
We could not find any results for:
Make sure your spelling is correct or try broadening your search.
| Share Name | Share Symbol | Market | Type |
|---|---|---|---|
| Impax Laboratories, Inc. (delisted) | NASDAQ:IPXL | NASDAQ | Common Stock |
| Price Change | % Change | Share Price | Bid Price | Offer Price | High Price | Low Price | Open Price | Shares Traded | Last Trade | |
|---|---|---|---|---|---|---|---|---|---|---|
| 0.00 | 0.00% | 18.30 | 18.30 | 18.55 | 0 | 01:00:00 |
HAYWARD, Calif., Aug. 26, 2016 /PRNewswire/ -- Impax Laboratories, Inc. (NASDAQ: IPXL) announced today that the Company had issued a voluntary nationwide retail level recall on August 19, 2016 for one lot of Lamotrigine Orally Disintegrating Tablet (ODT) 200 mg. The Company is issuing this press release to provide further heightened awareness of this recall and to provide instructions to consumers, pharmacists, and wholesalers in possession of the affected product. Details related to the recalled product are set forth below.
|
NDC Number |
Blister Pack |
Lot Number |
|
0115-1529-08 |
30 |
502240 |
Unit-of-use blister packs (a 10 count blister card contained in a single plastic shell-pack) may contain 100 mg product instead of 200 mg product. Each blister card within the unit-of-use blister pack is properly labeled as 100 mg ODT, however the plastic shell pack containing the 100 mg blister cards is labeled as 200 mg ODT. Shell-packs from the affected lot may contain 100 mg ODT instead of 200 mg ODT, and as a result, it is possible that consumers could take less than their intended lamotrigine dose.
Lamotrigine is indicated for the treatment of epilepsy or bipolar disorders. It is important for consumers to take the dose of lamotrigine prescribed by their physicians. A reduction in dose may lead to reduced therapeutic effects of lamotrigine and reemergence of epilepsy or bipolar disorder symptoms.
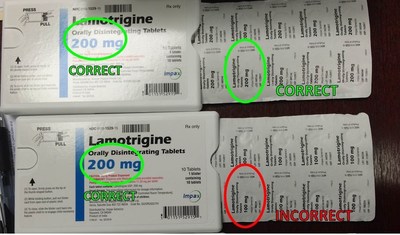
The affected lot was distributed between June 13, 2016 and August 10, 2016 to wholesale distributors and retail pharmacies nationwide. The lot number can be found on the side of the manufacturer's carton as well as on the blister cards within the unit-of-use blister packs. Lamotrigine ODT 200 mg tablets are off-white to white circular tablets, approximately 14 mm in diameter, debossed with "WPI" on one side and "3724" on the other, supplied in blisters of 10 (NDC 0115-1529-15) and in cartons of 30 (NDC 0115-1529-08).
Consumers are being asked to carefully inspect their medication. If they have the affected lot or any questions or concerns regarding this recall they should contact Stericycle at 1-866-300-2207 (Monday through Friday 8:00 a.m. through 5:00 p.m. EST). Consumers who are unsure if they have the affected lot number or have any concerns about their product should consult their pharmacy or health care professional.
Pharmacists and wholesalers are being asked to check their inventories for the affected lot, segregate any material from the lot, and to then contact Stericycle at 1-866-300-2207 for instructions on product return. Pharmacies that received the affected lot will receive a copy of this press release with their recall notification information to be prominently posted in the pharmacy area.
This voluntary recall is being made with the knowledge of the U.S. Food and Drug Administration.
About Impax Laboratories, Inc.
Impax Laboratories, Inc. (Impax) is a specialty pharmaceutical company applying its formulation expertise and drug delivery technology to the development of controlled-release and specialty generics in addition to the development of central nervous system disorder branded products. Impax markets its generic products through its Impax Generics division and markets its branded products through the Impax Specialty Pharma division. Additionally, where strategically appropriate, Impax develops marketing partnerships to fully leverage its technology platform and pursues partnership opportunities that offer alternative dosage form technologies, such as injectables, nasal sprays, inhalers, patches, creams, and ointments.
Contacts:
Consumer:
1-866-300-2207
Media:
Mark Donohue
1-215-558-4526
Photo - http://photos.prnewswire.com/prnh/20160826/401865
To view the original version on PR Newswire, visit:http://www.prnewswire.com/news-releases/impax-laboratories-inc--issues-voluntary-nationwide-recall-for-one-lot-of-lamotrigine-orally-disintegrating-tablet-200-mg-due-to-the-potential-for-100-mg-blister-cards-being-packaged-in-200-mg-containers-300318991.html
SOURCE Impax Laboratories, Inc.

Copyright 2016 PR Newswire
1 Year Impax Laboratories, Inc. (delisted) Chart |
1 Month Impax Laboratories, Inc. (delisted) Chart |

It looks like you are not logged in. Click the button below to log in and keep track of your recent history.
Support: +44 (0) 203 8794 460 | support@advfn.com
By accessing the services available at ADVFN you are agreeing to be bound by ADVFN's Terms & Conditions