
We could not find any results for:
Make sure your spelling is correct or try broadening your search.
| Share Name | Share Symbol | Market | Type |
|---|---|---|---|
| MiroMatrix Medical Inc | NASDAQ:MIRO | NASDAQ | Common Stock |
| Price Change | % Change | Share Price | Bid Price | Offer Price | High Price | Low Price | Open Price | Shares Traded | Last Trade | |
|---|---|---|---|---|---|---|---|---|---|---|
| 0.00 | 0.00% | 3.39 | 3.50 | 3.67 | 0 | 00:00:00 |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM
(Mark One)
EXCHANGE ACT OF 1934
For the quarterly period ended
or
EXCHANGE ACT OF 1934
For the transition period from to
Commission File Number:
(Exact name of registrant as specified in its charter)
(State or other jurisdiction of incorporation) | (I.R.S. Employer Identification Number) |
(Address of principal executive offices, including zip code)
(
(Registrant’s telephone number, including area code)
Not Applicable
(Former Name, Former Address and Former Fiscal Year, if Changed Since Last Report)
Securities registered pursuant to Section 12(b) of the Act:
Title of each class | Trading symbol | Name of exchange on which registered |
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days.
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files).
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
Large accelerated filer | ☐ | Accelerated filer | ☐ | ||
☒ | Smaller reporting company | Emerging growth company |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act.
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes
As of August 9, 2023, there were
TABLE OF CONTENTS
Page | ||
|---|---|---|
3 | ||
3 | ||
4 | ||
5 | ||
6 | ||
7 | ||
Management’s Discussion and Analysis of Financial Condition and Results of Operations | 16 | |
24 | ||
24 | ||
25 | ||
25 | ||
26 | ||
26 | ||
26 | ||
26 | ||
27 | ||
2
PART I —FINANCIAL INFORMATION
Item 1. Financial Statements
MIROMATRIX MEDICAL INC.
Condensed Balance Sheets
June 30, | December 31, | |||||
| 2023 | 2022 | ||||
(unaudited) |
| |||||
Assets | ||||||
Current assets: | ||||||
Cash and cash equivalents | $ | | $ | | ||
Restricted cash | | | ||||
Short-term investments | | | ||||
Employee retention credit receivable | | — | ||||
Receivable from Reprise Biomedical, Inc. |
| | | |||
Interest receivable | | | ||||
Prepaid expenses and other current assets |
| | | |||
Total current assets |
| | | |||
Deferred offering costs |
| — | | |||
Right of use asset | | | ||||
Property and equipment, net |
| | | |||
Total assets | $ | | $ | | ||
Liabilities and Shareholders' Equity | ||||||
Current liabilities: | ||||||
Current portion of deferred royalties | $ | | $ | | ||
Accounts payable |
| |
| | ||
Current portion of financing lease obligations | | | ||||
Current portion of lease liability | | | ||||
Accrued expenses |
| |
| | ||
Total current liabilities |
| |
| | ||
Deferred royalties, net |
| — |
| | ||
Long-term debt |
| |
| | ||
Financing lease obligations, net | | | ||||
Lease liability, net | | | ||||
Accrued interest |
| |
| | ||
Total liabilities |
| |
| | ||
Commitments and contingencies | ||||||
Shareholders’ equity: | ||||||
Common stock, par value $ |
| |
| | ||
Additional paid-in capital |
| |
| | ||
Accumulated deficit |
| ( |
| ( | ||
Total shareholders’ equity |
| |
| | ||
Total liabilities and shareholders’ equity | $ | | $ | | ||
The accompanying notes are an integral part of these condensed financial statements.
3
MIROMATRIX MEDICAL INC.
Condensed Statements of Operations
(Unaudited)
| Three Months Ended |
| Six Months Ended | |||||||||
June 30, | June 30, | |||||||||||
2023 |
| 2022 |
| 2023 |
| 2022 | ||||||
$ | | $ | | $ | | $ | | |||||
| |
| |
| |
| | |||||
Gross loss |
| ( |
| ( |
| ( |
| ( | ||||
Operating expenses: |
|
|
|
|
|
|
|
| ||||
Research and development |
| |
| |
| |
| | ||||
Regulatory and clinical |
| |
| |
| |
| | ||||
Quality |
| |
| |
| |
| | ||||
General and administration |
| |
| |
| |
| | ||||
Total operating expenses |
| |
| |
| |
| | ||||
Operating loss |
| ( |
| ( |
| ( |
| ( | ||||
Other income (expense) | ||||||||||||
Interest income |
| |
| |
| |
| | ||||
Interest expense |
| ( |
| ( |
| ( |
| ( | ||||
Employee retention credit | — | — | | — | ||||||||
Total other income | | | | | ||||||||
Net loss | $ | ( | $ | ( | $ | ( | $ | ( | ||||
Net loss per share, basic and diluted | $ | ( | $ | ( | $ | ( | $ | ( | ||||
Weighted average shares used in computing net loss per share, basic and diluted |
| |
| |
| |
| | ||||
The accompanying notes are an integral part of these condensed financial statements.
4
MIROMATRIX MEDICAL INC.
Condensed Statements of Shareholders’ Equity
(Unaudited)
Additional | Total | |||||||||||||
Common Stock | Paid-In | Accumulated | Shareholders’ | |||||||||||
| Shares |
| Amount |
| Capital |
| Deficit |
| Equity | |||||
Balance at March 31, 2023 | | $ | | $ | | $ | ( | $ | | |||||
Stock-based compensation expense |
| — |
| — |
| |
| — |
| | ||||
Issuance of restricted shares | | |
| ( |
| — |
| — | ||||||
Net loss |
| — |
| — |
| — |
| ( |
| ( | ||||
Balance at June 30, 2023 |
| | $ | | $ | | $ | ( | $ | | ||||
Balance at March 31, 2022 | | $ | | $ | | $ | ( | $ | | |||||
Stock-based compensation expense |
| — | — | | — |
| | |||||||
Exercise of stock options |
| | — | | — | | ||||||||
Exercise of stock warrants | | | | — | | |||||||||
Issuance of restricted shares | | — | — | — | — | |||||||||
Net loss |
| — |
| — |
| — |
| ( |
| ( | ||||
Balance at June 30, 2022 |
| | $ | | $ | | $ | ( | $ | | ||||
Additional | Total | |||||||||||||
Common Stock | Paid-In | Accumulated | Shareholders’ | |||||||||||
| Shares |
| Amount |
| Capital |
| Deficit |
| Equity | |||||
Balance at December 31, 2022 | | $ | | $ | | $ | ( | $ | | |||||
Stock-based compensation expense |
| — |
| — |
| |
| — |
| | ||||
Issuance of restricted shares | | |
| ( |
| — |
| — | ||||||
Sales of common stock, net of expenses | | | | — | | |||||||||
Tax withholdings related to net share settlements of stock-based compensation awards | — | — | ( | — | ( | |||||||||
Net loss |
| — |
| — |
| — |
| ( |
| ( | ||||
Balance at June 30, 2023 |
| | $ | | $ | | $ | ( | $ | | ||||
Balance at December 31, 2021 | | $ | | $ | | $ | ( | $ | | |||||
Stock-based compensation expense |
| — | — | | — |
| | |||||||
Exercise of stock options | | | | — | | |||||||||
Exercise of stock warrants | | | | — | | |||||||||
Issuance of restricted shares | | — | — | — | — | |||||||||
Net loss |
| — | — | — | ( |
| ( | |||||||
Balance at June 30, 2022 |
| | $ | | $ | | $ | ( | $ | | ||||
The accompanying notes are an integral part of these condensed financial statements.
5
MIROMATRIX MEDICAL INC.
Condensed Statements of Cash Flows
(Unaudited)
Six Months Ended June 30, | ||||||
2023 | 2022 | |||||
|
| |||||
Cash flows from operating activities: |
|
|
|
| ||
Net loss | $ | ( | $ | ( | ||
Adjustments to reconcile net loss to net cash used in operating activities: |
|
|
|
| ||
Depreciation and amortization |
| |
| | ||
Stock-based compensation |
| |
| | ||
Loss on disposal of property and equipment | — | | ||||
Non-cash interest income | | ( | ||||
Amortization of premium/discount on investments | ( | | ||||
Write-off of deferred offering costs | | — | ||||
Employee retention credit | ( | — | ||||
Changes in operating assets and liabilities: |
|
|
|
| ||
Receivable from Reprise Biomedical, Inc. |
| |
| | ||
Prepaid expenses |
| |
| | ||
Operating lease right of use asset | | ( | ||||
Tenant improvement receivable reimbursement | — | | ||||
Accounts payable and accrued expenses | ( | ( | ||||
Accrued interest |
| |
| | ||
Operating lease liability | ( | | ||||
Net cash used in operating activities |
| ( |
| ( | ||
Cash flows from investing activities: |
|
|
|
| ||
Purchase of investments | ( | ( | ||||
Proceeds from maturity of investments | | — | ||||
Purchases of property and equipment |
| ( |
| ( | ||
Net cash provided by (used in) investing activities |
| |
| ( | ||
Cash flows from financing activities: |
|
|
|
| ||
Payments on long-term debt |
| — |
| ( | ||
Payments on financing lease obligations | ( | ( | ||||
Payments on offering costs | — | ( | ||||
Proceeds from sale of common stock, net | | — | ||||
Employee taxes paid for shares withheld | ( | — | ||||
Proceeds from stock warrant exercises |
| — |
| | ||
Proceeds from stock option exercises |
| — |
| | ||
Net cash provided by financing activities |
| |
| | ||
Net decrease in cash and cash equivalents |
| ( |
| ( | ||
Cash, cash equivalents and restricted cash at beginning of period |
| |
| | ||
Cash, cash equivalents and restricted cash at end of period | $ | | $ | | ||
Cash and cash equivalents | $ | | $ | | ||
Restricted cash | | | ||||
Cash, cash equivalents and restricted cash at end of period | $ | | $ | | ||
Supplemental disclosure of cash flow information: |
|
|
| |||
Interest paid | $ | | $ | | ||
Purchases of property and equipment in accounts payable and accrued expenses | $ | — | $ | | ||
Accrued expenses related to deferred offering costs and financing | $ | — | $ | | ||
Leased assets obtained in exchange for new operating lease liabilities | $ | — | $ | | ||
The accompanying notes are an integral part of these condensed financial statements.
6
MIROMATRIX MEDICAL INC.
Notes to Condensed Financial Statements
(Unaudited)
NOTE 1 — DESCRIPTION OF BUSINESS AND SIGNIFICANT ACCOUNTING POLICIES
Description of Business
Miromatrix Medical Inc. (the “Company”) is a life sciences company pioneering a novel technology for bioengineering fully transplantable organs to help save and improve patients’ lives. Founded in 2009, the Company is one of a small group of companies at the forefront of developing alternatives to human-donor organ transplants, and within this small group of companies there are important differences between the technologies being developed. The Company’s proprietary technology is a scalable platform that uses a two-step method of decellularization and recellularization designed to remove the porcine cells from the organs obtained from pigs and replace them with unmodified human cells. The Company’s initial development focus is on bioengineering livers and kidneys, and the Company’s technology platform is also applicable to bioengineering other organs including hearts, lungs and pancreases. The Company has collaborations with Baxter International Inc. (“Baxter”), CareDx, Inc. (“CareDx”), the Mayo Clinic, the Mount Sinai Health System and the Texas Heart Institute, and we have received strategic investments from Baxter, CareDx and DaVita Inc.
Basis of Preparation
The accompanying unaudited condensed financial statements of the Company have been prepared in accordance with accounting principles generally accepted in the United States of America (“U.S. GAAP”) and the rules of the United States (“U.S.”) Securities and Exchange Commission applicable to interim reports of companies filing as a smaller reporting company. These condensed financial statements should be read in conjunction with the audited financial statements and notes thereto contained in the Company’s Annual Report on Form 10-K for the fiscal year ended December 31, 2022.
In the opinion of management, the accompanying condensed financial statements reflect all normal recurring adjustments necessary to present fairly the financial position, results of operations, stockholders’ equity and cash flows for the interim periods but are not necessarily indicative of the results of operations or cash flows to be anticipated for the full year 2023 or any future period. The Company has evaluated subsequent events occurring after the date of the condensed financial statements for events requiring recording or disclosure in the condensed financial statements.
Reclassifications
Certain reclassifications to previously reported financial information on the Condensed Balance Sheets and Condensed Statements of Operations have been made to conform to the current period presentation.
Use of Estimates
The preparation of financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates.
Employee Retention Credit
Under the provisions of the extension of the Coronavirus Aid, Relief, and Economic Security Act, the Company is eligible for a refundable employee retention credit subject to satisfaction of certain eligibility criteria. The Company qualified for the employee retention credit for the first three quarters of 2020 and the second and third quarters of 2021. The Company recognized $
7
Emerging Growth Company Status
The Company is an emerging growth company, as defined in the Jumpstart Our Business Startups Act of 2012 (“JOBS Act”). Under the JOBS Act, emerging growth companies can delay adopting new or revised accounting standards issued subsequent to the enactment of the JOBS Act, until such time as those standards apply to private companies. The Company has elected to use this extended transition period for complying with new or revised accounting standards that have different effective dates for public and private companies until the earlier of the date that it (i) is no longer an emerging growth company or (ii) affirmatively and irrevocably opts out of the extended transition period provided in the JOBS Act. As a result, these condensed financial statements may not be comparable to companies that comply with the new or revised accounting pronouncements as of public company effective dates.
Recent Accounting Pronouncements
Recently Adopted Accounting Pronouncements
The FASB issued ASU No. 2016-13, Measurement of Credit Losses on Financial Instruments and an updated ASU 2018-19 that clarifies the scope of the standard in the amendments in ASU 2016-13. This guidance introduces a new model for recognizing credit losses on financial instruments based on an estimate of current expected credit losses. Financial instruments impacted are trade and other receivables, held-to-maturity debt securities, loans and other instruments. The Company adopted the standard effective January 1, 2023, using the modified retrospective approach. The adoption did not have an impact on the Company's financial statements.
NOTE 2 — GOING CONCERN
The accompanying condensed financial statements have been prepared on the basis that the Company will continue as a going concern. The Company has incurred losses since inception, negative cash flows from operations, and had an accumulated deficit of approximately $
NOTE 3 — FAIR VALUE MEASUREMENT
The fair value of the Company’s financial instruments reflects the amount that the Company estimates that it would receive in connection with the sale of an asset or paid in connection with the transfer of a liability in an orderly transaction between market participants at the measurement date (exit price). The Company uses a three-tier valuation hierarchy based upon observable and non-observable inputs to measure fair value:
Level 1: Inputs that include quoted prices in active markets for identical assets and liabilities.
Level 2: Inputs other than Level 1 that are observable, either directly or indirectly, such as quoted prices for similar assets or liabilities, quoted prices in markets that are not active; or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the assets or liabilities.
Level 3: Unobservable inputs that are supported by little or no market activity and that are significant to the fair value of the assets or liabilities.
The Company classifies cash and cash equivalents, as well as restricted cash, as Level 1 in the fair value hierarchy.
8
The Company classifies its investments in U.S. Treasury notes as Level 1 in the fair value hierarchy. While the market for these securities are highly liquid and active, quoted prices for these securities may at times be derived from pricing models which use observable inputs such as benchmark yields, reported trades, broker/dealer quotes, issuer spreads, two-sided markets, benchmark securities, bids, offers and other reference data including market research publications.
NOTE 4 — INVESTMENTS
The Company currently invests its excess cash in U.S. Treasury securities. The Company intends and has the ability to hold these investments to maturity. Securities with original maturity dates of more than three months are reported as held-to-maturity investments and are recorded at amortized cost, which approximates fair value due to the negligible risk of changes in value due to interest rates. All investments held as of June 30, 2023 had contractual maturities of less than one year.
The amortized cost and estimated fair values of the Company’s investments as of June 30, 2023 are as follows:
Amortized | Unrealized | Unrealized | Fair | |||||||||
Cost | Holding Gains | Holding Losses | Value | |||||||||
Short-term: |
|
|
|
| ||||||||
U.S. Treasury notes |
| $ | |
| $ | — |
| $ | |
| $ | |
Total | $ | | $ | — | $ | | $ | | ||||
NOTE 5 — PROPERTY AND EQUIPMENT, NET
Property and equipment consisted of the following as of:
| June 30, |
| December 31, | |||
2023 | 2022 | |||||
Lab equipment | $ | | $ | | ||
Leasehold improvements |
| |
| | ||
Furniture, fixtures and computers |
| |
| | ||
| |
| | |||
Less accumulated depreciation and amortization |
| ( |
| ( | ||
$ | | $ | | |||
Depreciation and amortization expense was $
NOTE 6 — ACCRUED EXPENSES
Accrued expenses consisted of the following as of:
| June 30, |
| December 31, | |||
2023 | 2022 | |||||
Wages | $ | | $ | | ||
Pre-clinical study costs | | | ||||
Research and development consulting | | — | ||||
Taxes | | | ||||
Legal |
| |
| | ||
Key opinion leader compensation |
| |
| | ||
Royalties |
| — |
| | ||
Other |
| |
| | ||
Accrued expenses | $ | | $ | | ||
9
NOTE 7 — DEBT
In January 2019, the Company issued the Regents of the University of Minnesota (the “University”) a promissory note in the amount of $
Future principal maturities for debt were as follows:
Amounts Due in the Twelve Months Ending June 30, |
| ||
2024 | $ | — | |
2025 |
| | |
Total future maturities payments | | ||
Less current portion | — | ||
Long-term debt | $ | | |
NOTE 8 — EQUITY
Common Stock
The Company is authorized to issue
In March 2023, the Company completed a public offering pursuant to which it sold an aggregate of
The Company previously capitalized $
As of June 30, 2023 and December 31, 2022, there were
Preferred Stock
The Company is authorized to issue
Equity Incentive Plans
In May 2021, the Company’s stockholders approved the 2021 Equity Incentive Plan (the “2021 Plan”). The 2021 Plan provides for the issuance of stock options, restricted stock units and other awards to employees, directors and consultants of the Company. Shares of common stock underlying outstanding awards under the 2019 Plan (defined below) and the 2021 Plan that expire, are forfeited, are retained by the Company to satisfy any exercise price or any tax withholding, repurchased by the Company at their original purchase price or settled in cash may be added to the number of shares of common stock available for issuance under the 2021 Plan. The number of shares reserved for issuance under the 2021 Plan
10
will automatically increase on the first day of each fiscal year, beginning January 1, 2022, in the amount equal to the lesser of (a)
The Company also maintains its prior stock option plans adopted in 2010 (the “2010 Plan”) and 2019 (the “2019 Plan”). The Company ceased making awards under the 2010 Plan upon adoption of the 2019 Plan and similarly under the 2019 Plan upon stockholder approval of the 2021 Plan.
As of June 30, 2023, there were options to purchase
As of June 30, 2023, there were options to purchase
As of June 30, 2023, there were
Stock Options
The Company recognizes stock option compensation expense based on the grant date fair value of the award. The Company issues new common shares for stock options exercised.
Stock option activity was as follows:
Weighted | |||||
Average | |||||
Exercise | |||||
| Shares |
| Price | ||
Options outstanding at December 31, 2022 | | $ | | ||
Granted | | $ | | ||
Exercised | — | $ | — | ||
Canceled or expired | ( | $ | | ||
Options outstanding at June 30, 2023 |
| | $ | | |
Options exercisable at June 30, 2023 |
| | $ | | |
Stock-based compensation expense related to stock options was $
Included in the stock-based compensation expense numbers above are stock options to be granted to key opinion leaders which are marked to market at each reporting period with the change in the accrued balance expensed through research and development operating expenses. Stock-based compensation related to the key opinion leaders increased by $
The weighted average fair value of options granted during the six months ended June 30, 2023 and 2022 was $
11
Restricted Stock Units
The Company recognizes restricted stock unit (“RSU”) compensation expense based on the grant date fair value of the award. Each RSU is eligible to vest over time and settle into
RSU activity was as follows:
Weighted | |||||
Average Grant | |||||
Date Fair | |||||
| Shares |
| Value | ||
Unvested at December 31, 2022 |
| |
| $ | |
Granted |
| |
| $ | |
Vested |
| ( |
| $ | |
Canceled |
| ( |
| $ | |
Unvested at June 30, 2023 |
| |
| $ | |
Stock-based compensation expense related to RSUs was $
Employee Stock Purchase Plan
The Company accounts for employee stock purchases made under its 2021 Employee Stock Purchase Plan (“ESPP”) using the estimated grant date fair value in accordance with Accounting Standards Codification, Topic 718, Stock Compensation. The Company values ESPP shares using the Black-Scholes model.
There were
There were
Stock Warrants
Stock warrant activity was as follows:
Weighted | |||||
Average | |||||
Exercise | |||||
Shares |
| Price | |||
Warrants outstanding December 31, 2022 | | $ | | ||
Granted | — | $ | — | ||
Exercised | — | $ | — | ||
Expired | ( | $ | | ||
Warrants outstanding June 30, 2023 | | $ | | ||
12
NOTE 9 — SIGNIFICANT CUSTOMERS
The Company had
NOTE 10 — COMMITMENTS AND CONTINGENCIES
Patent License Agreement
Under an Exclusive Patent License Agreement between the Company and the University, the Company is required to make minimum royalty payments to the University of $
NOTE 11 — LEASES
The Company leases its corporate headquarters, which houses its research and development operations and office space. The lease term began in August 2021 and is scheduled to terminate in May 2029. The Company has
The Company also leases pieces of equipment that are accounted for as financing leases. Financing lease assets are classified as lab equipment within property and equipment on the condensed balance sheets.
Supplemental condensed balance sheet information for the Company is as follows:
June 30, | December 31, | |||||||
Leases | Classification |
| 2023 | 2022 | ||||
Assets | ||||||||
Operating lease assets | Right of use asset | $ | | $ | | |||
Financing lease assets | $ | | $ | | ||||
Liabilities | ||||||||
Current | ||||||||
Operating | Current portion of lease liability | $ | | $ | | |||
Financing | Current portion of financing lease obligations | $ | | $ | | |||
Noncurrent | ||||||||
Operating | Lease liability, net | $ | | $ | | |||
Financing | Financing lease obligations, net | $ | | $ | | |||
13
Information on the Company’s lease costs is as follows:
Three Months Ended June 30, | ||||||||
Lease cost | Classification | 2023 | 2022 | |||||
Operating lease cost |
| Operating expenses: General and administrative |
| $ | | $ | | |
Financing lease cost |
|
|
|
| ||||
Amortization of leased assets |
| Depreciation and amortization |
| $ | | $ | | |
Interest on lease liabilities |
| Interest expense |
| $ | | $ | | |
Variable lease cost(1) |
| Operating expenses: General and administrative |
| $ | | $ | | |
| (1) | Variable lease costs consist primarily of taxes, insurance and common area maintenance costs for the Company’s operating lease. |
Six Months Ended June 30, | ||||||||
Lease cost | Classification | 2023 | 2022 | |||||
Operating lease cost |
| Operating expenses: General and administrative | $ | |
| $ | | |
Financing lease cost |
|
|
|
| ||||
Amortization of leased assets |
| Depreciation and amortization | $ | |
| $ | | |
Interest on lease liabilities |
| Interest expense | $ | |
| $ | | |
Variable lease cost(1) |
| Operating expenses: General and administrative | $ | |
| $ | | |
| (1) | Variable lease costs consist primarily of taxes, insurance and common area maintenance costs for the Company’s operating lease. |
Future payments for the Company’s leases are as follows:
Amounts Due in Years Ending |
| Total | |||||||
2023 |
| $ | | $ | | $ | | ||
2024 | | | | ||||||
2025 | | — | | ||||||
2026 | | — | | ||||||
2027 | | — | | ||||||
Thereafter | | — | | ||||||
Total lease payments | | | | ||||||
Less imputed interest | ( | ( | ( | ||||||
Present value of lease liabilities |
| $ | | $ | | $ | | ||
Additional information related to leases is as follows:
Lease term and discount rate | June 30, 2023 | ||
Weighted-average remaining term (years) |
| ||
Operating lease | |||
Financing leases |
| ||
Weighted-average discount rate | |||
Operating lease | % | ||
Financing leases | % | ||
14
NOTE 12 — NET LOSS PER SHARE
Basic net loss per share is calculated by dividing net income by the weighted average number of common shares outstanding during the period. Diluted net loss per common share is calculated by dividing the weighted average number of common shares outstanding, after taking into consideration all dilutive potential shares outstanding during the period. Due to the existence of net losses for the three and six months ended June 30, 2023 and 2022, basic and diluted net loss per share were the same, as the effect of potentially dilutive securities would have been anti-dilutive.
The following potentially dilutive securities outstanding have been excluded from the computations of diluted weighted average shares outstanding because such securities would have had an antidilutive impact due to losses reported for the periods presented:
Three and Six Months Ended June 30, | ||||
| 2023 |
| 2022 | |
Common stock options outstanding | |
| | |
Restricted stock units | | | ||
Common stock warrants | |
| | |
Total common stock equivalents | |
| | |
NOTE 13 — SUBSEQUENT EVENTS
On July 31, 2023, the Company received cash payments totaling $
15
Item 2. Management’s Discussion and Analysis of Financial Condition and Results of Operations
References in this report (the “Quarterly Report”) to “we,” “us,” “our” or the “Company” refer to Miromatrix Medical Inc. The following discussion and analysis of the Company’s financial condition and results of operations should be read in conjunction with the condensed financial statements and the notes thereto contained elsewhere in this Quarterly Report. Information contained in the discussion and analysis set forth below includes forward-looking statements that involve risks and uncertainties.
Special Note Regarding Forward-Looking Statements
This Quarterly Report includes “forward-looking statements” within the meaning of Section 27A of the Securities Act of 1933, as amended (the “Securities Act”) and Section 21E of the Securities Exchange Act of 1934, as amended (the “Exchange Act”) that are not historical facts and involve risks and uncertainties that could cause actual results to differ materially from those expected and projected. All statements, other than statements of historical fact included in this report including, without limitation, statements in this “Management’s Discussion and Analysis of Financial Condition and Results of Operations” the Company’s financial position, business strategy and the plans and objectives of management for future operations, are forward-looking statements. Words such as “expect,” “believe,” “anticipate,” “intend,” “estimate,” “seek” and variations and similar words and expressions are intended to identify such forward-looking statements. Such forward-looking statements relate to future events or future performance, but reflect management’s current beliefs, based on information currently available. A number of factors could cause actual events, performance or results to differ materially from the events, performance and results discussed in the forward-looking statements. For information identifying important factors that could cause actual results to differ materially from those anticipated in the forward-looking statements, please refer to the Risk Factors section of the Company’s Annual Report on Form 10-K for the fiscal year ended December 31, 2022 filed with the U.S. Securities and Exchange Commission (“SEC”). The Company’s securities filings can be accessed on the EDGAR section of the SEC’s website at www.sec.gov. Except as expressly required by applicable securities law, the Company disclaims any intention or obligation to update or revise any forward-looking statements whether as a result of new information, future events or otherwise.
Overview
We are a life sciences company pioneering a novel technology for bioengineering fully transplantable organs to help save and improve patients’ lives. Founded in 2009, we are one of a small group of companies at the forefront of developing alternatives to human-donor organ transplants, and within this small group of companies there are important differences between the technologies being developed. Our proprietary technology is a scalable platform that uses a two-step method of decellularization and recellularization designed to remove the porcine cells from the organs obtained from pigs and replace them with unmodified human cells. Our initial development focus is on bioengineering livers and kidneys, and our technology platform is also applicable to bioengineering other organs including hearts, lungs and pancreases. We have collaborations with the Mayo Clinic, Baxter, CareDx, Mount Sinai and the Texas Heart Institute, and have received strategic investments from Baxter, CareDx and DaVita.
Substantially all of our revenue to date has been generated by sales of and royalties we received from acellular biologic surgical products, which we spun out as Reprise Biomedical, Inc. (“Reprise”) effective June 30, 2019. We subsequently divested our minority ownership stake in Reprise in March 2021. We have continued to receive royalties on the sales of these products by Reprise. Our revenue for the three and six months ended June 30, 2023 was $8,517 and $16,498, respectively, consisting entirely of licensing revenue. Our net loss for the three and six months ended June 30, 2023 was $6,530,420 and $14,010,572, respectively. We have not been profitable since inception and as of June 30, 2023, we had an accumulated deficit of $118,022,483.
16
Our product pipeline consists of three active programs, as described below:
● | miroliverELAP®, our External Liver Assist Product (“ELAP”) designed to provide liver dialysis for acute liver failure patients. |
● | miroliver, our fully implantable bioengineered liver intended to treat patients with acute and chronic liver failure. |
● | mirokidney, our fully implantable bioengineered kidney intended to treat patients with end-stage renal disease. |
Recent Developments
In the fourth quarter of 2022, we filed an IND application for our miroliverELAP to the FDA. In response to the IND application, we received a clinical hold letter from the FDA in January 2023 identifying certain nonclinical and clinical deficiencies and requesting responsive information, including, among other things, new toxicology studies in an appropriate animal model, biocompatibility studies, as well as various validations to be provided in our IND application. We plan to submit our complete response to the clinical hold letter to the FDA in the second half of 2023. If our complete response addresses the deficiencies to the FDA’s satisfaction and does not raise new concerns regarding risks to subjects, we expect the FDA will lift the clinical hold and we then intend to initiate a first-in-human, phase 1 clinical trial shortly thereafter. We have reallocated resources previously intended for miroliver and mirokidney, our fully implantable bioengineered liver and kidney programs, to miroliverELAP, which will likely delay the preclinical development of miroliver and mirokidney.
Components of Our Results of Operations
Licensing Revenue
For the periods presented, all of our revenue consists of licensing revenue pursuant to our license agreement with Reprise. Revenue pursuant to this agreement is recognized at the later of (i) when the related sales occur after the minimum guarantee is satisfied, or (ii) when the performance obligation to which some or all of the royalty has been allocated has been satisfied (or partially satisfied). Due to future uncertainty regarding the collectability of the 2021 and 2023 minimum royalties from Reprise, we determined the contract did not meet the requirements of Accounting Standards Codification, Topic 606, Revenue from Contracts with Customers (“ASC 606”); therefore, we did not record revenues or a receivable.
Cost of Goods Sold
Cost of goods sold relates to our license agreement with the University of Minnesota (the “University”), pursuant to which we owe the University royalties on our revenues, which are subject to annual minimum payments.
Gross Loss
Our gross loss is calculated by subtracting our cost of goods sold from our revenue.
Research and Development Expenses
Research and development expenses consist primarily of engineering, product development, consulting services, materials, depreciation and other costs associated with products and technologies in development. These expenses include payroll and related expenses, consulting expenses, laboratory supplies, and amounts incurred under certain collaborative agreements. Expenditures for research and development activities are charged to operations as incurred.
We expect research and development expenses to increase in the future, as we continue the development of our current product candidates. Research and development expenses will be dependent upon such factors including the results of our preclinical and clinical trials, and the number of product candidates under development.
17
Regulatory and Clinical Expenses
Regulatory and clinical expenses include costs for developing our regulatory and clinical study strategies for our product candidates. These expenses include payroll and related expenses and consulting expenses. We expect regulatory and clinical expenses will be dependent on the size and duration of any clinical programs that we may initiate.
Quality Expenses
Quality expenses relate to costs of systems and procedures to develop a manufacturing facility that is compliant with Current Good Manufacturing Practices. These expenses include payroll and related expenses. We expect quality expenses to increase in future years as we continue to develop the process and systems needed to produce our product candidates.
General and Administrative Expenses
General and administrative expenses include costs for our executive, accounting and human resources functions. Costs consist primarily of payroll and related expenses, professional service fees related to accounting, legal and other contract and administrative services and related infrastructure expenses.
Interest Income
Interest income consists of interest earned on our cash and cash equivalents and U.S. Treasury securities.
Interest Expense
Interest expense consists of interest under our loan agreements. See “— Liquidity and Capital Resources.”
Results of Operations
Comparison of the Three Months Ended June 30, 2023 to the Three Months Ended June 30, 2022
| Three Months Ended |
| ||||||||||
June 30, | Change | |||||||||||
| 2023 |
| 2022 |
| Dollar |
| Percentage | |||||
Licensing revenue | $ | 8,517 | $ | 3,952 | $ | 4,565 | 115.5 | % | ||||
Cost of goods sold | 125,000 | 125,000 | — | — | ||||||||
Gross loss | (116,483) | (121,048) | 4,565 | (3.8) |
| |||||||
Operating expenses: |
|
|
|
|
|
|
|
|
| |||
Research and development |
|
| 3,621,224 |
| 4,988,233 |
| (1,367,009) |
| (27.4) | |||
Regulatory and clinical |
|
| 397,448 |
| 419,394 |
| (21,946) |
| (5.2) | |||
Quality |
|
| 515,575 |
| 517,333 |
| (1,758) |
| (0.3) | |||
General and administrative |
|
| 2,037,682 |
| 2,188,460 |
| (150,778) |
| (6.9) | |||
Total operating expenses |
|
| 6,571,929 |
| 8,113,420 |
| (1,541,491) |
| (19.0) | |||
Operating loss |
|
| (6,688,412) |
| (8,234,468) |
| 1,546,056 |
| (18.8) | |||
Other income (expense) | ||||||||||||
Interest income |
|
| 166,162 |
| 61,078 |
| 105,084 |
| 172.0 | |||
Interest expense |
|
| (8,170) |
| (8,799) |
| 629 |
| (7.1) | |||
Total other income (expense) | 157,992 | 52,279 | 105,713 | 202.2 | ||||||||
Net loss | $ | (6,530,420) | $ | (8,182,189) | $ | 1,651,769 |
| (20.2) | % | |||
18
Licensing Revenue
Licensing revenue was $8,517 for the three months ended June 30, 2023 and $3,952 for the three months ended June 30, 2022, an increase of $4,565, or 115.5%. The licensing revenue is a result of the license agreement with Reprise. The remainder of minimum royalties due from Reprise for 2023 are due in January 2024. The remainder of the minimum royalties due from Reprise for 2021 have been deferred to 2023. Due to the uncertainty regarding the collectability of the 2021 and 2023 minimum royalties from Reprise, we determined the contract did not meet the requirements of ASC 606; therefore, we did not record revenues or a receivable.
Cost of Goods Sold
Cost of goods sold was $125,000 for both the three months ended June 30, 2023 and 2022. Cost of goods sold relates to the minimum royalty due to the University under our license agreement.
Gross Loss
Gross loss was $116,483 for the three months ended June 30, 2023 and $121,048 for the three months ended June 30, 2022, a decrease of $4,565, or 3.8%.
Research and Development
Research and development expenses were $3,621,224 for the three months ended June 30, 2023 and $4,988,233 for the three months ended June 30, 2022, a decrease of $1,367,009, or 27.4%. The decrease was primarily due to a lab supply expense decrease of $1,315,041, consulting expense decrease of $234,266 and office expense decrease of $96,172. The decrease was partially offset by a headcount increase which resulted in an increase in payroll expense of $257,238 and other expense increase of $21,232.
Regulatory and Clinical
Regulatory and clinical expenses were $397,448 for the three months ended June 30, 2023 and $419,394 for the three months ended June 30, 2022, a decrease of $21,946, or 5.2%. The decrease was primarily due to lower consulting expenses.
Quality
Quality expenses were $515,575 for the three months ended June 30, 2023 and $517,333 for the three months ended June 30, 2022, a decrease of $1,758, or 0.3%.
General and Administrative
General and administrative expenses were $2,037,682 for the three months ended June 30, 2023 and $2,188,460 for the three months ended June 30, 2022, a decrease of $150,778, or 6.9%. The decrease was primarily due to professional service fee decrease of $85,450, office expense decrease of $44,218, travel and entertainment expense decrease of $34,549, consulting expense decrease of $29,037 and other expense decrease of $2,849. The decrease was partially offset by taxes and licenses expense increase of $45,325.
Interest Income
Interest income was $166,162 for the three months ended June 30, 2023 and $61,078 for the three months ended June 30, 2022, an increase of $105,084, or 172.0%. The increase was primarily due to U.S. Treasury securities purchased during the second quarter of 2022 and the second quarter of 2023 with cash received from our initial public offering and our secondary public offering, which was completed in the first quarter of 2023.
19
Interest Expense
Interest expense was $8,170 for the three months ended June 30, 2023 and $8,799 for the three months ended June 30, 2022, a decrease of $629, or 7.1%.
Comparison of the Six Months Ended June 30, 2023 to the Six Months Ended June 30, 2022
| Six Months Ended |
| ||||||||||
June 30, | Change | |||||||||||
| 2023 |
| 2022 |
| Dollar |
| Percentage | |||||
Licensing revenue | $ | 16,498 | $ | 10,720 | $ | 5,778 | 53.9 | % | ||||
Cost of goods sold | 250,000 | 250,000 | — | — | ||||||||
Gross loss | (233,502) | (239,280) | 5,778 | (2.4) |
| |||||||
Operating expenses: |
|
|
|
|
|
|
|
|
| |||
Research and development |
|
| 8,013,342 |
| 8,994,899 |
| (981,557) |
| (10.9) | |||
Regulatory and clinical |
|
| 803,763 |
| 774,632 |
| 29,131 |
| 3.8 | |||
Quality |
|
| 1,098,917 |
| 958,268 |
| 140,649 |
| 14.7 | |||
General and administrative |
|
| 4,637,917 |
| 4,461,017 |
| 176,900 |
| 4.0 | |||
Total operating expenses |
|
| 14,553,939 |
| 15,188,816 |
| (634,877) |
| (4.2) | |||
Operating loss |
|
| (14,787,441) |
| (15,428,096) |
| 640,655 |
| (4.2) | |||
Other income (expense) | ||||||||||||
Interest income |
|
| 268,139 |
| 61,848 |
| 206,291 |
| 333.5 | |||
Interest expense |
|
| (18,413) |
| (19,690) |
| 1,277 |
| (6.5) | |||
Employee retention credit | 527,143 | — | 527,143 |
| 100.0 | |||||||
Total other income (expense) |
|
| 776,869 |
| 42,158 |
| 734,711 |
| 1,742.8 | |||
Net loss | $ | (14,010,572) | $ | (15,385,938) | $ | 1,375,366 |
| (8.9) | % | |||
Licensing Revenue
Licensing revenue was $16,498 for the six months ended June 30, 2023 and $10,720 for the six months ended June 30, 2022, an increase of $5,778, or 53.9%. The licensing revenue is a result of the license agreement with Reprise. The remainder of minimum royalties due from Reprise for 2023 are due in January 2024. The remainder of the minimum royalties due from Reprise for 2021 have been deferred to 2023. Due to the uncertainty regarding the collectability of the 2021 and 2023 minimum royalties from Reprise, we determined the contract did not meet the requirements of ASC 606; therefore, we did not record revenues or a receivable.
Cost of Goods Sold
Cost of goods sold was $250,000 for both the six months ended June 30, 2023 and 2022. Cost of goods sold relates to the minimum royalty due to the University under our license agreement.
Gross Loss
Gross loss was $233,502 for the six months ended June 30, 2023 and $239,280 for the six months ended June 30, 2022, a decrease of $5,778, or 2.4%.
Research and Development
Research and development expenses were $8,013,342 for the six months ended June 30, 2023 and $8,994,899 for the six months ended June 30, 2022, a decrease of $981,557, or 10.9%. The decrease was primarily due to lab supply expense decrease of $1,512,769, contract services expense decrease of $151,181, consulting expense decrease of $87,436, office expense decrease of $77,418 and travel and entertainment expense decrease of $22,664. The decrease was partially offset by payroll expense increase of $806,397 and other expense increase of $63,514.
20
Regulatory and Clinical
Regulatory and clinical expenses were $803,763 for the six months ended June 30, 2023 and $774,632 for the six months ended June 30, 2022, an increase of $29,131, or 3.8%. The increase was primarily due to a headcount increase which resulted in an increase in payroll expense of $85,199, partially offset by consulting expense decrease of $30,940, office expense decrease of $7,951, contract services expense decrease of $7,658, travel and entertainment expense decrease of $6,087 and other expense decrease of $3,432.
Quality
Quality expenses were $1,098,917 for the six months ended June 30, 2023 and $958,268 for the six months ended June 30, 2022, an increase of $140,649, or 14.7%. The increase was primarily due to an increase in lab supply expense of $341,972 and other expense increase of $22,982. The increase was partially offset by a decrease in payroll expense of $143,708, consulting expense decrease of $70,882, contract services expense decrease of $7,652 and office expense decrease of $2,063.
General and Administrative
General and administrative expenses were $4,637,917 for the six months ended June 30, 2023 and $4,461,017 for the six months ended June 30, 2022, an increase of $176,900, or 4.0%. The increase was primarily due to deferred offering costs write-off of $221,254, payroll expense increase of $122,278, shareholder expense increase of $7,420 and professional service expense increase of $3,551. The increase was partially offset by travel and entertainment expense decrease of $59,875, office expense decrease of $45,842, insurance expense decrease of $34,059, consulting expense decrease of $31,604 and other expense decrease of $6,223.
Interest Income
Interest income was $268,139 for the six months ended June 30, 2023 and $61,848 for the six months ended June 30, 2022, an increase of $206,291, or 333.5%. The increase was primarily due to U.S. Treasury securities purchased during the second quarter of 2022 and the second quarter of 2023 with cash received from our initial public offering and our secondary public offering, which was completed in the first quarter of 2023.
Interest Expense
Interest expense was $18,413 for the six months ended June 30, 2023 and $19,690 for the six months ended June 30, 2022, a decrease of $1,277, or 6.5%.
Employee Retention Credit
Employee retention credit income was $527,143 for the six months ended June 30, 2023. This is a refundable credit against certain employment taxes recognized under the provisions of the Coronavirus Aid, Relief, and Economic Security Act.
Liquidity and Capital Resources
We have incurred net losses since our inception. For the three months ended June 30, 2023 and 2022, we incurred net losses of $6,530,420 and $8,182,189, respectively. For the six months ended June 30, 2023 and 2022, we incurred net losses of $14,010,572 and $15,385,938, respectively. As of June 30, 2023, we had an accumulated deficit of $118,022,483.
21
We expect to incur additional losses in the near future, and we expect our expenses to increase in connection with our ongoing activities, particularly as we continue to develop our bioengineered organs, conduct clinical trials and other studies for our bioengineered organs, seek regulatory clearances or approvals for miroliverELAP, mirokidney and miroliver, continue preparing, filing and prosecuting patent applications, maintaining and enforcing our intellectual property rights, invest in our infrastructure to support our future manufacturing and other activities, and incur costs associated with operating as a public company in the U.S. The timing and amount of our operating expenditures will depend largely on our ability to, among other things:
| ● | advance clinical development of our product candidates; |
| ● | manufacture, or have manufactured on our behalf, our preclinical and clinical materials and develop processes for commercial manufacturing of any product candidates that may receive regulatory approval; |
| ● | seek regulatory approvals for any product candidates that successfully complete clinical trials; |
| ● | establish a sales, marketing, medical affairs and distribution infrastructure to commercialize any product candidates for which we may obtain marketing approval and intend to commercialize on our own; |
| ● | establish collaborations to commercialize any product candidates for which we may obtain marketing approval but do not intend to commercialize on our own; |
| ● | expand our operational, financial and management systems and hire additional personnel, including personnel to support our clinical development, quality control, research and development, manufacturing and commercialization efforts, our general and administrative activities and our operations as a public company; and |
| ● | obtain new intellectual property and maintain, expand and protect our intellectual property portfolio. |
Sources of Liquidity
The accompanying financial statements have been prepared on a going concern basis, which contemplates the realization of assets and the satisfaction of liabilities and commitments in the ordinary course of business. To date, we have primarily financed our operations through equity and debt financings, including public offerings of our common stock, as well as research grants. We do not have adequate liquidity to fund our operations for at least twelve months from the issuance of these financial statements without raising additional capital and such actions are not solely within our control. If we are unable to raise additional capital, we believe planned expenditures may need to be reduced in order to extend the time period that existing resources can fund our operations. We have based this estimate on assumptions that may prove to be wrong, and we could use our available capital resources sooner than we currently expect. As of June 30, 2023, we had cash and cash equivalents of $4,571,777 and short-term investments of $15,828,281.
Until such time, if ever, as we can generate substantial revenue from sales of our bioengineered organs, we expect to finance our cash needs through a combination of equity offerings, debt financings, strategic partnerships and collaborations, as well as other sources. To the extent that we raise additional capital through the sale of equity or convertible debt securities, the ownership interest of our stockholders will be diluted, and the terms of these securities may include liquidation or other preferences that adversely affect the rights of our stockholders. Debt financing and preferred equity financing, if available, may involve agreements that include covenants limiting or restricting our ability to take specific actions, such as incurring additional debt, making capital expenditures or declaring dividends. Additional capital may not be available when needed, on reasonable terms, or at all, and our ability to raise additional capital may be adversely impacted by factors not solely within our control. If we are unable to raise additional funds through equity or debt financings or other arrangements when needed, we may be required to delay, curtail or discontinue our product development or future commercialization efforts, or grant rights to develop and market products that we would otherwise prefer to develop and market ourselves.
Debt Financing
In January 2019, we issued the University a promissory note in the amount of $385,997 in satisfaction of our minimum royalty obligation under the license agreement with the University for the year ended December 31, 2018. The note bears interest at 6% per annum, compounded annually, and is due on January 31, 2025. In addition, we issued the University a 10-year warrant to purchase 20,587 shares of our common stock at an exercise price of $3.75 per share. As of both June 30, 2023 and December 31, 2022, the principal outstanding on this loan was $385,997.
22
Registration Statement
We filed a Registration Statement on Form S-3 with the SEC on July 1, 2022, which was declared effective on July 11, 2022 (the “Registration Statement”). The Registration Statement registered the offer and sale of an indeterminate number of shares of common stock and preferred stock, an indeterminate principal amount of debt securities and an indeterminate number of warrants to purchase common stock, preferred stock, and various series of debt securities and warrants to purchase any of such securities, having an aggregate initial offering price of $200.0 million.
Equity Distribution Agreement
On July 1, 2022, we entered into an Equity Distribution Agreement with Piper Sandler & Co. (“Piper Sandler”). The Equity Distribution Agreement provides that, upon the terms and subject to the conditions set forth therein, we may issue and sell through Piper Sandler, acting as the sales agent, shares of our common stock having an aggregate offering price of up to $50.0 million. We have no obligation to sell any such shares under the Equity Distribution Agreement. The sale of the shares of our common stock by Piper Sandler, if any, will be effected pursuant to the Registration Statement. We did not issue any shares under the Equity Distribution Agreement in the six months ended June 30, 2023.
Public Offering
In March 2023, we completed a public offering pursuant to which we sold an aggregate of 6,250,000 shares of our common stock at a public offering price of $1.60 per share. The offering closed on March 10, 2023, resulting in net proceeds of approximately $8.8 million, after deducting underwriting discounts and commissions and other offering expenses.
Employee Retention Credit
On July 31, 2023, we received cash payments totaling $457,143 of the $527,143 employee retention receivable.
Cash Flows
The following table summarizes our sources and uses of cash for each of the periods presented:
Six Months Ended | ||||||
June 30, | ||||||
| 2023 |
| 2022 | |||
Net cash (used in) provided by: | ||||||
Operating activities | $ | (13,684,036) | $ | (13,790,978) | ||
Investing activities | 4,224,731 | (26,757,741) | ||||
Financing activities |
| 8,823,077 |
| 330,503 | ||
Net decrease in cash and cash equivalents | $ | (636,228) | $ | (40,218,216) | ||
Operating Activities
Net cash used in operating activities consisted of net losses adjusted for certain non-cash items and changes in operating assets and liabilities.
During the six months ended June 30, 2023, net cash used in operating activities was $13,684,036 and reflected (i) the net loss of $14,010,572, (ii) net non-cash usage items of $865,184, including stock-based compensation of $610,610, depreciation and amortization expense of $579,367, deferred offering costs write-off of $221,254 and non-cash interest income of $77,154, which was partially offset by employee retention credit of $527,143 and amortization of premium/discount on investments of $96,058, and (iii) a net cash outflow from changes in balances of operating assets and liabilities of $538,648. The most significant items comprising the changes in balances of operating assets and liabilities
23
were a cash inflow of the receivable from Reprise Biomedical, Inc. of $921,838 and a cash outflow of accounts payable and accrued expenses of $1,544,792.
During the six months ended June 30, 2022, net cash used in operating activities was $13,790,978 and reflected (i) the net loss of $15,385,938, (ii) net non-cash usage items of $1,027,437, including $600,371 of stock-based compensation, $536,507 of depreciation and amortization expense, amortization of premium/discount on investments of $6,734 and $758 of loss on disposal of property and equipment, partially offset by non-cash interest income of $116,933, and (iii) a net cash inflow from changes in balances of operating assets and liabilities of $567,523.
Investing Activities
During the six months ended June 30, 2023, net cash provided by investing activities was $4,224,731, driven by proceeds from the maturity of investments of $14,000,000, partially offset by the purchase of investments of $9,742,734 and property and equipment purchase of $32,535.
During the six months ended June 30, 2022, net cash used by investing activities was $26,757,741 and reflected the purchase of investments of $26,026,125 and property and equipment purchases of $731,616.
Financing Activities
During the six months ended June 30, 2023, net cash provided by financing activities was $8,823,077 and was primarily the result of proceeds from the sale of common stock of $8,867,082, partially offset by payments on financing lease obligations of $29,376 and payments on employee taxes for shares withheld of $14,629.
During the six months ended June 30, 2022, net cash provided by financing activities was $330,503 and was primarily the result of proceeds from stock warrant exercises of $414,098 and proceeds from stock option exercises of $256,883, partially offset by payments on long-term debt of $295,450, payments on financing lease obligations of $26,488 and payments on offering costs of $18,540.
Item 3. Quantitative and Qualitative Disclosures About Market Risk
Not required for smaller reporting companies.
Item 4. Controls and Procedures
Evaluation of Disclosure Controls and Procedures
Disclosure controls and procedures are designed to ensure that information required to be disclosed by us in our Exchange Act reports is recorded, processed, summarized, and reported within the time periods specified in the SEC’s rules and forms, and that such information is accumulated and communicated to our management, including our principal executive officer and principal financial officer or persons performing similar functions, as appropriate to allow timely decisions regarding required disclosure.
Under the supervision and with the participation of our management, including our principal executive officer and principal financial officer, we conducted an evaluation of the effectiveness of our disclosure controls and procedures as of the end of the fiscal quarter ended June 30, 2023, as such term is defined in Rules 13a-15(e) and 15d-15(e) under the Exchange Act. Based on this evaluation, our principal executive officer and principal financial officer have concluded that our disclosure controls and procedures were not effective as of June 30, 2023 because of our previously reported material weaknesses in our internal control over financial reporting, which we describe in Part II, Item 9A of our Annual Report on Form 10-K for the year ended December 31, 2022.
24
Management is committed to remediating its material weaknesses as promptly as possible and is in the process of implementing its remediation plan. We are in the process of designing, implementing, documenting and testing the effectiveness of our processes, procedures and internal controls over financial reporting. The material weakness cannot be considered completely remediated until the applicable internal controls over financial reporting have operated for a sufficient period of time and management has concluded, through testing, that these controls are operating effectively. We cannot reasonably assure we will be able to remediate the material weaknesses we have identified or avoid potential future material weaknesses.
Changes in Internal Control over Financial Reporting
As outlined above, due to the identification of the material weaknesses, we continued to design, implement, document and test the effectiveness of our processes, procedures and internal controls over financial reporting. We made no other changes in our internal control over financial reporting that occurred during the fiscal quarter of 2023 covered by this Quarterly Report on Form 10-Q that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
PART II — OTHER INFORMATION
Item 1. Legal Proceedings
We are not presently a party to any litigation the outcome of which, we believe, if determined adversely to us, would individually or taken together have a material adverse effect on our business, operating results, cash flows or financial condition.
Item 1A. Risk Factors
This Quarterly Report on Form 10-Q should be read in conjunction with the risk factors included in our Annual Report on Form 10-K for the fiscal year ended December 31, 2022 filed with the SEC on March 31, 2023. Other than as set forth below, there have been no material changes to the risk factors disclosed under the heading “Risk Factors” in our Annual Report on Form 10-K for the fiscal year ended December 31, 2022.
As a result of our limited financial liquidity, we have expressed substantial doubt regarding our ability to continue as a “going concern.”
As a result of our current limited financial liquidity, the notes accompanying our unaudited interim condensed financial statements, which are included as part of this report, contain a statement concerning our ability to continue as a “going concern.” Our limited liquidity could make it more difficult for us to secure additional financing or enter into strategic relationships on terms acceptable to us, if at all, and may materially and adversely affect the terms of any financing that we may obtain and our public stock price generally.
We have incurred losses since inception, negative cash flows from operations, and had an accumulated deficit of approximately $118.0 million as of June 30, 2023. The Company does not have adequate liquidity to fund its operations for at least twelve months from the issuance of these financial statements without raising additional capital and such actions are not solely within the control of the Company. If the Company is unable to raise additional capital, management believes planned expenditures may need to be reduced in order to extend the time period that existing resources can fund the Company’s operations. To date, the Company has funded its operations through the issuance of equity and debt securities, and the receipt of grants. The Company intends to fund ongoing operations by utilizing its current cash, cash equivalents and short-term investments on hand, and by exploring various dilutive and non-dilutive sources of funding, including equity financings, debt financings, strategic partnerships and collaborations, as well as other sources. If the Company is unable to obtain additional capital on commercially reasonable terms, or at all, it would have a material adverse effect on the operations of the Company and the development of its technology, and the Company may have to cease operations altogether. These factors raise substantial doubt about the Company’s ability to continue as a going concern.
25
Item 2. Unregistered Sales of Equity Securities and Use of Proceeds
Unregistered Sales of Equity Securities
None.
Use of Proceeds
On June 28, 2021, we completed our initial public offering on common stock (the “IPO”) in which we sold 5,520,000 shares of common stock at a public offering price of $9.00 per share. The offer and sale of all of the shares in the IPO were registered under the Securities Act pursuant to the Company’s registration statement on Form S-1 (File No. 333-256649), as amended, which was declared effective on June 23, 2021 pursuant to Rule 462(b) under the Securities Act. Craig-Hallum Capital Group acted as sole managing underwriter for the IPO.
We received net proceeds of approximately $44.5 million from the IPO, after paying $342,500 of fees and expenses of Craig-Hallum. We are using the net proceeds from the IPO as follows:
| ● | between approximately $34.8 million to $40.0 million to fund our research and development activities, including, but not limited to, our Phase I trial for the miroliverELAP product and certain pre-clinical trials for our bioengineered organs; |
| ● | between approximately $3.0 million and $4.0 million to fund the full cost of constructing a new facility; and |
| ● | the remaining funds for working capital and general corporate purposes. |
Pending the uses of proceeds above, we have invested in a variety of capital preservation instruments, including short-term, interest-bearing obligations, investment-grade instruments, certificates of deposit or direct or guaranteed obligations of the United States government.
Item 3. Defaults Upon Senior Securities
None.
Item 4. Mine Safety Disclosures
Not applicable.
Item 5. Other Information
During the three months ended June 30, 2023, no director or officer of the Company
26
Item 6. Exhibits
Exhibit No. | Description | |
Second Amended and Restated Certificate of Incorporation of Miromatrix Medical Inc. | Incorporated by reference to Exhibit 3.1 of the Company’s Annual Report on Form 10-K, filed March 30, 2022. | |
Incorporated by reference to Exhibit 3.2 of the Company’s Current Report on Form 8-K, filed June 28, 2021. | ||
Filed herewith. | ||
Filed herewith. | ||
Certification of Chief Executive Officer Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 | Filed herewith. | |
Certification of Chief Financial Officer Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 | Filed herewith. | |
Furnished herewith. | ||
Furnished herewith. | ||
101.INS | Inline XBRL Instance Document – the instance document does not appear in the Interactive Data File because its XBRL tags are embedded within the Inline XBRL document | Filed herewith. |
101.SCH | Inline XBRL Taxonomy Extension Schema Document | Filed herewith. |
101.CAL | Inline XBRL Taxonomy Extension Calculation Linkbase Document | Filed herewith. |
101.DEF | Inline XBRL Taxonomy Extension Definition Linkbase Document | Filed herewith. |
101.LAB | Inline XBRL Taxonomy Extension Label Linkbase Document | Filed herewith. |
101.PRE | Inline XBRL Taxonomy Extension Presentation Linkbase Document | Filed herewith. |
104 | Cover Page Interactive Data File (embedded within the inline XBRL document) | Filed herewith. |
27
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereto, duly authorized.
MIROMATRIX MEDICAL INC. | ||
Dated: August 14, 2023 | By: | /s/ Jeffrey Ross |
Name: | Jeffrey Ross | |
Title: | Chief Executive Officer | |
(on behalf of Registrant) | ||
Dated: August 14, 2023 | By: | /s/ James Douglas |
Name: | James Douglas | |
Title: | Chief Financial Officer | |
(Principal Financial and Accounting Officer) | ||
28
Exhibit 10.1
LICENSE AGREEMENT
AMENDMENT #12
WHEREAS, effective as of the 1st day of December, 2024, and as further amended, Miromatrix Medical Inc., a corporation organized under the laws of the State of Delaware and having an office 10399 West 70th Street, Eden Prairie, MN 55344 (“Miromatrix”) and Mayo Foundation for Medical Education and Research, a not for profit corporation with an address at 200 First Street SW, Rochester, MN 55905 (“Mayo”) executed a License Agreement (the “Agreement”) for Services as outlined in the Agreement.
WHEREAS, Miromatrix and Mayo wish to amend said Agreement.
NOW, THEREFORE, Miromatrix and Mayo agree as follows:
| 1. | Miromatrix has agreed to provide additional funding. Accordingly, the attached Exhibit B-5 shall amend Section 3 of the Agreement. |
| 2. | This Agreement may be executed in any number of counterparts which, when taken together, will constitute one original, and photocopy, facsimile, electronic, or other copies shall have the same effect for all purposes as an ink-signed original. Each Party hereto consents to be bound by photocopy, facsimile or electronic signatures of such Party’s representation hereto. |
| 3. | Except as specifically provided herein, the terms and conditions of the Agreement shall remain in force. |
IN WITNESS WHEREOF, the Parties have executed this Amendment which shall be effective as of the last dated signature below.
MIROMATRIX MEDICAL INC.MAYO FOUNDATION FOR MEDICAL
EDUCATION AND RESEARCH
By: /s/ Jeff Ross By: /s/ Brenda L. Burns
Name: Jeff Ross Name: Brenda L. Burns
Title: CEO Title: Operations Manager, Legal Contract Administration
Date: 5/7/2023 Date: 4/25/2023

Budget – Exhibit B-5
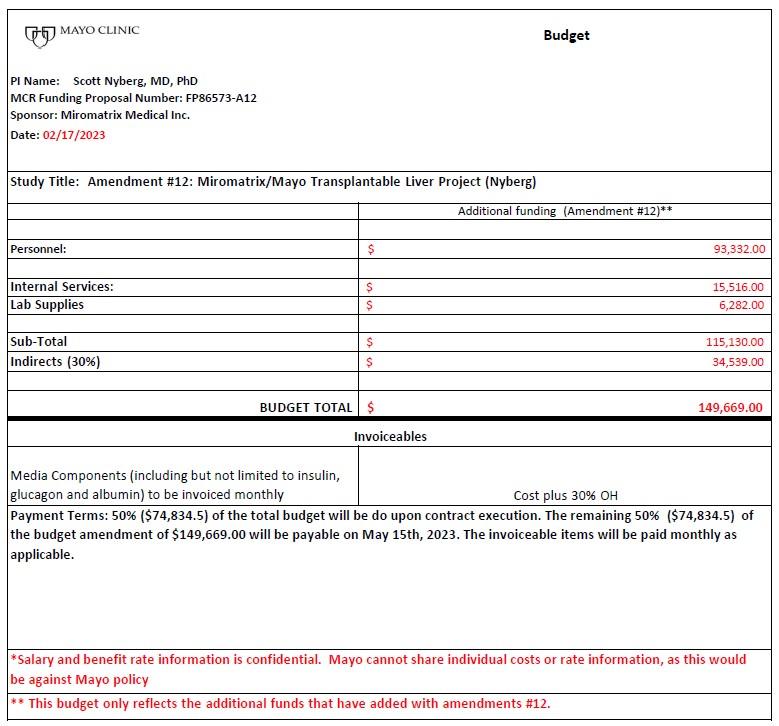
Exhibit 10.2
LICENSE AGREEMENT
AMENDMENT #13
WHEREAS, effective as of December 1, 2014, and as further amended, Miromatrix Medical Inc., a corporation organized under the laws of the State of Delaware and having an office 10399 West 70th Street, Eden Prairie, MN 55344 (“Miromatrix”) and Mayo Foundation for Medical Education and Research, a not for profit corporation with an address at 200 First Street SW, Rochester, MN 55905 (“Mayo”) executed a License Agreement (the “Agreement”) for Services as outlined in the Agreement.
WHEREAS, Miromatrix and Mayo wish to amend said Agreement.
NOW, THEREFORE, Miromatrix and Mayo agree as follows:
| 1. | The Term of the Agreement shall be extended to December 31, 2023. |
| 2. | Except as specifically provided herein, the terms and conditions of the Agreement shall remain in force. |
IN WITNESS WHEREOF, the Parties have executed this Amendment which shall be effective as of the last dated signature below.
MIROMATRIX MEDICAL INC.MAYO FOUNDATION FOR MEDICAL
EDUCATION AND RESEARCH
By: /s/ Jeff Ross By: /s/ Julie Johnson
Name: Jeff Ross Name: Julie Johnson
Title: CEO Title: Operations Manager
Date: 7/17/2023 Date: 7/14/2023

Budget – Exhibit B-5
U MAYO CLINIC
PI Name: Scott Nyberg, MD, PhD
MCR Funding Proposal Number: FP86573-A10
Sponsor: Miromatrix Medical Inc.
Date: 10/5/2022
Study Title: Amendment #11: Miromatrix/Mayo Transplantable Liver Project (Nyberg)
| | | Additional funding to extend project through 6/30/2023 (Amendment #11)** |
| | | |
Personnel: | | $ | 57,467.00 |
| | | |
Lab Supplies & Internal Services: | | $ | 208,341.00 |
| | | |
Sub-Total | | $ | 265,808.00 |
Indirects (30%) | | $ | 79,742.00 |
| | | |
BUDGET TOTAL (excluding fees) | | $ | 345,550.00 |
One-time, non-refundable fees: | | | |
IACUC Initial Review Fee | | $ | 2,000.00 |
Total Fees: | | $ | 2,000.00 |
BUDGET TOTAL | | $ | 347,550.00 |
Payment Terms: The total budget will be payable in three payments. The first payment of $117,183.33 which includes the $2,000 non-refundable Initial IACUC Review Fee, will be due upon the execution of contract amendment #11, the second payment of $115,183.33 will be due three months after the execution of contract amendment #11. The final payment of $115,183.33 will be due 6 months after the execution of contract amendment #11
*Salary and benefit rate information is confidential. Mayo cannot share individual costs or rate information, as this would be against Mayo policy
**This budget only reflects the additional funds that have added with amendments #11.
Exhibit 31.1
CERTIFICATION OF CHIEF EXECUTIVE OFFICER
I, Jeffrey Ross, certify that:
1. | I have reviewed this quarterly report on Form 10-Q of Miromatrix Medical Inc.; |
2. | Based on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report; |
3. | Based on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of operations and cash flows of the registrant as of, and for, the periods presented in this report; |
4. | The registrant’s other certifying officer and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) and internal control over financial reporting (as defined in Exchange Act Rules 13a-15(f) and 15d-15(f)) for the registrant and have: |
(a) | Designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the registrant, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this report is being prepared; |
(b) | Designed such internal controls over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles; |
(c) | Evaluated the effectiveness of the registrant’s disclosure controls and procedures and presented in this report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this report based on such evaluation; and |
(d) | Disclosed in this report any change in the registrant’s internal control over financial reporting that occurred during the registrant’s most recent fiscal quarter (the registrant’s fourth fiscal quarter in the case of an annual report) that has materially affected, or is reasonably likely to materially affect, the registrant’s internal control over financial reporting; and |
5. | The registrant’s other certifying officer and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the registrant’s auditors and the audit committee of the registrant’s board of directors (or persons performing the equivalent functions): |
(a) | All significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the registrant’s ability to record, process, summarize and report financial information; and |
(b) | Any fraud, whether or not material, that involves management or other employees who have a significant role in the registrant’s internal control over financial reporting. |
Date: August 14, 2023
| By: | /s/ Jeffrey Ross |
| Name: | Jeffrey Ross |
| Title: | Chief Executive Officer |
Exhibit 31.2
CERTIFICATION OF CHIEF FINANCIAL OFFICER
I, James Douglas, certify that:
1. | I have reviewed this quarterly report on Form 10-Q of Miromatrix Medical Inc.; |
2. | Based on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report; |
3. | Based on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of operations and cash flows of the registrant as of, and for, the periods presented in this report; |
4. | The registrant’s other certifying officer and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) and internal control over financial reporting (as defined in Exchange Act Rules 13a-15(f) and 15d-15(f)) for the registrant and have: |
(a) | Designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the registrant, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this report is being prepared; |
(b) | Designed such internal controls over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles; |
(c) | Evaluated the effectiveness of the registrant’s disclosure controls and procedures and presented in this report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this report based on such evaluation; and |
(d) | Disclosed in this report any change in the registrant’s internal control over financial reporting that occurred during the registrant’s most recent fiscal quarter (the registrant’s fourth fiscal quarter in the case of an annual report) that has materially affected, or is reasonably likely to materially affect, the registrant’s internal control over financial reporting; and |
5. | The registrant’s other certifying officer and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the registrant’s auditors and the audit committee of the registrant’s board of directors (or persons performing the equivalent functions): |
(a) | All significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the registrant’s ability to record, process, summarize and report financial information; and |
(b) | Any fraud, whether or not material, that involves management or other employees who have a significant role in the registrant’s internal control over financial reporting. |
Date: August 14, 2023
| By: | /s/ James Douglas |
| Name: | James Douglas |
| Title: | Chief Financial Officer |
Exhibit 32.1
Certification of CEO Pursuant to 18 U.S.C. Section 1350,
As Adopted Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002
In connection with the quarterly report of Miromatrix Medical Inc. (the “Company”) on Form 10-Q for the period ended June 30, 2023 (the “Report”), as filed with the Securities and Exchange Commission on the date hereof, I, the undersigned, certify, pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002, to the best of my knowledge, that:
(1) | The Report fully complies with the requirements of Section 13(a) or Section 15(d) of the Securities Exchange Act of 1934; and |
(2) | The information contained in the Report fairly presents, in all material respects, the financial condition and results of operations of the Company. |
Date: August 14, 2023
| | |
| By: | /s/ Jeffrey Ross |
| Name: | Jeffrey Ross |
| Title: | Chief Executive Officer |
Exhibit 32.2
Certification of CFO Pursuant to 18 U.S.C. Section 1350,
As Adopted Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002
In connection with the quarterly report of Miromatrix Medical Inc. (the “Company”) on Form 10-Q for the period ended June 30, 2023 (the “Report”), as filed with the Securities and Exchange Commission on the date hereof, I, the undersigned, certify, pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002, to the best of my knowledge, that:
(1) | The Report fully complies with the requirements of Section 13(a) or Section 15(d) of the Securities Exchange Act of 1934; and |
(2) | The information contained in the Report fairly presents, in all material respects, the financial condition and results of operations of the Company. |
Date: August 14, 2023
| | |
| By: | /s/ James Douglas |
| Name: | James Douglas |
| Title: | Chief Financial Officer |
Balance Sheets (Parenthetical) - $ / shares |
Jun. 30, 2023 |
Dec. 31, 2022 |
|---|---|---|
| Balance Sheets | ||
| Common stock, par value (in dollars per share) | $ 0.00001 | $ 0.00001 |
| Common stock, authorized (in shares) | 190,000,000 | 190,000,000 |
| Common stock, issued (in shares) | 27,310,553 | 20,944,109 |
| Common stock, outstanding (in shares) | 27,310,553 | 20,944,109 |
Statements of Operations - USD ($) |
3 Months Ended | 6 Months Ended | ||
|---|---|---|---|---|
Jun. 30, 2023 |
Jun. 30, 2022 |
Jun. 30, 2023 |
Jun. 30, 2022 |
|
| Statements of Operations | ||||
| Licensing revenue | $ 8,517 | $ 3,952 | $ 16,498 | $ 10,720 |
| Revenue from Contract with Customer, Product and Service | us-gaap:LicenseMember | us-gaap:LicenseMember | us-gaap:LicenseMember | us-gaap:LicenseMember |
| Cost of goods sold | $ 125,000 | $ 125,000 | $ 250,000 | $ 250,000 |
| Cost, Product and Service | us-gaap:LicenseMember | us-gaap:LicenseMember | us-gaap:LicenseMember | us-gaap:LicenseMember |
| Gross profit (loss) | $ (116,483) | $ (121,048) | $ (233,502) | $ (239,280) |
| Operating expenses: | ||||
| Research and development | 3,621,224 | 4,988,233 | 8,013,342 | 8,994,899 |
| Regulatory and clinical | 397,448 | 419,394 | 803,763 | 774,632 |
| Quality | 515,575 | 517,333 | 1,098,917 | 958,268 |
| General and administration | 2,037,682 | 2,188,460 | 4,637,917 | 4,461,017 |
| Total operating expenses | 6,571,929 | 8,113,420 | 14,553,939 | 15,188,816 |
| Operating loss | (6,688,412) | (8,234,468) | (14,787,441) | (15,428,096) |
| Other income (expense) | ||||
| Interest income | 166,162 | 61,078 | 268,139 | 61,848 |
| Interest expense | (8,170) | (8,799) | (18,413) | (19,690) |
| Employee retention credit | (527,143) | |||
| Total other income | 157,992 | 52,279 | 776,869 | 42,158 |
| Net loss | $ (6,530,420) | $ (8,182,189) | $ (14,010,572) | $ (15,385,938) |
| Net loss per share, basic (in dollar per share) | $ (0.24) | $ (0.40) | $ (0.56) | $ (0.75) |
| Net loss per share, diluted (in dollar per share) | $ (0.24) | $ (0.40) | $ (0.56) | $ (0.75) |
| Weighted average shares used in computing net loss per share, basic (in shares) | 27,250,573 | 20,615,218 | 24,897,796 | 20,547,070 |
| Weighted average shares used in computing net loss per share, diluted (in shares) | 27,250,573 | 20,615,218 | 24,897,796 | 20,547,070 |
DESCRIPTION OF BUSINESS AND SIGNIFICANT ACCOUNTING POLICIES |
6 Months Ended |
|---|---|
Jun. 30, 2023 | |
| DESCRIPTION OF BUSINESS AND SIGNIFICANT ACCOUNTING POLICIES | |
| DESCRIPTION OF BUSINESS AND SIGNIFICANT ACCOUNTING POLICIES | NOTE 1 — DESCRIPTION OF BUSINESS AND SIGNIFICANT ACCOUNTING POLICIES Description of Business Miromatrix Medical Inc. (the “Company”) is a life sciences company pioneering a novel technology for bioengineering fully transplantable organs to help save and improve patients’ lives. Founded in 2009, the Company is one of a small group of companies at the forefront of developing alternatives to human-donor organ transplants, and within this small group of companies there are important differences between the technologies being developed. The Company’s proprietary technology is a scalable platform that uses a two-step method of decellularization and recellularization designed to remove the porcine cells from the organs obtained from pigs and replace them with unmodified human cells. The Company’s initial development focus is on bioengineering livers and kidneys, and the Company’s technology platform is also applicable to bioengineering other organs including hearts, lungs and pancreases. The Company has collaborations with Baxter International Inc. (“Baxter”), CareDx, Inc. (“CareDx”), the Mayo Clinic, the Mount Sinai Health System and the Texas Heart Institute, and we have received strategic investments from Baxter, CareDx and DaVita Inc. Basis of Preparation The accompanying unaudited condensed financial statements of the Company have been prepared in accordance with accounting principles generally accepted in the United States of America (“U.S. GAAP”) and the rules of the United States (“U.S.”) Securities and Exchange Commission applicable to interim reports of companies filing as a smaller reporting company. These condensed financial statements should be read in conjunction with the audited financial statements and notes thereto contained in the Company’s Annual Report on Form 10-K for the fiscal year ended December 31, 2022. In the opinion of management, the accompanying condensed financial statements reflect all normal recurring adjustments necessary to present fairly the financial position, results of operations, stockholders’ equity and cash flows for the interim periods but are not necessarily indicative of the results of operations or cash flows to be anticipated for the full year 2023 or any future period. The Company has evaluated subsequent events occurring after the date of the condensed financial statements for events requiring recording or disclosure in the condensed financial statements. Reclassifications Certain reclassifications to previously reported financial information on the Condensed Balance Sheets and Condensed Statements of Operations have been made to conform to the current period presentation. Use of Estimates The preparation of financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates. Employee Retention Credit Under the provisions of the extension of the Coronavirus Aid, Relief, and Economic Security Act, the Company is eligible for a refundable employee retention credit subject to satisfaction of certain eligibility criteria. The Company qualified for the employee retention credit for the first three quarters of 2020 and the second and third quarters of 2021. The Company recognized $527,143 during the six months ended June 30, 2023, consistent with guidance from International Accounting Standards 20, Accounting for Government Grants and Disclosure of Government Assistance, where the Company must have substantially met the program’s eligibility conditions to record revenue. It was reported as an employee retention credit receivable on the Condensed Balance Sheet and employee retention credit other income on the Condensed Statement of Operations for the six months ended June 30, 2023. Emerging Growth Company Status The Company is an emerging growth company, as defined in the Jumpstart Our Business Startups Act of 2012 (“JOBS Act”). Under the JOBS Act, emerging growth companies can delay adopting new or revised accounting standards issued subsequent to the enactment of the JOBS Act, until such time as those standards apply to private companies. The Company has elected to use this extended transition period for complying with new or revised accounting standards that have different effective dates for public and private companies until the earlier of the date that it (i) is no longer an emerging growth company or (ii) affirmatively and irrevocably opts out of the extended transition period provided in the JOBS Act. As a result, these condensed financial statements may not be comparable to companies that comply with the new or revised accounting pronouncements as of public company effective dates. Recent Accounting Pronouncements Recently Adopted Accounting Pronouncements The FASB issued ASU No. 2016-13, Measurement of Credit Losses on Financial Instruments and an updated ASU 2018-19 that clarifies the scope of the standard in the amendments in ASU 2016-13. This guidance introduces a new model for recognizing credit losses on financial instruments based on an estimate of current expected credit losses. Financial instruments impacted are trade and other receivables, held-to-maturity debt securities, loans and other instruments. The Company adopted the standard effective January 1, 2023, using the modified retrospective approach. The adoption did not have an impact on the Company's financial statements. |
GOING CONCERN |
6 Months Ended |
|---|---|
Jun. 30, 2023 | |
| GOING CONCERN | |
| GOING CONCERN | NOTE 2 — GOING CONCERN The accompanying condensed financial statements have been prepared on the basis that the Company will continue as a going concern. The Company has incurred losses since inception, negative cash flows from operations, and had an accumulated deficit of approximately $118.0 million as of June 30, 2023. The Company does not have adequate liquidity to fund its operations for at least twelve months from the issuance of these financial statements without raising additional capital and such actions are not solely within the control of the Company. If the Company is unable to raise additional capital, management believes planned expenditures may need to be reduced in order to extend the time period that existing resources can fund the Company’s operations. To date, the Company has funded its operations through the issuance of equity and debt securities, and the receipt of grants. The Company intends to fund ongoing operations by utilizing its current cash, cash equivalents and short-term investments on hand, and by exploring various dilutive and non-dilutive sources of funding, including equity financings, debt financings, strategic partnerships and collaborations, as well as other sources. If the Company is unable to obtain additional capital, it would have a material adverse effect on the operations of the Company and the development of its technology, and the Company may have to cease operations altogether. These factors raise about the Company’s ability to continue as a going concern. |
FAIR VALUE MEASUREMENT |
6 Months Ended |
|---|---|
Jun. 30, 2023 | |
| FAIR VALUE MEASUREMENT | |
| FAIR VALUE MEASUREMENT | NOTE 3 — FAIR VALUE MEASUREMENT The fair value of the Company’s financial instruments reflects the amount that the Company estimates that it would receive in connection with the sale of an asset or paid in connection with the transfer of a liability in an orderly transaction between market participants at the measurement date (exit price). The Company uses a three-tier valuation hierarchy based upon observable and non-observable inputs to measure fair value: Level 1: Inputs that include quoted prices in active markets for identical assets and liabilities. Level 2: Inputs other than Level 1 that are observable, either directly or indirectly, such as quoted prices for similar assets or liabilities, quoted prices in markets that are not active; or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the assets or liabilities. Level 3: Unobservable inputs that are supported by little or no market activity and that are significant to the fair value of the assets or liabilities. The Company classifies cash and cash equivalents, as well as restricted cash, as Level 1 in the fair value hierarchy. The Company classifies its investments in U.S. Treasury notes as Level 1 in the fair value hierarchy. While the market for these securities are highly liquid and active, quoted prices for these securities may at times be derived from pricing models which use observable inputs such as benchmark yields, reported trades, broker/dealer quotes, issuer spreads, two-sided markets, benchmark securities, bids, offers and other reference data including market research publications. |
INVESTMENTS |
6 Months Ended | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Jun. 30, 2023 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| INVESTMENTS | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| INVESTMENTS | NOTE 4 — INVESTMENTS The Company currently invests its excess cash in U.S. Treasury securities. The Company intends and has the ability to hold these investments to maturity. Securities with original maturity dates of more than three months are reported as held-to-maturity investments and are recorded at amortized cost, which approximates fair value due to the negligible risk of changes in value due to interest rates. All investments held as of June 30, 2023 had contractual maturities of less than one year. The amortized cost and estimated fair values of the Company’s investments as of June 30, 2023 are as follows:
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PROPERTY AND EQUIPMENT |
6 Months Ended | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Jun. 30, 2023 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PROPERTY AND EQUIPMENT, NET | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LAB EQUIPMENT, FURNITURE, LEASEHOLD IMPROVEMENTS AND CONSTRUCTION IN PROGRESS | NOTE 5 — PROPERTY AND EQUIPMENT, NET Property and equipment consisted of the following as of:
Depreciation and amortization expense was $289,439 and $273,610 for the three months ended June 30, 2023 and 2022, respectively, and $579,367 and $536,507 for the six months ended June 30, 2023 and 2022, respectively. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
ACCRUED EXPENSES |
6 Months Ended | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Jun. 30, 2023 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ACCRUED EXPENSES | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ACCRUED EXPENSES | NOTE 6 — ACCRUED EXPENSES Accrued expenses consisted of the following as of:
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
DEBT |
6 Months Ended | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Jun. 30, 2023 | |||||||||||||||||||||||||||||
| DEBT | |||||||||||||||||||||||||||||
| DEBT | NOTE 7 — DEBT In January 2019, the Company issued the Regents of the University of Minnesota (the “University”) a promissory note in the amount of $385,997 in satisfaction of the Company’s minimum royalty obligation for the year ended December 31, 2018. The note bears interest at 6.0% per annum, compounded annually, and is due on January 31, 2025. As of both June 30, 2023 and December 31, 2022, the balance outstanding on this loan was $385,997. In addition, the Company issued the University a 10-year warrant to purchase 20,587 shares of the Company’s common stock at an exercise price of $3.75 per share, which remained outstanding through June 30, 2023. Future principal maturities for debt were as follows:
|
||||||||||||||||||||||||||||
EQUITY |
6 Months Ended | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Jun. 30, 2023 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| EQUITY | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| EQUITY | NOTE 8 — EQUITY Common Stock The Company is authorized to issue 190,000,000 shares of common stock, with a par value of $0.00001. Holders of common stock are entitled to one vote for each share held on all matters submitted to a vote of common stockholders. Subject to preferences that may be applicable to any outstanding preferred shares, each share of common stock is entitled to share pro rata in any distributions. In any distribution of capital assets, holders of common stock are entitled to receive pro rata the assets remaining after payment of liabilities and liquidation preferences on any outstanding preferred stock. In March 2023, the Company completed a public offering pursuant to which it sold an aggregate of 6,250,000 shares of the Company’s common stock at a public offering price of $1.60 per share, resulting in gross proceeds of $10 million. The offering closed on March 10, 2023, resulting in net proceeds of approximately $8.8 million, after deducting underwriting discounts and commissions and other offering expenses. The Company previously capitalized $232,899 of deferred offering costs relating to the $200 million shelf registration statement declared effective on July 11, 2022. When the Company completed the public offering in March 2023, $11,645 was reclassified to additional paid-in capital on the Balance Sheet and $221,254 was written-off to general and administrative expenses in the Statement of Operations, on a pro-rata basis. As of June 30, 2023 and December 31, 2022, there were 27,310,553 and 20,944,109 shares of common stock issued and outstanding, respectively. Preferred Stock The Company is authorized to issue 10,000,000 shares of preferred stock, with a par value of $0.00001. As of June 30, 2023 and December 31, 2022, there were no shares of preferred stock issued and outstanding. Equity Incentive Plans In May 2021, the Company’s stockholders approved the 2021 Equity Incentive Plan (the “2021 Plan”). The 2021 Plan provides for the issuance of stock options, restricted stock units and other awards to employees, directors and consultants of the Company. Shares of common stock underlying outstanding awards under the 2019 Plan (defined below) and the 2021 Plan that expire, are forfeited, are retained by the Company to satisfy any exercise price or any tax withholding, repurchased by the Company at their original purchase price or settled in cash may be added to the number of shares of common stock available for issuance under the 2021 Plan. The number of shares reserved for issuance under the 2021 Plan will automatically increase on the first day of each fiscal year, beginning January 1, 2022, in the amount equal to the lesser of (a) 4.5% of the total number of shares of common stock outstanding as of December 31 of the immediately preceding calendar year, (b) 600,000 shares of common stock, or (c) such lesser number of shares as determined by the Board of Directors. On January 1, 2023, the number of shares reserved for issuance under the 2021 Plan automatically increased by 600,000 shares of common stock. The Company also maintains its prior stock option plans adopted in 2010 (the “2010 Plan”) and 2019 (the “2019 Plan”). The Company ceased making awards under the 2010 Plan upon adoption of the 2019 Plan and similarly under the 2019 Plan upon stockholder approval of the 2021 Plan. As of June 30, 2023, there were options to purchase 2,309,853 and 266,500 shares of common stock outstanding under the 2010 Plan and 2019 Plan, respectively. As of June 30, 2023, there were options to purchase 1,474,833 shares of common stock and restricted stock units eligible to vest and settle into 474,271 shares of common stock outstanding under the 2021 Plan. As of June 30, 2023, there were 775,410 shares of common stock available for issuance under future awards granted under the 2021 Plan. Stock Options The Company recognizes stock option compensation expense based on the grant date fair value of the award. The Company issues new common shares for stock options exercised. Stock option activity was as follows:
Stock-based compensation expense related to stock options was $150,849 and $141,773 for the three months ended June 30, 2023 and 2022, respectively. Stock-based compensation expense related to stock options was $333,055 and $332,589 for the six months ended June 30, 2023 and 2022, respectively. Included in the stock-based compensation expense numbers above are stock options to be granted to key opinion leaders which are marked to market at each reporting period with the change in the accrued balance expensed through research and development operating expenses. Stock-based compensation related to the key opinion leaders increased by $2,650 and $1,400 for the three months ended June 30, 2023 and 2022, respectively, and decreased by $6,050 and $11,475 for the six months ended June 30, 2023 and 2022, respectively. The weighted average fair value of options granted during the six months ended June 30, 2023 and 2022 was $2.16 and $1.48 per share, respectively. Restricted Stock Units The Company recognizes restricted stock unit (“RSU”) compensation expense based on the grant date fair value of the award. Each RSU is eligible to vest over time and settle into one newly issued share of Company common stock. RSU activity was as follows:
Stock-based compensation expense related to RSUs was $130,452 and $126,017 for the three months ended June 30, 2023 and 2022, respectively. Stock-based compensation expense related to RSUs was $271,505 and $256,307 for the six months ended June 30, 2023 and 2022, respectively. Employee Stock Purchase Plan The Company accounts for employee stock purchases made under its 2021 Employee Stock Purchase Plan (“ESPP”) using the estimated grant date fair value in accordance with Accounting Standards Codification, Topic 718, Stock Compensation. The Company values ESPP shares using the Black-Scholes model. There were 300,000 shares of common stock initially reserved for issuance under the ESPP. In addition, the ESPP contains a provision which provides for an automatic annual share increase on January 1 of each year, in an amount equal to the lesser of (i) 1% of the total number of shares outstanding as of December 31 of the immediately preceding calendar year, (ii) 200,000 shares or (iii) such number of shares as determined by the Board. As of December 31, 2022, there were 500,000 shares of common stock available for issuance under the ESPP. On January 1, 2023, the number of shares reserved for issuance under the ESPP automatically increased by 200,000 shares of common stock. There were no shares issued under the ESPP during the six months ended June 30, 2023. Stock Warrants Stock warrant activity was as follows:
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
SIGNIFICANT CUSTOMERS |
6 Months Ended |
|---|---|
Jun. 30, 2023 | |
| SIGNIFICANT CUSTOMERS | |
| SIGNIFICANT CUSTOMERS | NOTE 9 — SIGNIFICANT CUSTOMERS The Company had one customer that accounted for 100% of total revenue for the three and six months ended June 30, 2023 and 2022. The current receivable for this customer is included in Receivable from Reprise Biomedical, Inc. (“Reprise”) on the condensed balance sheets. The Company received $8,517 and $3,952 for the three months ended June 30, 2023 and 2022, respectively, and $16,498 and $10,720 for the six months ended June 30, 2023 and 2022, respectively, as royalties related from the spin-out of the Acellular Business to Reprise. As of June 30, 2023 and December 31, 2022, the Company had receivables related to the minimum royalties of $700,436 and $466,934, respectively, from Reprise, but due to the uncertainty regarding collectability the Company fully reserved against the receivable. |
COMMITMENTS AND CONTINGENCIES |
6 Months Ended |
|---|---|
Jun. 30, 2023 | |
| COMMITMENTS AND CONTINGENCIES | |
| COMMITMENTS AND CONTINGENCIES | NOTE 10 — COMMITMENTS AND CONTINGENCIES Patent License Agreement Under an Exclusive Patent License Agreement between the Company and the University, the Company is required to make minimum royalty payments to the University of $500,000 per year. Under the Patent and Know-How License Agreement with Reprise, Reprise has minimum royalty obligations to the Company of $500,000 per year. |
LEASES |
6 Months Ended | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Jun. 30, 2023 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LEASES | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LEASES | NOTE 11 — LEASES The Company leases its corporate headquarters, which houses its research and development operations and office space. The lease term began in August 2021 and is scheduled to terminate in May 2029. The Company has one option to extend the term for a period of five years. The depreciable life of assets and leasehold improvements is limited by the expected lease term. The lease provided a tenant improvement allowance of $1,256,950, which was received by the Company during the first quarter of 2022. The tenant improvement allowance is included in the calculation of the right of use asset and lease liability. The Company also leases pieces of equipment that are accounted for as financing leases. Financing lease assets are classified as lab equipment within property and equipment on the condensed balance sheets. Supplemental condensed balance sheet information for the Company is as follows:
Information on the Company’s lease costs is as follows:
Future payments for the Company’s leases are as follows:
Additional information related to leases is as follows:
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
NET LOSS PER SHARE |
6 Months Ended | |||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Jun. 30, 2023 | ||||||||||||||||||||||||||||||||||||
| NET LOSS PER SHARE | ||||||||||||||||||||||||||||||||||||
| NET LOSS PER SHARE | NOTE 12 — NET LOSS PER SHARE Basic net loss per share is calculated by dividing net income by the weighted average number of common shares outstanding during the period. Diluted net loss per common share is calculated by dividing the weighted average number of common shares outstanding, after taking into consideration all dilutive potential shares outstanding during the period. Due to the existence of net losses for the three and six months ended June 30, 2023 and 2022, basic and diluted net loss per share were the same, as the effect of potentially dilutive securities would have been anti-dilutive. The following potentially dilutive securities outstanding have been excluded from the computations of diluted weighted average shares outstanding because such securities would have had an antidilutive impact due to losses reported for the periods presented:
|
|||||||||||||||||||||||||||||||||||
SUBSEQUENT EVENTS |
6 Months Ended |
|---|---|
Jun. 30, 2023 | |
| SUBSEQUENT EVENTS | |
| SUBSEQUENT EVENTS | NOTE 13 — SUBSEQUENT EVENTS On July 31, 2023, the Company received cash payments totaling $457,143 of the $527,143 employee retention receivable. |
DESCRIPTION OF BUSINESS AND SIGNIFICANT ACCOUNTING POLICIES (Policies) |
6 Months Ended |
|---|---|
Jun. 30, 2023 | |
| DESCRIPTION OF BUSINESS AND SIGNIFICANT ACCOUNTING POLICIES | |
| Basis of Preparation | Basis of Preparation The accompanying unaudited condensed financial statements of the Company have been prepared in accordance with accounting principles generally accepted in the United States of America (“U.S. GAAP”) and the rules of the United States (“U.S.”) Securities and Exchange Commission applicable to interim reports of companies filing as a smaller reporting company. These condensed financial statements should be read in conjunction with the audited financial statements and notes thereto contained in the Company’s Annual Report on Form 10-K for the fiscal year ended December 31, 2022. In the opinion of management, the accompanying condensed financial statements reflect all normal recurring adjustments necessary to present fairly the financial position, results of operations, stockholders’ equity and cash flows for the interim periods but are not necessarily indicative of the results of operations or cash flows to be anticipated for the full year 2023 or any future period. The Company has evaluated subsequent events occurring after the date of the condensed financial statements for events requiring recording or disclosure in the condensed financial statements. |
| Reclassifications | Reclassifications Certain reclassifications to previously reported financial information on the Condensed Balance Sheets and Condensed Statements of Operations have been made to conform to the current period presentation. |
| Use of Estimates | Use of Estimates The preparation of financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates. |
| Employee Retention Credit - CARES Act | Employee Retention Credit Under the provisions of the extension of the Coronavirus Aid, Relief, and Economic Security Act, the Company is eligible for a refundable employee retention credit subject to satisfaction of certain eligibility criteria. The Company qualified for the employee retention credit for the first three quarters of 2020 and the second and third quarters of 2021. The Company recognized $527,143 during the six months ended June 30, 2023, consistent with guidance from International Accounting Standards 20, Accounting for Government Grants and Disclosure of Government Assistance, where the Company must have substantially met the program’s eligibility conditions to record revenue. It was reported as an employee retention credit receivable on the Condensed Balance Sheet and employee retention credit other income on the Condensed Statement of Operations for the six months ended June 30, 2023. |
| Emerging Growth Company Status | Emerging Growth Company Status The Company is an emerging growth company, as defined in the Jumpstart Our Business Startups Act of 2012 (“JOBS Act”). Under the JOBS Act, emerging growth companies can delay adopting new or revised accounting standards issued subsequent to the enactment of the JOBS Act, until such time as those standards apply to private companies. The Company has elected to use this extended transition period for complying with new or revised accounting standards that have different effective dates for public and private companies until the earlier of the date that it (i) is no longer an emerging growth company or (ii) affirmatively and irrevocably opts out of the extended transition period provided in the JOBS Act. As a result, these condensed financial statements may not be comparable to companies that comply with the new or revised accounting pronouncements as of public company effective dates. |
| Recently Adopted Accounting Pronouncements | Recent Accounting Pronouncements Recently Adopted Accounting Pronouncements The FASB issued ASU No. 2016-13, Measurement of Credit Losses on Financial Instruments and an updated ASU 2018-19 that clarifies the scope of the standard in the amendments in ASU 2016-13. This guidance introduces a new model for recognizing credit losses on financial instruments based on an estimate of current expected credit losses. Financial instruments impacted are trade and other receivables, held-to-maturity debt securities, loans and other instruments. The Company adopted the standard effective January 1, 2023, using the modified retrospective approach. The adoption did not have an impact on the Company's financial statements. |
INVESTMENTS (Tables) |
6 Months Ended | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Jun. 30, 2023 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| INVESTMENTS | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Schedule of amortized cost and estimated fair values of investments |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PROPERTY AND EQUIPMENT (Tables) |
6 Months Ended | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Jun. 30, 2023 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PROPERTY AND EQUIPMENT, NET | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Schedule of lab equipment, furniture, leasehold improvements and construction in progress |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
ACCRUED EXPENSES (Tables) |
6 Months Ended | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Jun. 30, 2023 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ACCRUED EXPENSES | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Schedule of current accrued expenses |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
DEBT (Tables) |
6 Months Ended | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Jun. 30, 2023 | |||||||||||||||||||||||||||||
| DEBT | |||||||||||||||||||||||||||||
| Schedule of future principal maturities for debt |
|
||||||||||||||||||||||||||||
EQUITY (Tables) |
6 Months Ended | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Jun. 30, 2023 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Share-based compensation | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Schedule of stock option activity | Stock option activity was as follows:
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Schedule of stock warrant activity |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Restricted stock units | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Share-based compensation | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Summary of restricted stock unit activity | RSU activity was as follows:
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
LEASES (Tables) |
6 Months Ended | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Jun. 30, 2023 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LEASES | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Schedule of supplemental condensed balance sheet information |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Schedule of lease costs |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Schedule of future payments, Operating leases | Future payments for the Company’s leases are as follows:
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Schedule of future payments, Financing leases |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Schedule of lease term and discount rate |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
NET LOSS PER SHARE (Tables) |
6 Months Ended | |||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Jun. 30, 2023 | ||||||||||||||||||||||||||||||||||||
| NET LOSS PER SHARE | ||||||||||||||||||||||||||||||||||||
| Schedule of potentially dilutive securities |
|
|||||||||||||||||||||||||||||||||||
DESCRIPTION OF BUSINESS AND SIGNIFICANT ACCOUNTING POLICIES - Employee Retention Credit (Details) |
6 Months Ended |
|---|---|
|
Jun. 30, 2023
USD ($)
| |
| DESCRIPTION OF BUSINESS AND SIGNIFICANT ACCOUNTING POLICIES | |
| Employee retention credit | $ 527,143 |
GOING CONCERN (Details) - USD ($) |
6 Months Ended | |
|---|---|---|
Jun. 30, 2023 |
Dec. 31, 2022 |
|
| GOING CONCERN | ||
| Accumulated deficit | $ (118,022,483) | $ (104,011,911) |
| Substantial Doubt about Going Concern, within One Year | true |
INVESTMENTS - Amortized Cost and Estimated Fair Value (Details) |
Jun. 30, 2023
USD ($)
|
|---|---|
| Investments | |
| Amortized Cost | $ 15,828,281 |
| Unrealized Holding Losses | 50,181 |
| Fair Value | 15,778,100 |
| U.S. Treasury notes, Short-term | |
| Investments | |
| Amortized Cost | 15,828,281 |
| Unrealized Holding Losses | 50,181 |
| Fair Value | $ 15,778,100 |
PROPERTY AND EQUIPMENT, NET (Details) - USD ($) |
3 Months Ended | 6 Months Ended | |||
|---|---|---|---|---|---|
Jun. 30, 2023 |
Jun. 30, 2022 |
Jun. 30, 2023 |
Jun. 30, 2022 |
Dec. 31, 2022 |
|
| Property and equipment, net | |||||
| Property and equipment, gross | $ 7,425,085 | $ 7,425,085 | $ 7,392,550 | ||
| Less accumulated depreciation and amortization | (2,426,223) | (2,426,223) | (1,846,856) | ||
| Property and equipment, net | 4,998,862 | 4,998,862 | 5,545,694 | ||
| Depreciation and amortization | 289,439 | $ 273,610 | 579,367 | $ 536,507 | |
| Lab equipment | |||||
| Property and equipment, net | |||||
| Property and equipment, gross | 2,009,971 | 2,009,971 | 1,977,436 | ||
| Leasehold improvements | |||||
| Property and equipment, net | |||||
| Property and equipment, gross | 3,392,220 | 3,392,220 | 3,392,220 | ||
| Furniture and fixtures & computers | |||||
| Property and equipment, net | |||||
| Property and equipment, gross | $ 2,022,894 | $ 2,022,894 | $ 2,022,894 | ||
ACCRUED EXPENSES (Details) - USD ($) |
Jun. 30, 2023 |
Dec. 31, 2022 |
|---|---|---|
| ACCRUED EXPENSES | ||
| Wages | $ 1,174,792 | $ 1,434,675 |
| Pre-clinical study costs | 393,103 | 200,000 |
| Research and development consulting | 109,200 | |
| Taxes | 75,000 | 112,974 |
| Legal | 25,000 | 80,794 |
| Key opinion leader compensation | 12,650 | 18,700 |
| Royalties | 3,422 | |
| Other | 126,359 | 97,811 |
| Accrued expenses | $ 1,916,104 | $ 1,948,376 |
DEBT - Agreements (Details) - USD ($) |
1 Months Ended | ||
|---|---|---|---|
Jan. 31, 2019 |
Jun. 30, 2023 |
Dec. 31, 2022 |
|
| Debt | |||
| Long-term debt outstanding | $ 385,997 | ||
| Warrants, University of Minnesota | |||
| Debt | |||
| Term of warrant | 10 years | ||
| Number of warrants issued | 20,587 | ||
| Warrant exercise price (in dollars per share) | $ 3.75 | ||
| Promissory Note, University of Minnesota, Due January 31, 2025 | |||
| Debt | |||
| Face value of debt | $ 385,997 | ||
| Interest rate (as a percent) | 6.00% | ||
| Long-term debt outstanding | $ 385,997 | $ 385,997 |
DEBT - Debt Maturities (Details) - USD ($) |
Jun. 30, 2023 |
Dec. 31, 2022 |
|---|---|---|
| Amounts Due in the Twelve Months Ending June 30 | ||
| 2025 | $ 385,997 | |
| Total future maturities payments | 385,997 | |
| Long-term debt | $ 385,997 | $ 385,997 |
EQUITY - Preferred Stock (Details) - $ / shares |
Jun. 30, 2023 |
Dec. 31, 2022 |
|---|---|---|
| EQUITY | ||
| Preferred stock , authorized (in shares) | 10,000,000 | 10,000,000 |
| Preferred stock, par value (in dollars per share) | $ 0.00001 | $ 0.00001 |
| Preferred stock, issued (in shares) | 0 | 0 |
EQUITY - Equity Incentive Plans (Details) - shares |
Jan. 01, 2023 |
Jan. 01, 2022 |
Jun. 30, 2023 |
Dec. 31, 2022 |
|---|---|---|---|---|
| Share-based compensation | ||||
| Options outstanding (in shares) | 4,051,186 | 3,831,686 | ||
| 2010 Plan | ||||
| Share-based compensation | ||||
| Options outstanding (in shares) | 2,309,853 | |||
| 2019 Plan | ||||
| Share-based compensation | ||||
| Options outstanding (in shares) | 266,500 | |||
| 2021 Plan | ||||
| Share-based compensation | ||||
| Maximum annual increase in shares reserved for issuance, as a percent of total shares of common stock outstanding | 4.50% | |||
| Maximum number of additional shares reserved for issuance annually | 600,000 | |||
| Increase in number of shares reserved for issuance during the period | 600,000 | |||
| Options outstanding (in shares) | 1,474,833 | |||
| Number of shares of common stock available for issuance | 775,410 | |||
| 2021 Plan | Restricted stock units | ||||
| Share-based compensation | ||||
| Units outstanding (in shares) | 474,271 |
EQUITY - Restricted Stock Unit Activity (Details) - Restricted stock units - USD ($) |
3 Months Ended | 6 Months Ended | |||
|---|---|---|---|---|---|
Jun. 30, 2023 |
Jun. 30, 2022 |
Jun. 30, 2023 |
Jun. 30, 2022 |
Dec. 31, 2022 |
|
| Share-based compensation | |||||
| Number of common shares issued upon vesting of each RSU | 1 | ||||
| Summary of RSU activity, Shares | |||||
| Unvested at beginning of the year | 239,198 | ||||
| Granted (in shares) | 381,005 | ||||
| Vested (in shares) | (116,444) | ||||
| Canceled (in shares) | (29,488) | ||||
| Unvested at end of the year | 474,271 | 474,271 | |||
| Summary of RSU activity, Weighted Average Grant Date Fair Value | |||||
| Granted, Weighted average grant date fair value (in dollars per share) | $ 1.60 | ||||
| Vested, Weighted average grant date fair value (in dollars per share) | 3.62 | ||||
| Canceled, Weighted average grant date fair value (in dollars per share) | 4.59 | ||||
| Unvested, Weighted average grant date fair value (in dollars per share) | $ 2.23 | $ 2.23 | $ 4.20 | ||
| Stock based compensation | $ 130,452 | $ 126,017 | $ 271,505 | $ 256,307 | |
EQUITY - Employee Stock Purchase Plan (Details) - 2021 ESPP - shares |
6 Months Ended | 12 Months Ended | ||
|---|---|---|---|---|
Jan. 01, 2023 |
Jun. 30, 2023 |
Dec. 31, 2021 |
Dec. 31, 2022 |
|
| Share-based compensation | ||||
| Number of shares reserved for issuance | 300,000 | |||
| Maximum annual increase in shares reserved for issuance, as a percent of total shares of common stock outstanding | 1.00% | |||
| Maximum number of additional shares reserved for issuance annually | 200,000 | |||
| Stock available for issuance (in shares) | 500,000 | |||
| Automatic increase in shares reserved for issuance (in shares) | 200,000 | |||
| Number of shares issued, ESPP | 0 |
EQUITY - Stock Warrant Activity (Details) - $ / shares |
6 Months Ended | |
|---|---|---|
Jun. 30, 2023 |
Dec. 31, 2022 |
|
| Summary of stock warrant activity, Shares | ||
| Warrants outstanding, beginning balance (in shares) | 599,191 | |
| Expired (in shares) | (2,604) | |
| Warrants outstanding, ending balance (in shares) | 596,587 | |
| Summary of stock warrant activity, Weighted Average Exercise Price | ||
| Expired (in dollars per share) | $ 2.50 | |
| Outstanding (in dollars per share) | $ 6.18 | $ 6.16 |
SIGNIFICANT CUSTOMERS (Details) |
3 Months Ended | 6 Months Ended | |||
|---|---|---|---|---|---|
|
Jun. 30, 2023
USD ($)
customer
|
Jun. 30, 2022
USD ($)
customer
|
Jun. 30, 2023
USD ($)
customer
|
Jun. 30, 2022
USD ($)
customer
|
Dec. 31, 2022
USD ($)
|
|
| SIGNIFICANT CUSTOMERS | |||||
| Net sales | $ 8,517 | $ 3,952 | $ 16,498 | $ 10,720 | |
| Reprise Biomedical, Inc. | Patent and Know-How License Agreement | |||||
| SIGNIFICANT CUSTOMERS | |||||
| Net sales | 8,517 | $ 3,952 | 16,498 | $ 10,720 | |
| Long term receivables | $ 700,436 | $ 700,436 | $ 466,934 | ||
| Revenue | Customer | One customer | |||||
| SIGNIFICANT CUSTOMERS | |||||
| Number of customers | customer | 1 | 1 | 1 | 1 | |
| Concentration risk, percentage | 100.00% | 100.00% | 100.00% | 100.00% | |
COMMITMENTS AND CONTINGENCIES - Patent License Agreement (Details) |
Jun. 30, 2023
USD ($)
|
|---|---|
| University | Patent License Agreement | |
| Agreements | |
| Minimum royalty payments to be paid per year | $ 500,000 |
| Reprise Biomedical, Inc. | Patent and Know-How License Agreement | |
| Agreements | |
| Minimum royalty payments to be received per year from related party | $ 500,000 |
LEASES - Agreements (Details) - Building |
1 Months Ended | 3 Months Ended |
|---|---|---|
|
Aug. 31, 2021
item
|
Mar. 31, 2022
USD ($)
|
|
| Leases | ||
| Number of options to extend | item | 1 | |
| Option to extend term | 5 years | |
| Tenant improvement allowance received during the period | $ | $ 1,256,950 |
LEASES - Balance Sheet Information (Details) - USD ($) |
Jun. 30, 2023 |
Dec. 31, 2022 |
|---|---|---|
| Assets | ||
| Operating lease assets, Right of use asset | $ 1,570,837 | $ 1,673,575 |
| Financing lease assets | $ 62,366 | 81,325 |
| Finance Lease, Right-of-Use Asset, Statement of Financial Position | Property and equipment, net | |
| Liabilities | ||
| Current Operating - Current portion of lease liability | $ 405,566 | 389,649 |
| Current Financing - Current portion of financing lease obligations | 22,506 | 44,157 |
| Noncurrent Operating - Lease liability, net | 2,512,062 | 2,720,781 |
| Noncurrent Financing - Financing lease obligations, net | $ 3,964 | $ 11,689 |
LEASES - Lease costs (Details) - USD ($) |
3 Months Ended | 6 Months Ended | ||
|---|---|---|---|---|
Jun. 30, 2023 |
Jun. 30, 2022 |
Jun. 30, 2023 |
Jun. 30, 2022 |
|
| Lease cost | ||||
| Operating lease cost - Operating expenses: General and administrative | $ 82,885 | $ 82,885 | $ 165,771 | $ 163,569 |
| Financing lease cost | ||||
| Amortization of leased assets - Depreciation and amortization | 9,479 | 9,479 | 18,959 | 18,959 |
| Interest on lease liabilities - Interest expense | 613 | 1,228 | 1,463 | 2,943 |
| Variable lease cost - Operating expenses: General and administrative | $ 56,145 | $ 57,090 | $ 96,245 | $ 96,319 |
LEASES - Lease payments (Details) |
Jun. 30, 2023
USD ($)
|
|---|---|
| Operating Leases | |
| Remainder of 2023 | $ 255,835 |
| 2024 | 527,020 |
| 2025 | 542,830 |
| 2026 | 559,115 |
| 2027 | 575,889 |
| Thereafter | 847,731 |
| Total lease payments | 3,308,420 |
| Less imputed interest | (390,792) |
| Present value of lease liabilities | $ 2,917,628 |
| Operating Lease, Liability, Statement of Financial Position | Current portion of lease liability, Lease liability, net |
| Financing Leases | |
| 2023, Remainder of fiscal year | $ 15,450 |
| 2024 | 12,030 |
| Total lease payments | 27,480 |
| Less imputed interest | (1,010) |
| Present value of lease liabilities | $ 26,470 |
| Finance Lease, Liability, Statement of Financial Position | Current portion of financing lease obligations, Financing lease obligations, net |
| Total Operating and Financing Leases | |
| 2023, Remainder of fiscal year | $ 271,285 |
| 2024 | 539,050 |
| 2025 | 542,830 |
| 2026 | 559,115 |
| 2027 | 575,889 |
| Thereafter | 847,731 |
| Total lease payments | 3,335,900 |
| Less imputed interest | (391,802) |
| Present value of lease liabilities | $ 2,944,098 |
LEASES - Additional information (Details) |
Jun. 30, 2023 |
|---|---|
| LEASES | |
| Weighted-average remaining term (in years) - Operating leases | 5 years 10 months 24 days |
| Weighted-average remaining term (in years) - Financing leases | 10 months 24 days |
| Weighted-average discount rate - Operating lease (as a percent) | 4.20% |
| Weighted-average discount rate - Financing lease (as a percent) | 6.90% |
NET LOSS PER SHARE (Details) - shares |
6 Months Ended | |
|---|---|---|
Jun. 30, 2023 |
Jun. 30, 2022 |
|
| NET LOSS PER SHARE | ||
| Potentially dilutive securities | 5,122,044 | 4,746,482 |
| Employee Stock Option [Member] | ||
| NET LOSS PER SHARE | ||
| Potentially dilutive securities | 4,051,186 | 3,954,756 |
| Restricted stock units | ||
| NET LOSS PER SHARE | ||
| Potentially dilutive securities | 474,271 | 192,535 |
| Common stock warrants | ||
| NET LOSS PER SHARE | ||
| Potentially dilutive securities | 596,587 | 599,191 |
SUBSEQUENT EVENTS (Details) - USD ($) |
Jul. 31, 2023 |
Jun. 30, 2023 |
|---|---|---|
| SUBSEQUENT EVENTS | ||
| Employee retention credit receivable | $ 527,143 | |
| Subsequent event | ||
| SUBSEQUENT EVENTS | ||
| Cash proceeds related to employee retention receivable | $ 457,143 |
Pay vs Performance Disclosure - USD ($) |
3 Months Ended | 6 Months Ended | ||
|---|---|---|---|---|
Jun. 30, 2023 |
Jun. 30, 2022 |
Jun. 30, 2023 |
Jun. 30, 2022 |
|
| Pay vs Performance Disclosure | ||||
| Net Income (Loss) | $ (6,530,420) | $ (8,182,189) | $ (14,010,572) | $ (15,385,938) |
Insider Trading Arrangements |
3 Months Ended |
|---|---|
Jun. 30, 2023 | |
| Trading Arrangements, by Individual | |
| Rule 10b5-1 Arrangement Adopted | false |
| Non-Rule 10b5-1 Arrangement Adopted | false |
| Rule 10b5-1 Arrangement Terminated | false |
| Non-Rule 10b5-1 Arrangement Terminated | false |
1 Year MiroMatrix Medical Chart |
1 Month MiroMatrix Medical Chart |

It looks like you are not logged in. Click the button below to log in and keep track of your recent history.
Support: +44 (0) 203 8794 460 | support@advfn.com
By accessing the services available at ADVFN you are agreeing to be bound by ADVFN's Terms & Conditions