
We could not find any results for:
Make sure your spelling is correct or try broadening your search.
| Share Name | Share Symbol | Market | Type |
|---|---|---|---|
| Eloxx Pharmaceuticals Inc | NASDAQ:ELOX | NASDAQ | Common Stock |
| Price Change | % Change | Share Price | Bid Price | Offer Price | High Price | Low Price | Open Price | Shares Traded | Last Trade | |
|---|---|---|---|---|---|---|---|---|---|---|
| 0.00 | 0.00% | 3.82 | 3.60 | 3.75 | 0 | 00:00:00 |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported):
(Exact name of registrant as specified in its charter)
|
(State or other jurisdiction of incorporation) |
(Commission File Number) |
(I.R.S. Employer Identification No.) |
|
|
||
| (Address of principal executive offices) | (Zip Code) |
(Registrant’s telephone number,
including area code): (
N/A
(Former name or former address, if changed since last report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class | Trading Symbol(s) |
Name of each exchange on which registered |
| The |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging
growth company
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ¨
| Item 8.01 | Other Events. |
As previously announced, on June 27, 2023, Eloxx Pharmaceuticals, Inc. (the “Company”) hosted an investor and analyst call (“KOL Day”) featuring key opinion leaders to review insights on Alport syndrome and to review the Company’s additional data from its ELX-02 Phase 2 clinical trial for the potential treatment of Alport syndrome. Select slides included in the presentation materials used at the KOL Day are filed as Exhibit 99.1 hereto and incorporated by reference herein.
| Item 9.01 | Financial Statements and Exhibits. |
(d) Exhibits.
|
Exhibit |
Description | |
| 99.1 | Slides from KOL Day, dated June 27, 2023. | |
| 104 | Cover Page Interactive Data File (embedded within the inline XBRL document). | |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| Date: July 13, 2023 | ELOXX PHARMACEUTICALS, INC. | |
| By: |
/s/ Sumit Aggarwal | |
| Name: | Sumit Aggarwal | |
| Title: | President and Chief Executive Officer | |
Exhibit 99.1

/ 1 Baseline characteristics of patients that have completed therapy Patient Age Sex COl4 Gene Affected Nonsense Mutation RAAS Block dose Cr (mg/dL) Proteinuria (mg/g) 4401 - 01 13 Male COL4A4 c.2906C>G*; p.Ser969X Enalapril 2.5 mg QD 0.7 1299 4401 - 02 13 Male COL4A4 c.2906C>G*; p.Ser969X Enalapril 32.5 mg QD 0.5 1646 4402 - 01 19 Female COL4A4 c.2906C>G*; p.Ser969X Enalapril 5 mg QD 1.31 1645 * Most common mutation in the UK Patients had autosomal recessive disease with differing levels of background RAAS blockade

/ 2 Phase 2 Alport patient results to date 539 678 1,299 1,646 1,659 1,799 850 2,209 0 500 1000 1500 2000 2500 3000 3500 4401-01 4401-02 4402-01 Urine Protein/Urine Creatinine (mg/g) -1 year Baseline Average of treatment period* * UPCR averaged over 6 values collected in 8 weeks for 4401 - 01 and 4401 - 02. UPCR values collected for 4401 - 01 and 4401 - 02 at wee k 6 were excluded as they were deemed to be unreliable due to inconsistent processing during Easter holidays and inconsistency with the clinical presentation. All 8 UPCR values inc lud ed for 4401 - 02 Remission in one Alport patient with an approx. 50% reduction from baseline p=0.189 - 49% p=0.009 Patient 4401 - 02 achieved partial remission after completing 8 weeks of treatment • Average reduction of baseline ~50% • 5 out of 8 UPCR readings were on average 53% below baseline p=0.15
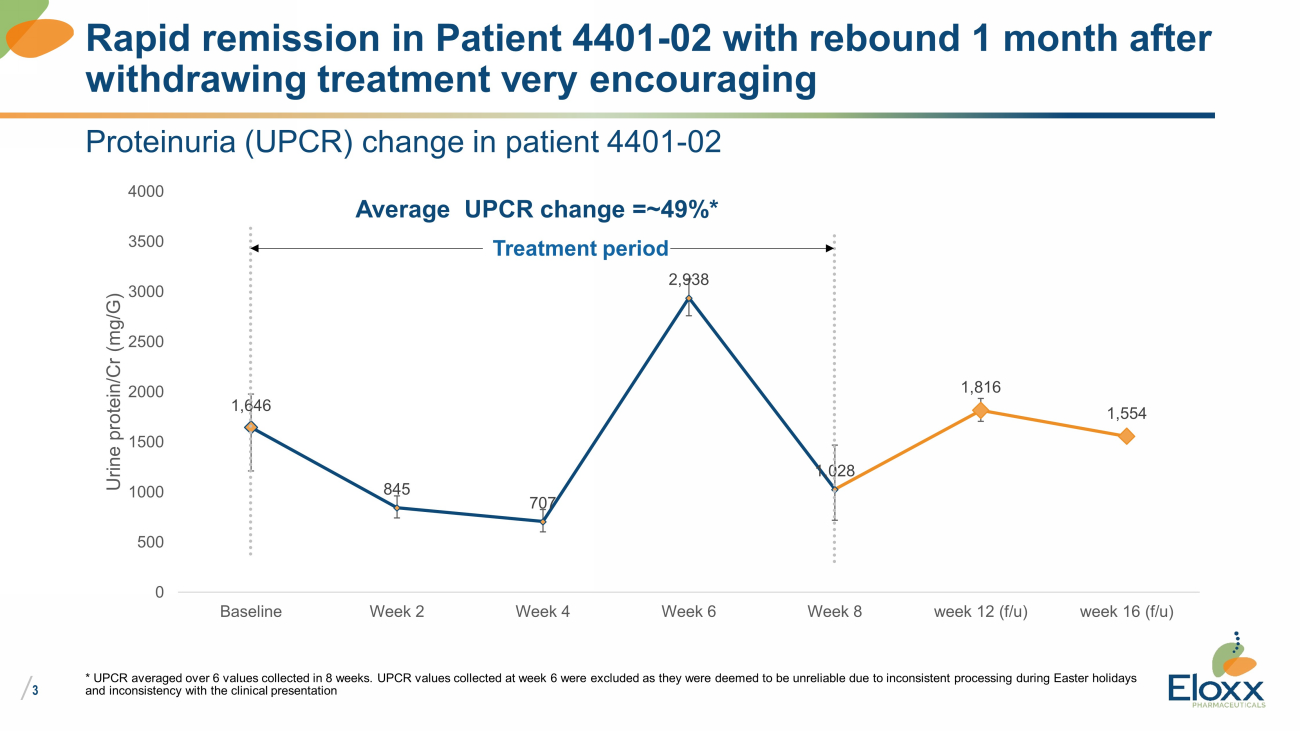
/ 3 Proteinuria (UPCR) change in patient 4401 - 02 1,646 845 707 2,938 1,028 1,816 1,554 0 500 1000 1500 2000 2500 3000 3500 4000 Baseline Week 2 Week 4 Week 6 Week 8 week 12 (f/u) week 16 (f/u) Urine protein/Cr (mg/G) * UPCR averaged over 6 values collected in 8 weeks. UPCR values collected at week 6 were excluded as they were deemed to be u nre liable due to inconsistent processing during Easter holidays and inconsistency with the clinical presentation Rapid remission in Patient 4401 - 02 with rebound 1 month after withdrawing treatment very encouraging Treatment period Average UPCR change =~49%*

/ 4 Proteinuria change in patient 4401 - 01 and 4402 - 01 Patient 4401 - 01 UPCR change over treatment Patient 4402 - 01 UPCR change over treatment 1,659 1,403 3,522 1,828 2,635 1,280 0 500 1000 1500 2000 2500 3000 3500 4000 Baseline Week 2 Week 4 Week 6 Week 8 Week 12 f/u Urine protein/Cr (mg/G) No change in other 2 patients after ELX - 02 treatment 1,299 1,216 1,943 2,155 2,466 2,018 1,846 0 500 1000 1500 2000 2500 3000 3500 4000 Baseline Week 2 Week 4 Week 6 Week 8 Week 12 f/u Week 16 f/u Urine protein/Cr (mg/G) Treatment period Treatment period
Cover |
Jul. 13, 2023 |
|---|---|
| Cover [Abstract] | |
| Document Type | 8-K |
| Amendment Flag | false |
| Document Period End Date | Jul. 13, 2023 |
| Entity File Number | 001-31326 |
| Entity Registrant Name | Eloxx Pharmaceuticals, Inc. |
| Entity Central Index Key | 0001035354 |
| Entity Tax Identification Number | 84-1368850 |
| Entity Incorporation, State or Country Code | DE |
| Entity Address, Address Line One | 480 Arsenal Way, Suite 130 |
| Entity Address, City or Town | Watertown |
| Entity Address, State or Province | MA |
| Entity Address, Postal Zip Code | 02451 |
| City Area Code | 781 |
| Local Phone Number | 577-5300 |
| Written Communications | false |
| Soliciting Material | false |
| Pre-commencement Tender Offer | false |
| Pre-commencement Issuer Tender Offer | false |
| Title of 12(b) Security | Common Stock, $0.01 par value per share |
| Trading Symbol | ELOX |
| Security Exchange Name | NASDAQ |
| Entity Emerging Growth Company | false |
1 Year Eloxx Pharmaceuticals Chart |
1 Month Eloxx Pharmaceuticals Chart |

It looks like you are not logged in. Click the button below to log in and keep track of your recent history.
Support: +44 (0) 203 8794 460 | support@advfn.com
By accessing the services available at ADVFN you are agreeing to be bound by ADVFN's Terms & Conditions