal year 2015 and 2014 were fully vested when issued or vested over a twelve month period. Stock option expense for stock options granted to consultants was zero and $11,000 for fiscal year 2015 and 2014, respectively. For the fiscal years 2015 and 2014, all stock options were issued at or above fair market value of a share of common stock. The weighted-average grant-date fair value of options granted during fiscal years 2015 and 2014 was $0.25 and $0.26, respectively. A summary of the status of non-vested shares for the fiscal years ended September 30 was:
The total unrecognized compensation expense for outstanding stock options was $24,000 as of September 30, 2015, which will be recognized over a weighted average period of five months. The total fair value of shares vested during fiscal years 2015 and 2014 was $156,000 and $688,000, respectively. The details of stock options for the fiscal year ended September 30, 2015 are as follows:
Stock-based compensation expense recognized in the statement of operations is as follows (in thousands):
The fair value of the options associated with the above compensation expense was determined at the date of the grant using the Black-Scholes option pricing model with the following weighted average assumptions:
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
J. Income Taxes |
12 Months Ended | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Sep. 30, 2015 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Income Tax Disclosure [Abstract] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| J. Income Taxes |
As of September 30, 2015 and 2014, the Company had federal net operating loss ("NOL") carry-forwards of $115,515,000 and $113,321,000, respectively and state operating loss carry-forwards of $38,916,000 and $35,244,000, respectively. The use of these federal and state NOL carry-forwards might be subject to limitation under the rules regarding a change in stock ownership as determined by the Internal Revenue Code (the "Code"). The Company may have had a change of control under Section 382 of the Code during fiscal 2004 and 2006; however, a complete analysis of the limitation of the NOL carry-forwards will not be completed until the time the Company projects it will be able to utilize such NOLs. The federal net operating and the state net operating losses began to expire in 2010. Additionally, the Company had federal research and development carry-forwards as of September 30, 2015 and 2014 of $4,127,000 and $3,990,000, respectively. The Company had state research and development carry-forwards as of September 30, 2015 and 2014 of $1,452,000 and $1,316,000, respectively. Significant components of the Company's deferred tax assets at September 30, 2015 and 2014 consisted of the following (in thousands):
Due to the uncertainty surrounding the realization of the favorable tax attributes in future tax returns, all of the deferred tax assets have been fully offset by a valuation allowance. The change in the Taxes computed at the statutory federal income tax rate of 34% are reconciled to the provision for income taxes as follows (dollars in thousands):
At September 30, 2015, we had unrecognized tax benefits of $5,085,000, all of which would impact the effective tax rate if recognized, however a valuation allowance would be recorded against this amount. During fiscal 2014, unrecognized tax benefits increased to $4,859,000 as a result of the tax positions taken in prior years and current year. No interest and penalties have been provided for with respect to the unrecognized tax benefits. A reconciliation of the beginning and ending amount of unrecognized tax benefits is as follows:
The Company's federal income tax returns for the tax years 2011 to 2013 remain open to examination. The Company's California income tax returns for the tax years 2010 to 2013 remain open to examination. A reconciliation of the current year tax provision is as follows:
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
K. Contingencies |
12 Months Ended |
|---|---|
Sep. 30, 2015 | |
| Notes to Financial Statements | |
| K. Contingencies | Duke Licenses The Company has obtained exclusive worldwide licenses (the "Duke Licenses") from Duke University ("Duke") to develop, make, have made, use and sell products using certain technology in the field of free radical and antioxidant research, developed by certain scientists at Duke. Future discoveries in the field of antioxidant research from these scientists' laboratories at Duke are also covered by the Duke Licenses. The Duke Licenses require the Company to use its best efforts to pursue development of products using the licensed technology and compounds. These efforts are to include the manufacture or production of products for testing, development and sale. Aeolus is also obligated to use its best efforts to have the licensed technology cleared for marketing in the United States by the U.S. Food and Drug Administration and in other countries in which Aeolus intends to sell products using the licensed technology. Aeolus will pay royalties to Duke on net product sales during the terms of the Duke Licenses, and milestone payments upon certain regulatory approvals and annual sales levels. In addition, Aeolus is obligated under the Duke Licenses to pay all or a portion of patent prosecution, maintenance and defense costs. Unless earlier terminated, the Duke Licenses continue until the expiration of the last to expire issued patent on the licensed technology. National Jewish Medical and Research Center Agreements Aeolus has an exclusive worldwide license ("NJH License") from National Jewish Health to develop, make, have made, use and sell products using certain technology developed by certain scientists at NJH. The NJH License requires Aeolus to use commercially reasonable efforts to diligently pursue the development and government approval of products using the licensed technology. Aeolus will be obligated to pay royalties to NJH on net product sales during the term of the NJH License and a milestone payment upon regulatory approval, if obtained. In addition, Aeolus is obligated under the NJH License to pay all or a portion of patent prosecution, maintenance and defense costs. Unless earlier terminated, the NJH License continues until the expiration of the last to expire issued patent on the licensed technology. |
L. Agreements |
12 Months Ended |
|---|---|
Sep. 30, 2015 | |
| Notes to Financial Statements | |
| L. Agreements | Elan Corporation, plc In May 2002, the Company entered into a collaboration transaction with affiliates of Elan Corporation, plc for the development of the Company's catalytic antioxidant compounds as a treatment for tissue damage from cancer radiation and chemotherapy. Although Elan and the Company terminated this collaboration in January 2003, the Company will pay Elan a royalty on net sales of its catalytic antioxidant products sold, if any, for the prevention and treatment of radiation-induced and chemotherapy-induced tissue damage. |
M. Litigation |
12 Months Ended |
|---|---|
Sep. 30, 2015 | |
| Commitments and Contingencies Disclosure [Abstract] | |
| M. Litigation | From time to time we are subject to various lawsuits and claims with respect to matters arising out of the normal course of our business. Due to the uncertainty of the ultimate outcome of these matters, the impact on future financial results is not subject to reasonable estimates. |
N. Quarterly Financial Data (unaudited) |
12 Months Ended | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Sep. 30, 2015 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Quarterly Financial Information Disclosure [Abstract] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| N. Quarterly Financial Data (unaudited) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
O. Recently Issued Accounting Pronouncements |
12 Months Ended |
|---|---|
Sep. 30, 2015 | |
| Notes to Financial Statements | |
| O. Recently Issued Accounting Pronouncements |
From time to time, new accounting pronouncements are issued by the Financial Accounting Standards Board that are adopted by us as of the specified effective date. Unless otherwise discussed, we believe that the impact of recently issued standards that are not yet effective will not have a material impact on our financial position or results of operations upon adoption. In May 2014, the Financial Accounting Standards Board ("FASB") issued Accounting Standards Update ("ASU") 2015-14 Revenue, which deferred the effective date of ASU 2014-09 Revenue from Contracts with Customers (ASC 606), which updates the principles for recognizing revenue. ASU 2014-09 also amends the required disclosures of the nature, amount, timing and uncertainty of revenue and cash flows arising from contracts with customers. ASU 2014-09 is now effective for annual reporting periods beginning after December 15, 2017, including interim periods within that reporting period. The Company is evaluating the potential impacts of the new standard on its existing revenue recognition policies and procedures. In August 2014, the FASB issued ASU 2014-15, Disclosure of Uncertainties about an Entity's Ability to Continue as a Going Concern. ASU 2014-15 requires that an entity's management evaluate whether there are conditions or events that raise substantial doubt about the entity's ability to continue as a going concern within one year after the date that the financial statements are issued. ASU 2014-15 is effective for annual periods beginning after December 15, 2016 and for interim periods thereafter. The Company is evaluating the potential impacts of this new standard on its quarterly reporting process. |
B. Organization, Business and Summary of Significant Accounting Policies (Policies) |
12 Months Ended | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Sep. 30, 2015 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| B. Organization Business And Summary Of Significant Accounting Policies Policies | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Organization | The accompanying audited consolidated financial statements include the accounts of Aeolus Pharmaceuticals, Inc. and its wholly-owned subsidiary, Aeolus Sciences, Inc. (collectively "we," "us," "Company" or "Aeolus"). All significant intercompany accounts and transactions have been eliminated in consolidation. Aeolus is a Delaware corporation. The Company's primary operations are located in Mission Viejo, California. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Business | Aeolus is developing a new class of broad-spectrum, catalytic antioxidant compounds based on technology discovered at Duke University and National Jewish Health. The Company's lead compound, 10150, is a metalloporphyrin specifically designed to neutralize reactive oxygen and nitrogen species. The Company is developing 10150 as a medical countermeasure against the pulmonary effects of radiation exposure under a contract ("BARDA Contract") valued at up to $118.4 million with the Biomedical Advanced Research and Development Authority ("BARDA"), a division of the Department of Health and Human Services ("HHS"). Additionally, Aeolus receives development support from the National Institutes of Health ("NIH") for development of the compound as a medical countermeasure against radiation and chemical exposure. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Basis of Presentation | The consolidated financial statements include the accounts of Aeolus and its wholly owned subsidiary. All significant intercompany accounts and transactions have been eliminated. The Company uses the equity method to account for its 35% ownership interest in CPEC, which is further discussed in Note D. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Use of Estimates | The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosures of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Such estimates include revenue recognition, allowance for doubtful accounts and stock-based compensation. Actual results could differ from those estimates. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cash and Cash Equivalents | The Company invests available cash in short-term bank deposits. Cash and cash equivalents include investments with maturities of three months or less at the date of purchase. The carrying value of cash and cash equivalents approximate their fair market value at September 30, 2015 and 2014 due to their short-term nature. Also, the Company maintains cash balances with financial institutions in excess of federally insured limits. The Company does not anticipate any losses with such cash balances. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Significant customer and accounts receivable | For the year ended September 30, 2015, the Company's only customer was BARDA. For the year ended September 30, 2015, revenues from BARDA comprised 100% of total revenues. As of September 30, 2015, the Company's receivable balances were comprised 100% from this customer. Unbilled accounts receivable, included in accounts receivable, totaling $589,000 as of September 30, 2015 relate to work that has been performed, though invoicing has not yet occurred. All of the unbilled receivables are expected to be billed and collected within the next three months. Accounts receivable are stated at invoice amounts and consist primarily of amounts due from HHS as well as amounts due under reimbursement contracts with other government entities and non-government and philanthropic organizations. If necessary, the Company records a provision for doubtful receivables to allow for any amounts which may be unrecoverable. This provision is based upon an analysis of the Company's prior collection experience, customer creditworthiness and current economic trends. As of September 30, 2015 and 2014, an allowance for doubtful accounts was not recorded as the collection history from the Company's customers indicated that collection was probable. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Concentrations of credit risk | Financial instruments that potentially subject the Company to concentrations of credit risk consist primarily of cash and cash equivalents and accounts receivable. The Company places its cash and cash equivalents with high quality financial institutions. Management believes that the financial risks associated with its cash and cash equivalents and investments are minimal. Because accounts receivable consist entirely of amounts due from the U.S. federal government agencies, management deems there to be minimal credit risk. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Revenue Recognition | Aeolus recognizes revenue in accordance with the authoritative guidance for revenue recognition. Revenue is recognized when all of the following criteria are met: (i) persuasive evidence of an arrangement exists, (ii) delivery (or passage of title) has occurred or services have been rendered, (iii) the seller's price to the buyer is fixed or determinable, and (iv) collectability is reasonably assured.
The BARDA Contract is classified as a "cost-plus-fixed-fee" contract. Aeolus recognizes government contract revenue in accordance with the authoritative guidance for revenue recognition including the authoritative guidance specific to federal government contracts. Reimbursable costs under the contract primarily include direct labor, subcontract costs, materials, equipment, travel, and indirect costs.
Aeolus estimates subcontractor costs, materials and related revenue for some vendors when invoices are not received timely. We receive regular updates from our subcontractors regarding estimated completion of individual projects. Management evaluates the status of each project with respect to budgeted work completed, actual work completed, and cost of actual work completed. We are required to provide BARDA with monthly reports in addition to our bi-weekly conference calls with BARDA regarding the progress of each project.
In addition, we receive a fixed fee under the BARDA Contract, which is unconditionally earned as allowable costs are incurred and is not contingent on success factors. Reimbursable costs under this BARDA Contract, including the fixed fee, are generally recognized as revenue in the period the reimbursable costs are incurred and become billable. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Fair Value of Financial Instruments | The carrying amounts of our short-term financial instruments, which include cash and cash equivalents, accounts receivable, accounts payable, accrued liabilities, debt, and redemption liability, approximate their fair values due to their short maturities.
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Fair Value Measurements | The Company adopted Accounting Standards Codification ("ASC") Topic 820, Fair Value Measurements and Disclosures, for financial and non-financial assets and liabilities.
ASC 820 discusses valuation techniques, such as the market approach (comparable market prices), the income approach (present value of future income or cash flow) and the cost approach (cost to replace the service capacity of an asset or replacement cost). The Company utilizes the market approach. The statement utilizes a fair value hierarchy that prioritizes the inputs to valuation techniques used to measure fair value into three broad levels. The following is a brief description of those three levels:
The redemption liability, discussed further at Note F. Debt, is measured at fair market value on a recurring basis as of September 30, 2015 and is summarized below (in thousands):
The following table summarizes, as of September 30, 2015, the redemption liability activity subject to Level 1 inputs which are measured on a recurring basis:
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Research and Development | Research and development costs are expensed in the period incurred. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Leases | The Company leases office space and office equipment under month to month operating lease agreements. For the years ended September 30, 2015 and 2014, total rent expense was approximately $42,000 and $42,000, respectively. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Income Taxes | The Company recognizes liabilities or assets for the deferred tax consequences of temporary differences between the tax bases of assets or liabilities and their reported amounts in the financial statements. These temporary differences will result in taxable or deductible amounts in future years when the reported amounts of the assets or liabilities are recovered or settled. A valuation allowance is established when management determines that it is more likely than not that all or a portion of a deferred tax asset will not be realized. Management evaluates the Company's ability to realize its net deferred tax assets on a quarterly basis and valuation allowances are provided, as necessary. During this evaluation, management reviews its forecasts of income in conjunction with other positive and negative evidence surrounding the Company's ability to realize its deferred tax assets to determine if a valuation allowance is required. Adjustments to the valuation allowance will increase or decrease the Company's income tax provision or benefit. Management also applies the relevant guidance to determine the amount of income tax expense or benefit to be allocated among continuing operations, discontinued operations, and items charged or credited directly to stockholders' equity (deficit). A tax position must meet a minimum probability threshold before a financial statement benefit is recognized. The minimum threshold is a tax position that is more likely than not to be sustained upon examination by the applicable taxing authority, including resolution of any related appeals or litigation process, based on the technical merits of the position. The Company recognizes interest and penalties related to uncertain tax positions in income tax expense. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Net Income (Loss) Per Common Share | The Company computes basic net income (loss) per weighted average share attributable to common stockholders using the weighted average number of shares of common stock outstanding during the period. The Company computes diluted net income (loss) per weighted average share attributable to common stockholders using the weighted average number of shares of common and dilutive potential common shares outstanding during the period. Potential common shares outstanding consist of stock options, warrants and convertible preferred stock using the treasury stock method and are excluded if their effect is anti-dilutive. Diluted weighted average common shares did not include any incremental shares for the fiscal year ended September 30, 2015 and 2014. Diluted weighted average common shares excluded incremental shares from common and preferred shares and warrants of approximately 29,611,000 and 29,048,000 for the fiscal year 2015 and 2014, respectively, due to their anti-dilutive effect.
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Accounting for Stock-Based Compensation | The Company recognizes stock-based compensation expense in the statement of operations based upon the fair value of the equity award amortized over the vesting period. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Segment Reporting | The Company currently operates in one segment. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
B. Organization, Business and Summary of Significant Accounting Policies (Tables) |
12 Months Ended | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Sep. 30, 2015 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| B. Organization Business And Summary Of Significant Accounting Policies Tables | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Fair Value Measurements |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Fair value measurements of warrants using significant unobservable inputs |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Schedule of Earnings Per Share, Basic and Diluted |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
G. Stockholder's Equity (Tables) |
12 Months Ended | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Sep. 30, 2015 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| G. Stockholders Equity Tables | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Warrants for Common Stock Outstanding | As of September 30, 2015, warrants to purchase an aggregate of 15,949,627 shares of common stock were outstanding. Details of the warrants for common stock outstanding at September 30, 2015 were as follows:
As of September 30, 2014, warrants to purchase an aggregate of 16,029,627 shares of common stock were outstanding. Details of the warrants for common stock outstanding at September 30, 2014 were as follows:
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Summary Of Warrant Activity |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
I. Stock-Based Compensation (Tables) |
12 Months Ended | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Sep. 30, 2015 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| I. Stock-based Compensation Tables | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Summary Of Stock Option Activity |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Schedule of non-vested shares |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details Of Stock Options |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Stock-based compensation expense |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Assumptions Of Stock Based Compensation |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
J. Income Taxes (Tables) |
12 Months Ended | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Sep. 30, 2015 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| J. Income Taxes Tables | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Deferred tax assets |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Tax rate reconciliation |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Unrecognized tax benefits |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reconciliation of the current year tax provision |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
N. Quarterly Financial Data (unaudited) (Tables) |
12 Months Ended | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Sep. 30, 2015 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| N. Quarterly Financial Data Tables | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Quarterly Financial Data |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
B. Summary of Significant Accounting Policies - Fair Value (Details) |
Sep. 30, 2015
shares
|
|---|---|
| Fair Value Inputs Level 1 Member | |
| Fair Value Measurements | |
| Redemption liability | 275 |
| Fair Value Inputs Level 2 Member | |
| Fair Value Measurements | |
| Redemption liability | 0 |
| Fair Value Inputs Level 3 Member | |
| Fair Value Measurements | |
| Redemption liability | 0 |
B. Summary of Significant Accounting Policies - Warrant Fair Value (Details 1) $ in Thousands |
12 Months Ended |
|---|---|
|
Sep. 30, 2015
USD ($)
| |
| Fair value measurements of redemption liability | |
| Beginning balance | $ 0 |
| Issuance of convertible promissory notes with redemption feature | 275 |
| Change in fair value of redemption liability | 0 |
| Ending balance | $ 275 |
C Liquidity (Details Narrative) - USD ($) $ in Thousands |
12 Months Ended | |
|---|---|---|
Sep. 30, 2015 |
Sep. 30, 2014 |
|
| C Liquidity Details Narrative | ||
| Cash (outflows) inflows from operations | $ (2,443) | $ 323 |
| Net Income (loss) | $ (2,628) | $ (80) |
D. Investments (Details Narrative) |
Sep. 30, 2015 |
|---|---|
| D. Investments Details Narrative | |
| Equity method investment ownership interest | 35.00% |
G. Stockholders' Equity - Warrants (Details) - shares |
12 Months Ended | |
|---|---|---|
Sep. 30, 2015 |
Sep. 30, 2014 |
|
| Number of Shares | 15,949,627 | 16,029,627 |
| $0.50 | ||
| Number of Shares | 50,000 | 50,000 |
| Expiration Date | May-16 | May-16 |
| $0.50 | ||
| Number of Shares | 50,000 | 50,000 |
| Expiration Date | Jul-16 | Jul-16 |
| $1.00 | ||
| Number of Shares | 50,000 | 50,000 |
| Expiration Date | Jul-16 | Jul-16 |
| $1.50 | ||
| Number of Shares | 50,000 | 50,000 |
| Expiration Date | Jul-16 | Jul-16 |
| $2.00 | ||
| Number of Shares | 50,000 | 50,000 |
| Expiration Date | Jul-16 | Jul-16 |
| $2.50 | ||
| Number of Shares | 50,000 | 50,000 |
| Expiration Date | Jul-16 | Jul-16 |
| $0.40 | ||
| Number of Shares | 1,337,627 | 1,337,627 |
| Expiration Date | Mar-17 | Mar-17 |
| $0.40 | ||
| Number of Shares | 325,000 | 325,000 |
| Expiration Date | Apr-17 | Apr-17 |
| $0.258 | ||
| Number of Shares | 300,000 | 300,000 |
| Expiration Date | Jun-17 | Jun-17 |
| $0.26 | ||
| Number of Shares | 50,000 | 50,000 |
| Expiration Date | Jun-17 | Jun-17 |
| $0.35 | ||
| Number of Shares | 140,000 | 140,000 |
| Expiration Date | Oct-17 | Oct-17 |
| $0.25 | ||
| Number of Shares | 12,205,000 | 12,285,000 |
| Expiration Date | Feb-18 | Feb-18 |
| $0.25 | ||
| Number of Shares | 1,242,000 | 1,242,000 |
| Expiration Date | Mar-18 | Mar-18 |
| $0.49 | ||
| Number of Shares | 50,000 | |
| Expiration Date | Jan-20 | |
| $0.38 | ||
| Number of Shares | 50,000 | |
| Expiration Date | Apr-15 | |
G. Stockholders' Equity - Warrant Activity (Details 2) - Warrant [Member] - USD ($) $ / shares in Units, $ in Thousands |
12 Months Ended | |
|---|---|---|
Sep. 30, 2015 |
Sep. 30, 2014 |
|
| Number of Shares | ||
| Outstanding beginning balance | 16,925,664 | 18,775,664 |
| Granted | 50,000 | 50,000 |
| Exercised | (80,000) | (1,300,000) |
| Cancelled | (50,000) | (600,000) |
| Forfeited | 0 | 0 |
| Outstanding ending balance | 16,845,664 | 16,925,664 |
| Exercisable | 16,845,664 | |
| Exercise Price | ||
| Outstanding beginning balance | $ 0.27 | $ 0.29 |
| Granted | .49 | 0.26 |
| Exercised | .25 | 0.25 |
| Expired or Canceled | .38 | 0.86 |
| Forfeited | 0.00 | 0.00 |
| Outstanding ending balance | .27 | $ 0.27 |
| Exercisable | $ .27 | |
| Remaining Contractual Term (in years) | ||
| Outstanding beginning balance | 3 years 1 month 6 days | 4 years 1 month 6 days |
| Outstanding ending balance | 2 years 2 months 12 days | 3 years 1 month 6 days |
| Exercisable | 2 years 2 months 12 days | |
| Aggregate Intrinsic Value | ||
| Outstanding beginning balance | $ 215,048 | $ 693,340 |
| Exercised | 10,975 | 318,000 |
| Outstanding ending balance | 206,626 | $ 215,048 |
| Exercisable | $ 206,626 | |
I. Stock-Based Compensation - Stock Option Activity (Details) - Employee Stock Option [Member] - USD ($) $ / shares in Units, $ in Thousands |
12 Months Ended | |
|---|---|---|
Sep. 30, 2015 |
Sep. 30, 2014 |
|
| Number of Shares | ||
| Outstanding beginning balance | 11,596,591 | 11,214,898 |
| Granted | 775,000 | 775,000 |
| Exercised | 0 | 0 |
| Cancelled | (207,000) | (393,307) |
| Forfeited | 0 | 0 |
| Outstanding ending balance | 12,164,591 | 11,596,591 |
| Exercisable | 12,002,089 | |
| Exercise Price | ||
| Outstanding beginning balance | $ 0.43 | $ 0.52 |
| Granted | .27 | 0.26 |
| Exercised | 0.00 | 0.00 |
| Cancelled | .90 | 2.64 |
| Forfeited | 0.00 | 0.00 |
| Outstanding ending balance | .42 | $ 0.43 |
| Exercisable | $ .42 | |
| Remaining Contractual Term (in years) | ||
| Outstanding beginning balance | 6 years 1 month 6 days | 6 years 8 months 12 days |
| Outstanding ending balance | 5 years 4 months 24 days | 6 years 1 month 6 days |
| Exercisable | 5 years 4 months 24 days | |
| Aggregate Intrinsic Value | ||
| Outstanding beginning balance | $ 1 | $ 2 |
| Outstanding ending balance | 1 | $ 1 |
| Exercisable | $ 1 | |
I. Stock-Based Compensation - Nonvested Options (Details 1) - $ / shares |
12 Months Ended | |
|---|---|---|
Sep. 30, 2015 |
Sep. 30, 2014 |
|
| I. Stock-based Compensation - Nonvested Options Details 1 | ||
| Nonvested beginning | 168,752 | 1,482,286 |
| Granted | 775,000 | 775,000 |
| Vested | (781,250) | (2,088,534) |
| Forfeited | 0 | 0 |
| Nonvested ending | 162,502 | 168,752 |
| Weighted Average Grant-Date Fair Value, Nonvested beginning | $ .22 | $ 0.37 |
| Granted | .24 | 0.22 |
| Vested | .24 | 0.33 |
| Forfeited | 0.00 | 0.00 |
| Weighted Average Grant-Date Fair Value, Nonvested ending | $ .23 | $ 0.22 |
I. Stock-Based Compensation (Details 3) - USD ($) $ in Thousands |
12 Months Ended | |
|---|---|---|
Sep. 30, 2015 |
Sep. 30, 2014 |
|
| Stock-based compensation expense recognized in the statement of operations | ||
| Research and Development Expenses | $ 0 | $ 11 |
| General and Administrative Expenses | 178 | 624 |
| Total Stock-based compensation expense | $ 178 | $ 635 |
I. Stock-Based Compensation (Details 4) |
12 Months Ended | |
|---|---|---|
Sep. 30, 2015 |
Sep. 30, 2014 |
|
| Notes to Financial Statements | ||
| Dividend yield | 0.00% | 0.00% |
| Unvested forfeiture rate | 5.35% | 0.00% |
| Expected volatility | 137.00% | 122.00% |
| Risk-free interest rate | 1.70% | 1.54% |
| Expected term | 5 years 3 months 7 days | 5 years 3 months 7 days |
I. Stock-Based Compensation (Details Narrative) $ in Thousands |
12 Months Ended |
|---|---|
|
Sep. 30, 2015
USD ($)
| |
| I. Stock-based Compensation Details Narrative | |
| Unrecognized compensation expense | $ 24 |
| Period for recognition | 5 months |
J. Income Taxes (Details) - USD ($) $ in Thousands |
Sep. 30, 2015 |
Sep. 30, 2014 |
|---|---|---|
| J. Income Taxes Details | ||
| Accrued payroll related liabilities | $ 1,036 | $ 1,103 |
| Depreciation and amortization | 586 | 704 |
| Total deferred tax assets | 1,622 | 1,807 |
| State Taxes | (113) | (126) |
| Total deferred tax liabilities | (113) | (126) |
| Deferred tax liabilities | 0 | 0 |
| Valuation allowance for deferred assets | (1,509) | (1,681) |
| Net deferred tax asset | $ 0 | $ 0 |
J. Income Taxes (Details 1) - USD ($) $ in Thousands |
12 Months Ended | |
|---|---|---|
Sep. 30, 2015 |
Sep. 30, 2014 |
|
| J. Income Taxes Details 1 | ||
| Effective income tax rate | 0.00% | 0.00% |
| United States Federal income tax at statutory rate | $ (891) | $ (25) |
| State income taxes (net of federal benefit) | 1 | 1 |
| NQSO forfeiture | 234 | 0 |
| Prior year deferred true up | 39 | (280) |
| Change in valuation reserves | (153) | 96 |
| FIN 48 | 883 | 354 |
| Other | (111) | (144) |
| Provision for income taxes | $ 2 | $ 2 |
J. Income Taxes (Details 2) $ in Thousands |
12 Months Ended |
|---|---|
|
Sep. 30, 2015
USD ($)
| |
| J. Income Taxes Tables | |
| Unrecognized tax benefits at October 1, 2014 | $ 4,859 |
| Additions for tax positions related to current year | 226 |
| Additions/reductions for tax positions taken in prior years | 0 |
| Settlements | 0 |
| Lapse of Limitations | 0 |
| Unrecognized tax benefits at September 30, 2015 | $ 5,085 |
J. Income Taxes (Details 3) - USD ($) $ in Thousands |
12 Months Ended | |
|---|---|---|
Sep. 30, 2015 |
Sep. 30, 2014 |
|
| J. Income Taxes Details 3 | ||
| Current – Federal | $ 0 | |
| Current – State | 2 | |
| Total current | 2 | |
| Deferred - Federal | 134 | |
| Deferred – State | 38 | |
| Total Deferred | 172 | |
| Less: Valuation Allowance | (172) | |
| Total Deferred | 0 | |
| Total current and deferred | $ 2 | $ 2 |
1 Year Aeolus Pharmaceuticals (CE) Chart |
1 Month Aeolus Pharmaceuticals (CE) Chart |

It looks like you are not logged in. Click the button below to log in and keep track of your recent history.
Support: +44 (0) 203 8794 460 | support@advfn.com
By accessing the services available at ADVFN you are agreeing to be bound by ADVFN's Terms & Conditions