
We could not find any results for:
Make sure your spelling is correct or try broadening your search.
| Share Name | Share Symbol | Market | Type |
|---|---|---|---|
| DMK Pharmaceuticals Corporation | NASDAQ:DMK | NASDAQ | Common Stock |
| Price Change | % Change | Share Price | Bid Price | Offer Price | High Price | Low Price | Open Price | Shares Traded | Last Trade | |
|---|---|---|---|---|---|---|---|---|---|---|
| 0.00 | 0.00% | 0.232 | 0.242 | 0.25 | 0 | 00:00:00 |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM
CURRENT REPORT
PURSUANT TO SECTION 13 OR 15(d) OF THE
SECURITIES EXCHANGE ACT OF 1934
Date of report (Date of earliest event reported):
(Exact Name of Registrant as Specified in Charter)
| (State or other jurisdiction of incorporation) | (Commission File Number) | (IRS Employer Identification No.) |
|
|
||
| (Address of Principal Executive Offices) | (Zip Code) |
Registrant’s telephone number, including
area code:
(Former name or Former Address, if Changed Since Last Report.)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions (see General Instruction A.2. below):
| Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) | |
| Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) | |
| Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) | |
| Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Exchange Act:
| Title of each class | Trading Symbol(s) | Name of each exchange on which registered | ||
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Item 7.01 Regulation FD Disclosure.
On January 3, 2024, DMK Pharmaceuticals Corporation (the “Company”) began making presentations to investors using slides containing the information attached to this Current Report on Form 8-K as Exhibit 99.1 (the “Investor Presentation”). The Company expects to use the Investor Presentation, in whole or in part, and possibly with modifications, in connection with presentations to investors, analysts and others during the fiscal year ending December 31, 2024.
The information furnished pursuant to this Item 7.01 shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), nor shall it be deemed incorporated by reference in any filing under the Securities Act of 1933, as amended, or the Exchange Act, except as expressly set forth by specific reference in such a filing. The information furnished pursuant to this Item 7.01 contains “forward looking statements” within the meaning of the safe harbor provisions of the federal securities laws. It should be read in conjunction with the risk factors included in the Company’s periodic reports filed with the Securities and Exchange Commission and the other public announcements that the Company may make, by press release or otherwise, from time to time.
Item 9.01. Exhibits.
(d)
| Exhibit 99.1 | Investor Presentation |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| DMK PHARMACEUTICALS CORPORATION | ||
| Dated: January 3, 2024 | By: | /s/ Seth A. Cohen |
| Name: | Seth A. Cohen | |
| Title: | Chief Financial Officer | |
EXHIBIT INDEX
| Exhibit No. | Description |
| 99.1 | Investor Presentation |
DMK Pharmaceuticals Corporation 8-K
Exhibit 99.1

DMK Pharmaceuticals Corporation Focused on reducing deaths and addiction January 2024

2

3 Dr. Versi appointed CEO in May 2023 • Large (Lilly, Pfizer, Astellas) and small companies (Odyssey, Plethora, Auxilium, Mt. Cook) • Medical lead at Pfizer – team that created the overactive bladder market o Current OAB market >$5B • Planned and executed clinical studies for multiple approved products • Authored over 100 scientific publications and inventor of several patents EBOO VERSI M.D., Ph.D. CEO Founder, Chairman and CEO Seth Cohen, MBA, CFO Founder, Chairman and CEO Mr. Cohen appointed CFO in October 2023 • 30+ Years in financial management, including multiple CFO roles in public, private equity - backed and private corporations • Former trustee for City of New York’s public pension systems, $100+ billion in assets New management team installed in Oct 2023

4 New Management in October 2023 • Change in direction and strategy: Treatment of substance use disorder. o Focus on opioid crisis by commercializing ZIMHI® and developing DPI - 125 with non - dilutive funding • Business development activities to monetize non - core assets Laser focus on revenue generation & growth to drive stockholder value • Increase U.S. sales of ZIMHI® our lead commercial product o White House and congressional initiatives to lift barriers to purchase ZIMHI with federal grants o Partnerships in US & ex - U.S. for ZIMHI • Out - license SYMJEPI® and other assets that are not core to the addiction business • Execute NIH funded program and continue to obtain non - dilutive government grants for development and discovery (~750 NCE) programs
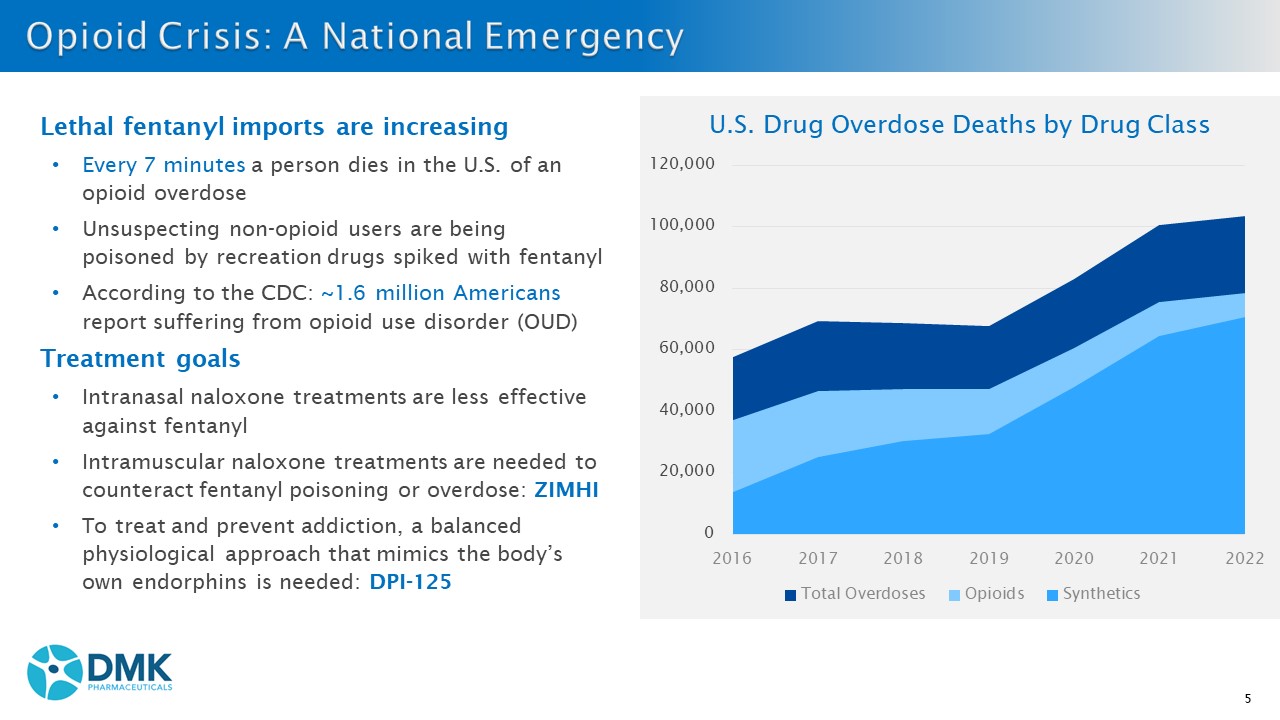
5 Lethal f entanyl imports are increasing • Every 7 minutes a person dies in the U.S. of an opioid overdose • Unsuspecting n on - opioid users are being poisoned by recreation drugs spiked with fentanyl • According to the CDC: ~1.6 million Americans report suffering from opioid use disorder (OUD) Treatment goals • I ntranasal naloxone treatments are less effective against fentanyl • Intramuscular naloxone treatments are needed to counteract fentanyl poisoning or overdose: ZIMHI • To treat and prevent addiction, a balanced physiological approach that mimics the body’s own endorphins is needed: DPI - 125 0 20,000 40,000 60,000 80,000 100,000 120,000 2016 2017 2018 2019 2020 2021 2022 U.S. Drug Overdose Deaths by Drug Class Total Overdoses Opioids Synthetics

6 Our mission: To prevent deaths due to poisoning, overdose, and addiction ZIMHI (naloxone) : T o rapidly reverse fentanyl overdose • M arket in US and Canada exceeds $725M with CAGR of 10.1% to 2032 o Significant market potential, with only two competitors • Intramuscular results in more rapid increase in blood levels than intranasal delivery o More rapid, sustained effect needing fewer administrations: cost effective. DPI - 125 transdermal system for treatment opioid use disorder • US market >$3B addressed by old Rx solutions with limitations of access, efficacy, and safety o NIH - sponsored studies show equal efficacy to treat withdrawal symptoms, with improved safety observed in pre - clinical studies • Opportunity to expand into treatment of acute moderate to severe pain globally o Global pain market >$22B Additional portfolio candidates • Indicated for urology (clinical stage) and Parkinson’s disease and other disorders o Opportunities for out - license revenues
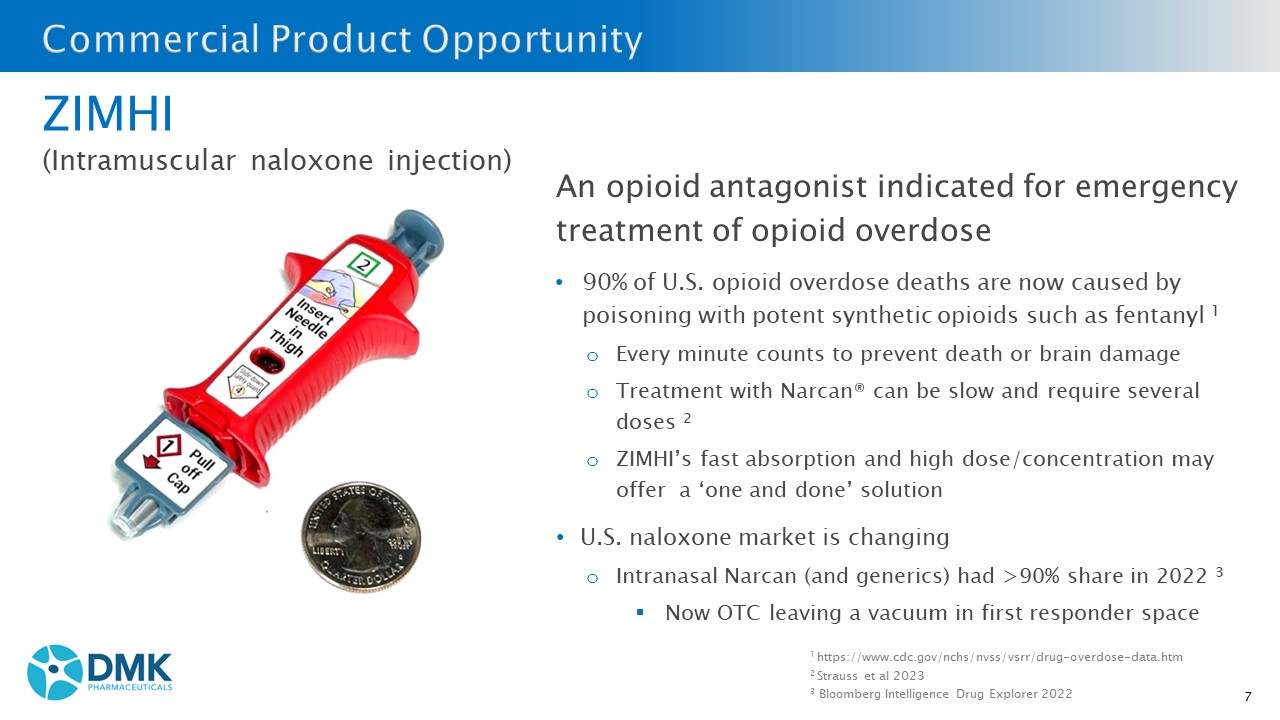
7 ZIMHI (Intramuscular naloxone injection) An opioid antagonist indicated for emergency treatment of opioid overdose • 90% of U.S. opioid overdose deaths are now caused by poisoning with potent synthetic opioids such as fentanyl 1 o Every minute counts to prevent death or brain damage o Treatment with Narcan® can be slow and require several doses 2 o ZIMHI’s fast absorption and high dose/concentration may offer a ‘one and done’ solution • U.S. naloxone market is changing o Intranasal Narcan (and generics) had >90% share in 2022 3 ▪ Now OTC leaving a vacuum in first responder space 1 https://www.cdc.gov/nchs/nvss/vsrr/drug - overdose - data.htm 2 Strauss et al 2023 3 Bloomberg Intelligence Drug Explorer 2022
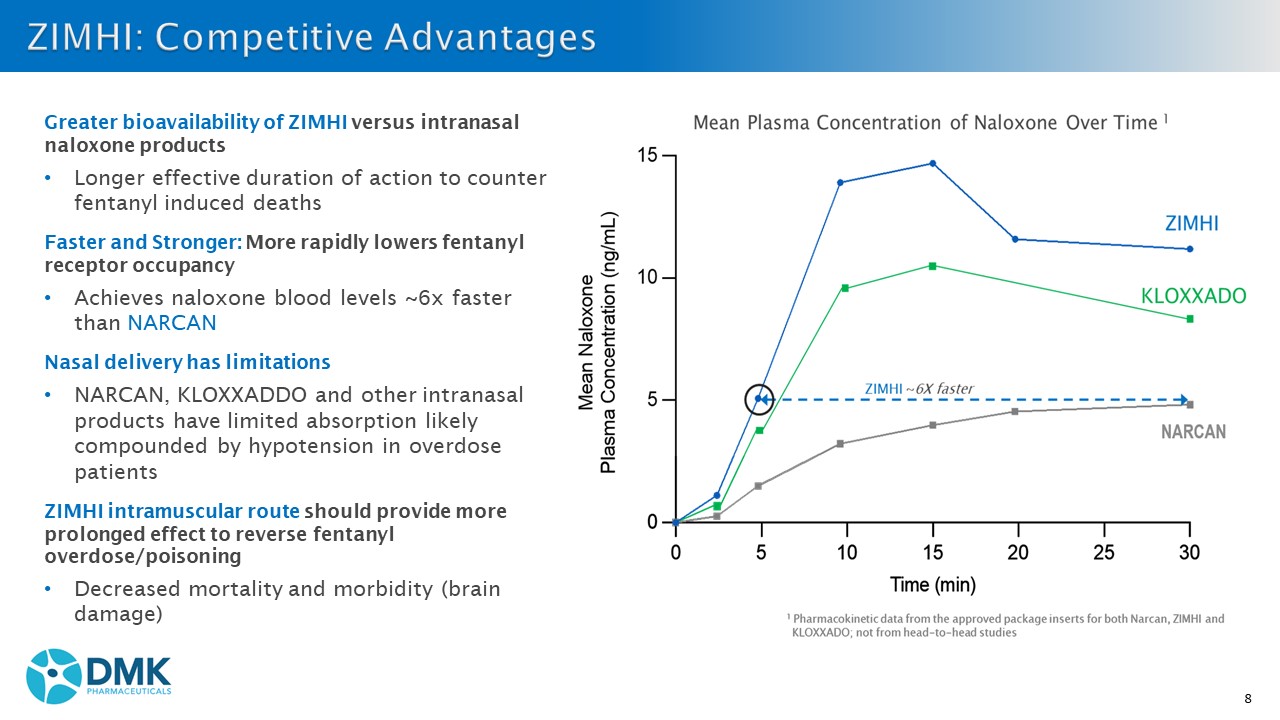
8 Greater bioavailability of ZIMHI versus intranasal naloxone products • Longer effective duration of action to counter fentanyl induced deaths Faster and Stronger: More rapidly lowers fentanyl receptor occupancy • Achieves naloxone blood levels ~6x faster than NARCAN Nasal delivery has limitations • NARCAN, KLOXXADDO and other intranasal products have limited absorption likely compounded by hypotension in overdose patients ZIMHI intramuscular route should provide more prolonged effect to reverse fentanyl overdose/poisoning • Decreased mortality and morbidity (brain damage)
9 Death due to respiratory depression 1 • The more potent the opioid, the more profound the potential for respiratory depression • Fentanyl and its analogues are the most potent opioids • Reversal of fentanyl induced respiratory depression requires higher blood levels of naloxone New data for fentanyl overdose reversal • Results of FDA funded study conducted by Dr. Albert Dahan compared recovery from fentanyl with 1, 2 or 4 doses of Narcan (generic equivalent) 2 o For the most effective recovery, 4 doses should be administered within 2.5 minutes (this is off - label use) o This data is currently in press to be published soon • DMK’s independent PK study suggests that a single dose of ZIMHI would be more effective than the standard of care 3 • Currently a head - to - head study of ZIMHI against Narcan (generic equivalent) as follow up to FDA funded study (Dr. Dahan ) is being performed to attempt to demonstrate superiority in fentanyl overdose reversal 1 Represents opinion of the speaker - based statements in the scientific literature 2 FDA presentation at the Reagan - Udall meeting 3 Represents opinion of the speaker, not based on any specific study

10 Anecdotal data from patients and first responders facing the prevalence of fentanyl poisonings “Prescription strength ZIMHI is the best remedy available for opioid overdose. If we had had some in the house when our son Sammy died, he would still be with us” - Samuel P. Chapman, Director - Parents for Safer Children 1 “…..but with fentanyl, we are needing to use about 3 doses [NARCAN] to achieve a recovery” - David B. Rausch, Director of the Tennessee Bureau of Investigation 1 “He wasn’t waking up, so I gave him a third [NARCAN]…” – Law enforcement perspective: 1 https://healthandjusticejournal.biomedcentral.com/articles/10.1186/s40352 - 022 - 00172 - y “…it can take three to four doses [of NARCAN] to revive somebody because the strength of the fentanyl that people are using right now” - Scott Kerman, Blanchet House Executive Director 1 “It has become apparent that many overdoses require much higher initial doses to reverse…as high as 10 - 12mg of naloxone” 1,2 “In Indiana, Ohio, and Michigan, we are seeing a lot more synthetic drugs and a need for repeat dosing. We are using naloxone more and more…for these super high potent derivatives, Narcan just isn't enough.” 1,2 1 Represents the opinion or observations of individuals and not based on any studies or systematic tests 2 From DMK sponsored third - party market research
11 Already launched strategic government relations campaign to facilitate market access • First win: Invited to private White House meeting with Director of National Drug Policy, Advisor to the President, Assistant Secretaries for HSS and SAMSA in June 2023 – goals and outcomes discussed include: o Implementing naloxone saturation policy o Increasing grant funding to states to purchase more naloxone o Creating guideline templates to roll out to states and municipalities to remove barriers to naloxone access o Director may issue a directive to mandate removal of barriers to naloxone access when using Federal funds 1 • Interactions with Capital Hill o Met with 19 congressional offices, Senators and House members of both parties to explain the cost savings and potential to reduce mortality with ZIMHI based on scientific data o The value proposition of ZIMHI was well received by all offices visited o HR 4007, introduced with bipartisan support, would remove barriers to naloxone access that currently favor NARCAN New targeted publicity campaigns to make purchasers aware of the value of ZIMHI • Leverage completed FDA funded study demonstrating need for higher doses of naloxone to treat fentanyl overdoses • Disseminate survey data on number of Narcan doses needed to achieve recovery • Publicize head - to - head data (ZIMHI vs Narcan/generic), when available 2 1 There is no assurance that any of these measures will actually be implemented 2 Assuming favorable results from this study

Development Candidate DPI - 125 Targets the Opioid Crisis

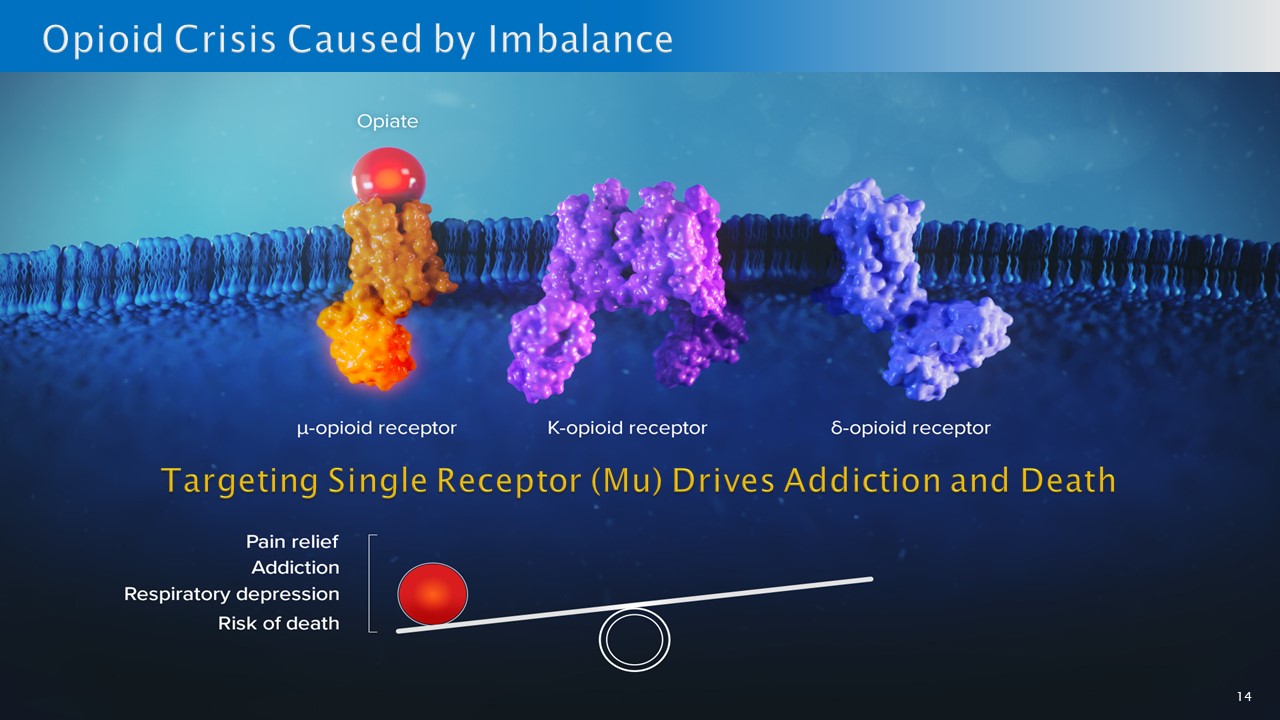
13 Opioid use is a worsening health crisis • Every 7 minutes a person dies in the U.S. of an opioid overdose 1 • According to the CDC: ~1.6 million Americans report suffering from opioid use disorder (OUD) 2 • Rx options include methadone, buprenorphine and naltrexone (all old), but 87% do not receive evidence - based medical treatment 3 Market concerns • Prescription and illicit opioids target the mu opioid receptor, which provides potent pain relief • However, binding only to the mu receptor can lead to addiction and death • A balanced physiological approach that mimics the body’s own endorphins is needed 1 www.cdc.gov/nchs/nvss/vsrr/drug - overdose - data.htm 2 https://www.cdc.gov/stopoverdose/stigma/index.html 3 https://www.sciencedirect.com/science/article/pii/S0955395922002031 4 U.S. Centers for Disease Control and Prevention
14
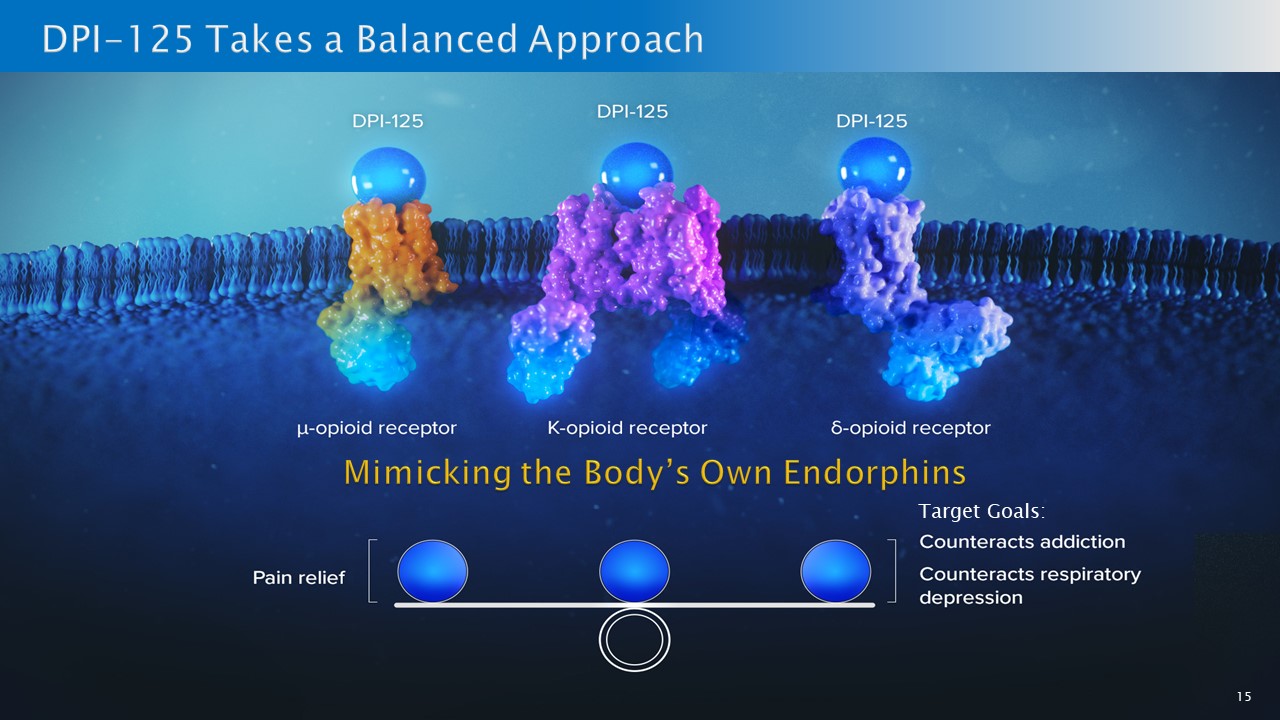
15 Target Goals:

16 New solution: DPI - 125 1 • Clinical stage asset • Animal studies have shown efficacy and safety in terms of respiratory depression and addiction with potent analgesia 2 Alternative treatment for OUD and pain • Current options methadone and buprenorphine (>40 years old) but they work and yet only ~ 13 % get this medical treatment o They are addictive, are difficult to access (drug scheduling) and patients have to undergo withdrawal symptoms. • DPI - 125 is not expected to have these issues so could significantly grow the >$3 billion OUD treatment market 3 o Potential to reduce the OUD crisis by prevention with a safer, effective, non - dependent pain treatment (100 million Americans: $22B market) 4 Assuming adequate funding to develop the product, successful trial results, FDA approval, and subject to market factors, competitive factors and other risks and uncertainties 2 Company’s interpretation of the data from rodent and non - human primate studies 3 Bloomberg Intelligence Drug Explorer 2022 4 SF Examiner, Overdose deaths in S.F. decline but one community hit hardest by crisis, Nov. 22
17 100 million Americans suffer with pain • The global prescription market for opioids in 2021 was greater than $22 billion 1 • For many, the treatment of pain, using currently marketed, single - receptor opioids was the root cause for their cycle of addiction DPI - 125 may also offer a safer treatment for acute and chronic pain 2 • Animal models have demonstrated DPI - 125 to be a powerful analgesic • However, mimicking the body’s natural endorphins by binding to all three receptors (delta, mu & kappa), may be a safer and less addictive option 1 Grand View Research Report ID: GVR - 2 - 68038 - 131 - 3 2 Assuming clinical trial results demonstrate safety compared to standard of care and product label reflects this positive data

18 Execute new partnership to sell ZIMHI • Focus on first responders by selling to government entities, hospitals and other institutions • Expand into Canada Fund product pipeline via non - dilutive funding (grants) • DMK previously received NIH funding, we have an active grant to explore portfolio for a novel treatment of alcohol use disorder and have just applied for a $11.7m follow up grant to further develop DPI - 125 Divest SYMJEPI • Low - cost, EpiPen - like product does not fit with current product mix but has value as intellectual property • Currently evaluating US and ex - US companies to e ither sell or out - license

Cover |
Jan. 03, 2024 |
|---|---|
| Cover [Abstract] | |
| Document Type | 8-K |
| Amendment Flag | false |
| Document Period End Date | Jan. 03, 2024 |
| Entity File Number | 0-26372 |
| Entity Registrant Name | DMK PHARMACEUTICALS CORPORATION |
| Entity Central Index Key | 0000887247 |
| Entity Tax Identification Number | 82-0429727 |
| Entity Incorporation, State or Country Code | DE |
| Entity Address, Address Line One | 11622 El Camino Real |
| Entity Address, Address Line Two | Suite 100 |
| Entity Address, City or Town | San Diego |
| Entity Address, State or Province | CA |
| Entity Address, Postal Zip Code | 92130 |
| City Area Code | (858) |
| Local Phone Number | 997-2400 |
| Written Communications | false |
| Soliciting Material | false |
| Pre-commencement Tender Offer | false |
| Pre-commencement Issuer Tender Offer | false |
| Title of 12(b) Security | Common Stock |
| Trading Symbol | DMK |
| Security Exchange Name | NASDAQ |
| Entity Emerging Growth Company | false |
1 Year DMK Pharmaceuticals Chart |
1 Month DMK Pharmaceuticals Chart |

It looks like you are not logged in. Click the button below to log in and keep track of your recent history.
Support: +44 (0) 203 8794 460 | support@advfn.com
By accessing the services available at ADVFN you are agreeing to be bound by ADVFN's Terms & Conditions